ABSTRACT
Mentalizing is the key socio-cognitive ability. Its heterogeneous structure may result from a variety of forms of mental state inference, which may be based on lower-level processing of cues encoded in the observable behavior of others, or rather involve higher-level computations aimed at understanding another person’s perspective. Here we aimed to investigate the representational content of the brain regions engaged in mentalizing. To this end, 61 healthy adults took part in an fMRI study. We explored ROI activity patterns associated with five well-recognized ToM tasks that induce either decoding of mental states from motion kinematics or belief-reasoning. By using multivariate representational similarity analysis, we examined whether these examples of lower- and higher-level forms of social inference induced common or distinct patterns of brain activity. Distinct patterns of brain activity related to decoding of mental states from motion kinematics and belief-reasoning were found in lTPJp and the left IFG, but not the rTPJp. This may indicate that rTPJp supports a general mechanism for the representation of mental states. The divergent patterns of activation in lTPJp and frontal areas likely reflect differences in the degree of involvement of cognitive functions which support the basic mentalizing processes engaged by the two task groups.
Introduction
Humans are an inherently social species. In order to navigate the social environment, we need to make sense of others’ behavior; to do so, we think of their actions in terms of mental states (e.g., beliefs or intentions). This ability is referred to as mentalizing or Theory of Mind (for consistency, we will use the former term here). Due to its importance for effective social functioning, mentalizing is seen as the core of social cognition and has been the subject of increasing scientific interest. Nevertheless, the neurocognitive mechanisms underlying this capacity are still a matter of debate (Happé et al., Citation2017; Quesque & Rossetti, Citation2020; Schaafsma et al., Citation2015; Warnell & Redcay, Citation2019).
According to the classic definitions, mentalizing encompasses the capacity to attribute mental states to conspecifics’ in order to predict their behavior (Premack & Woodruff, Citation1978). Although it is often considered as a unitary concept, the available evidence suggests that it might have a heterogeneous structure with complementary but functionally separate modules, including various forms of mental state inference (Happé et al., Citation2017; Quesque & Rossetti, Citation2020; Schaafsma et al., Citation2015; Schurz et al., Citation2014; Warnell & Redcay, Citation2019). Indeed, we mentalize on the basis of a variety of social cues. Others’ mental states might be either decoded from the low-level features of observed behavior, for example motion kinematics, or rather inferred through understanding of another person’s perspective, such as their false beliefs.
Belief-reasoning has been considered a key manifestation of mentalizing competence (Wellman, Citation2018), with false belief tasks (FBT) regarded as a litmus test (Dennett, Citation1978). In a classic FBT, participants must recognize that the protagonist of a story or a cartoon holds a false belief concerning the location of an object, taking into account that the person does not know that the object has been moved. The participant is then asked a direct question about the protagonist’s mental state and belief-based behavior (Wimmer & Perner, Citation1983). Various measures have emerged to capture the processes related to belief-reasoning (Dodell-Feder et al., Citation2011; Kovács et al., Citation2010; Wellman, Citation2018). For instance, in the non-verbal (implicit) versions of the FBT, tracking others’ mental states is spontaneous and uninstructed. According to the evidence, albeit not particularly robust, belief-tracking induced by both implicit and explicit false-belief tasks depend on the same neurocognitive basis (see, e.g., Bardi et al., Citation2017).
Decoding mental states from motion kinematics is considered a lower-level form of mentalizing (Gobbini et al., Citation2007). This has been applied in tasks that induce automatic attribution of intentions on the basis of either animated motion of geometric figures (Frith – Happé Animations Test; Abell et al., Citation2000; White et al., Citation2011) or the biological motion of actors recorded with the use of point-light displays (PLDs; Johansson, Citation1973). Such measures rely on non-complex visual input and therefore are very often used, especially in clinical groups, as efficient indicators of mental state inference (see, e.g., Centelles et al., Citation2013; Moessnang et al., Citation2020; Okruszek, Citation2018). However, the evidence from developmental studies suggests a dissociation between decoding of intentions from motion kinematics as a lower-level of reasoning and belief-reasoning as a higher-level of reasoning about others’ behavior (Quesque & Rossetti, Citation2020; Wellman, Citation2018). Already in the first year of life, long before passing verbal FBTs, infants can interpret others’ actions as intentional on the basis of observed actions (Csibra et al., Citation2003; Phillips & Wellman, Citation2005).
Indeed, understanding of motion kinematics relies on low-level processes enabling a direct interpretation of the perceptually available visuo-spatial data, i.a. recognition of a goal-directed movement characteristics (e.g., A is waving his hand in the direction of B). However, the results of studies on clinical groups suggest that success in inferring intentions from motion kinematics cannot be fully explained by the basic visuospatial processing. For instance, Okruszek et al. (Citation2015) showed that the performance in discriminating the specific intentions of the point-light agents in patients with schizophrenia was not accounted by nonsocial visuo-spatial skills. In fact, processing the motion kinematics might automatically trigger the mental state attribution (e.g., People wave their hands when they intend to say “hello” so A might intend to greet B; Centelles et al., Citation2011). In the case of belief-reasoning such an inference requires a purposeful shift to the others’ state of knowledge along with a greater involvement of working memory and executive functions, i.a. to identify and update others’ perspective in the changing reality (e.g., A is waving his hand, but B didn’t notice that; hence, B doesn’t know that A is waving; Perner & Roessler, Citation2012) or inhibit one’s own perspective (I know that A waves to say hello, but it is irrelevant to predict the behavior of B; Flynn, Citation2007).
Although they engage diverse cognitive functions, these two types of social reasoning could both involve a common process of representing the mental states of other agents in a general fashion (e.g., A intends to greet B; B believes that A hasn’t arrived yet). However, it has not been settled yet whether there exists a general neurocognitive mechanism which integrates the various forms of mental state inference.
Neuroimaging methods provide useful tools to address such issues (Happé et al., Citation2017; Schaafsma et al., Citation2015; Warnell & Redcay, Citation2019). For instance, it has been demonstrated that the bilateral temporo-parietal junction (TPJ) and the medial prefrontal cortex (mPFC), as the key nodes of the mentalizing network, are consistently engaged by different tasks that induce thinking about others’ mental states, independently of the type and format of social cues embedded in the stimuli (Gallagher & Frith, Citation2003; Molenberghs et al., Citation2016; Saxe & Kanwisher, Citation2003; Schurz et al., Citation2014). Therefore, under the assumption that common activations result from a common cognitive mechanism, the bilateral TPJ and mPFC might constitute the core of the broader mentalizing network, responsible for the general ability to represent mental states on the basis of different kinds of social information of varying complexity (Molenberghs et al., Citation2016; Schurz et al., Citation2014). However, another line of evidence suggests that various forms of mental state inference may differentially engage the mentalization network, including its core, which would rather suggest that they are linked to specific computational mechanisms (see, e.g., Schurz et al., Citation2020). Firstly, it has been shown that the roles of the left TPJ (lTPJ) and the right TPJ (rTPJ) might be dissociable (see, e.g., Dodell-Feder et al., Citation2016; Ogawa & Kameda, Citation2019). Moreover, studies that focused on the relationship between effortful higher-level and automatic lower-level social cognitive processes (exemplified by belief-reasoning and mental state decoding from motion kinematics as discussed above) have found contradictory results regarding the underlying neural mechanisms (Frith & Frith, Citation2008; Spunt & Lieberman, Citation2014; Van Overwalle & Baetens, Citation2009). Finally, the studies that have considered potential differences between various forms of mental state inference, and could thus provide insights into the underlying functional structure of the mentalizing ability, have been conducted mostly with the use of mass-univariate methods (e.g., Bardi et al., Citation2017; Gobbini et al., Citation2007; Jacoby et al., Citation2016; Thye et al., Citation2018). Such methods are the necessary first step in studying the neurocognitive mechanisms of mentalization by providing localization data. However, because of their reliance on the average magnitude of responses across multiple voxels, the univariate techniques provide limited data and therefore do not indicate how different categories of stimuli are represented in a given brain region (Haxby, Citation2012).
In order to fill this research gap, multivariate methods (Mur et al., Citation2009) are increasingly used to indicate and characterize the patterns of neural responses related to thinking about others’ cognitive and affective states (e.g., Koster-Hale et al., Citation2017; Tamir et al., Citation2016; Thornton & Mitchell, Citation2018). Nevertheless, it still remains unclear how the key mentalizing brain regions represent the information obtained through different forms of social reasoning. Better understanding of this issue could help to determine whether higher- and lower-level forms of mental state inference engage a common computational mechanism. This could be indicated by the level of similarity of multivoxel patterns of activity related to belief-reasoning and decoding intentions from motion kinematics. Hence, in the current fMRI study we aim to address this issue by combining classic whole-brain analysis and multivariate pattern analysis-representational similarity analysis (MVPA-RSA; Kriegeskorte et al., Citation2008; Popal et al., Citation2019). We compare the patterns evoked by five popular tasks which are all used as the mentalizing measures according to the literature (e.g., Fehlbaum et al., Citation2022). They, however, vary in terms of the social-cognitive processes engaged. The first three measures induced belief-reasoning of different levels of complexity, ranging from spontaneous, non-verbal belief-tracking to false-belief inference on the basis of verbal information derived from a relatively complex social context. The other two tasks engaged intention recognition on the basis of social cues coded in two types of observed actions: animated goal-directed movement and biological motion. We hypothesize that both belief-reasoning and decoding of mental states from motion kinematics rely on the common process of representing others’ mental states. Hence, we expect the multivoxel patterns of activity originating from belief and motion tasks to be similar in the structures known as the core of the mentalizing network (bilateral TPJ, mPFC). At the same time, we assume that the patterns in the remaining parietal (inferior parietal lobule), temporal (posterior middle temporal gyrus) and lateral frontal (middle temporal, anterior temporal, inferior temporal clusters) parts of the mentalizing network would be dissimilar which would reflect the functional diversity of these two forms of mental state inference (i.e., belief-reasoning and decoding of mental states from motion kinematics).
Methods
Participants
A total of 61 right handed participants with no history of neurological or psychiatric treatment were recruited to participate in the study. Four subjects did not complete the fMRI examination and were therefore excluded from the analysis. As a result, the final sample consisted of 57 participants (31 females; age: M = 27.07, SD = 7.52). All participants had normal or corrected-to-normal vision, provided written informed consent, and were financially compensated with 110 PLN (approximately 23 EUR). The experimental procedure was approved by the Ethical Committee of the Faculty of Psychology, University of Warsaw and was carried out in accordance with the Declaration of Helsinki.
Procedure
The total MRI procedure was divided into two sessions, each lasting 1 hour respectively, with a compulsory refreshment break outside the scanner (10 min). The first session comprised the resting-state fMRI sequence (15 min 18 sec; not reported in the current study), the Implicit False-Belief Task (19 min 14 sec), and the Social Perception and Interaction Task (22 min 52 sec). The second session consisted of the Explicit False-Belief Task (19 min 14 sec), HCP Social Task (6 min 52 sec), Understanding False-Belief Stories task (9 min 02 sec), T1-weighted (6 min 52 sec), T2-weighted (8 min 37 sec; not analyzed here) and diffusor tensor imaging sequences (14 min 54 sec; not analysed in the current study). Apart from the BOLD response, accuracy and response times were measured during task fMRI sequences.
Implicit false-belief task (Implicit FBT)
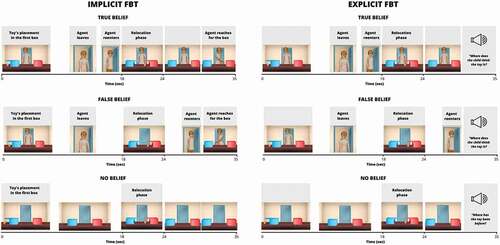
This task was designed on the basis of a classic change-of-location FBT (Wimmer & Perner, Citation1983). It should be noted that it was developed for the study of neurodevelopmental mechanisms of mentalizing, hence the child-friendly character of the stimuli. The stimuli consisted of temporally-tuned 3D animations (35 seconds each), depicting a child (four different female agents), two boxes, and four toys. After four familiarization animations, 24 animations assigned to one of three conditions (8 per condition) were presented in two runs of 12 trials each. In the False Belief (FB) condition, the agent observes the toy (moving in a self-propelled way) being placed in the first box and then, while the agent is absent, the toy moves itself to the second box (relocation phase, 18–24 sec). The agent comes back and reaches for one of the boxes in a manner consistent or inconsistent with her belief regarding the toy’s location. In the True Belief (TB) condition, the agent comes back earlier to witness the toy’s relocation, and therefore has a true belief regarding its location. In the No Belief (NB) control condition, there is no agent throughout the whole scene. The order of FB, TB, and NB trials, the side of the toy’s initial location (left or right), and the box into which the agent reaches (left or right) were pseudo-randomized. A static picture of the sun was displayed at the beginning of the run and between trials instead of a classic fixation cross (with jitter, lasting 9000–12500 ms). The task aimed to induce spontaneous tracking of mental states, so the subjects were only instructed to carefully follow what was happening on the screen.
Explicit false-belief task (Explicit FBT)
The explicit version of the change-of-location False-Belief Task was designed to be as similar as possible to the implicit version. The only difference was that instead of passively watching the story, participants were explicitly instructed to reason about the agent’s mental states and answer the previously recorded question (FB and TB: “Where does the child think the toy is?”, NB: “Where was the toy previously?”) by pressing the button corresponding to the proper box (left or right). To this end, in the explicit version the agent reaching for one of the boxes in the 30th second was removed and the trial finished with the static picture of the agent standing in the middle of the room, while participants heard the recorded question. (). The explicit FBT was always performed after the Implicit version, in a separate session.
Inferring communicative intentions from biological motion (SOPIT)
The task was based on the four types of vignettes (Interaction, Non-Interaction, Emotions, and Scrambled Motion) from the Social Perception and Interaction Database, which was designed to study higher-order processing of biological motion (Okruszek & Chrustowicz, Citation2020). During the four runs, participants were presented with 60 vignettes presented in ca. 10 second blocks of animations followed by a 3 second response screen, followed by an inter-block-interval of 8 seconds (Rest). During the tasks, the participant was asked to classify stimuli into one of four categories (Interaction/Non-Interaction/Emotions/Scrambled Motion). For the current study, the point-light vignettes depicting the use of communicative gestures between the two agents (e.g., waving to each other to say goodbye, Interaction) were contrasted with Scrambled Motion.
Inferring mental states from the social animations (HCP soc task)
The stimuli set was adapted to Polish from the social cognition task included in the fMRI battery for the Human Connectome Project (Barch et al., Citation2013; Castelli et al., Citation2000, Citation2002; White et al., Citation2011). Participants watch 10 animations (20 seconds) depicting the movement of geometric figures. The task consisted of two fMRI runs (5 animations per run). In the Mentalizing condition, the actions of the figures were goal-oriented and directed at each other, which was supposed to stimulate intention attribution processes. In the Random condition, the actions of the figures were entirely random, so that they should not induce any mental state inference. Each animation was followed by a forced-choice Question (3 seconds): the subjects were asked to indicate with a button press whether an interaction or no interaction was depicted; this was followed by a rest period (15 seconds).
Understanding false-belief stories (FB localizer)
We developed a Polish version of the False Belief (FB) Localizer by Dodell-Feder and colleagues (Dodell-Feder et al., Citation2011). Stimuli consisted of 20 short stories in two conditions (10 per condition). The stories were pseudo-randomly presented in two blocks (10 per block). In the Belief condition, the protagonists have an incorrect belief regarding the situation they were involved in. In the Photo (control) condition, an outdated photograph, map, or sign misrepresenting the current state of the world was presented. After reading each vignette (10 sec), participants assessed whether a statement related to the story was true or false (Belief question/Photo question) with a button press (4 sec), and then a fixation cross was displayed (12 sec).
fMRI data acquisition
The study took place in Bioimaging Research Center of Institute of Physiology and Pathology of Hearing in Kajetany/Warsaw, Poland. All tasks were displayed in a 3T MRI scanner using a VisualSystem HD (NordicNeurolab Inc.). MRI scanning was performed on a 3T Siemens Prisma MRI scanner equipped with a 64-channel phased-array RF head coil. Functional data for all tasks were acquired using a Multi-band (Simultaneous Multi-Slice) echo-planar-imaging (EPI) sequence (TR = 800 ms, TE = 38 ms, flip angle = 52°, FOV = 216x216 mm, 108 × 108 mm image matrix, 72 transversal slices of 2.00 mm slice thickness, voxel size of 2.0 x 2.0 x 2.0 mm, Slice Accel. Factor = 8, In-Plane Accel. Factor = 1, IPAT = 8). The following numbers of volumes were acquired for each run of the tasks: (Implicit FBT: TA = 9.34 min, 704 volumes; Explicit FBT: TA = 9.34 min, 704 volumes; SOPIT: TA = 5.43 min, 415 volumes, HCP Soc Task: TA = 3.26 min, 244 volumes; FB Localizer: TA = 4.31 min, 325 volumes). Although the Multi-band EPI sequence causes a 40% increase of the signal-to-noise ratio, it also introduces image distortions related to the accumulation of phase errors in k-space. This effect was accounted for by dividing all functional scans into even numbers of runs with the opposite phase coding direction (Anterior-Posterior and Posterior-Anterior). Structural images were collected with a T1-weighted 3D MP-Rage sequence (TR = 2400 ms, TI = 1000 ms, TE = 2.74 ms, 8° flip angle, FOV = 256×256 mm, image matrix 320 × 320 mm, voxel size of 0.80 x 0.80 x 0.80 mm, 240 slices of 0.80 mm slice thickness, TA = 6.52 min).
fMRI data preprocessing
Preprocessing of the fMRI data was carried out with the use of SPM12 (SPM; WellcomeTrust Centre for Neuroimaging, London, UK) and in-house code. The FSL topup() tool (Andersson et al., Citation2003) based on the average representation of the images in both directions (Anterior-Posterior and Posterior-Anterior) was used to correct the spatial distortions. The functional data were spatially realigned to the mean image and co-registered to the individual structural images. High-resolution structural images were segmented and normalized to the common MNI space with resampling to 1 mm isometric voxels. The obtained transformation parameters were applied to the functional volumes with resampling to 2 mm isometric voxels. Spatial smoothing with a Gaussian kernel of full-width half-maximum (FWHM) of 6 mm was performed on the normalised functional images. Additional high-pass filters were used for the functional data: Explicit FBT, Implicit FBT and SOPIT (cutoff: 256 seconds), HCP Soc Task (cutoff: 512 seconds), and FB Localizer (cutoff: 128 seconds).
fMRI data analysis
Whole-brain analysis
The whole-brain analyses were performed using SPM12 and the general linear model (GLM) approach. First-level models were created subject-wise for each task. For Implicit and Explicit FBTs, the entire timeline of the story was divided into consecutive events within each condition (see ). The toy’s relocation in the FB and TB conditions was assumed to overlap with the phase of belief attribution to the agent (belief formation phase; see Kovács et al., Citation2010). In the No Belief (NB) control condition, there was no agent during the whole scene as this condition should not evoke belief attribution. Thus, although all events were added to the GLM models as regressors, the change-of-location (6 sec) corresponding to the belief formation phase was used as the time window of interest in further analysis. For the other tasks, the stimuli were also divided into phases (SOPIT: Interaction/No-interaction/Emotions/Scrambled motion/Rest; HCP Soc Task: Mentalizing/Random/Question; FB Localizer: Belief/Photo/Belief question/Photo question) which were modeled as regressors and convolved with the canonical HRF. Furthermore, six subject-specific movement regressors calculated during the realignment step of preprocessing were added per run to account for head motion. Second-level random effects analysis was performed separately for each task on the key contrast images generated from the first-level models: SOPIT: Interaction > Scrambled Motion; HCP Soc Task: Mentalizing > Random; FB Localizer: Belief > Photo). FB-change-of-location > NB-change-of-location was the contrast of interest for the Implicit and Explicit FBT. The rationale for this choice was as follows: 1) These two conditions do involve tracking the sequence of changing states of reality, but in the case of NB, this does not require the representation of mental states. Therefore, this should result in different activation patterns (FB should activate the mentalization network, NB does not); 2) FB-change-of-location and NB-change-of-location are perceptually identical (the agent is absent) in the belief formation phase which is not the case for the TB condition (the agent is present); 3) using the FB-change-of-location > NB-change-of-location contrast makes the analysis comparable to the FB Localizer Task, in which the condition requiring false belief understanding (Belief) is contrasted with the stimuli that engage reasoning about the content of a nonsocial representation of reality (Photo; Saxe, Citation2006). The results for FB-change-of-location > TB-change-of-location and TB-change-of-location > NB-change-of-location are presented in the Supplementary Materials (Table S4).
We report the whole-brain analysis results above which survived the family-wise error correction threshold with cluster extent-based correction (FWEc) at the p < .05 level of significance (cluster-forming threshold p < .001). The Bspmview toolbox, based on the Anatomy Toolbox, was used for automated anatomical labeling of the results (http://www.bobspunt.com/bspmview/).
Representational similarity analysis
This method is based on the assumption that stimuli evoking similar neural processes will produce similar voxel activation patterns (Kriegeskorte et al., Citation2008). We applied RSA to compare brain representations of different types of mental state inference engaged by 2 groups of tasks inducing either belief-reasoning (Implicit and Explicit FBT, FB Localizer) or decoding of mental states from motion kinematics (HCP Soc Task, SOPIT).
We aimed to explore how these types of reasoning are represented in structures that previous literature has reported as being the key mentalizing regions. Accordingly, the analyses were performed on 18 a priori Regions of Interest (ROIs) created as spheres (8 mm radius) centered around the peak coordinates reported by Schurz et al. in a meta-analysis of functional neuroimaging studies on mentalizing (Schurz et al., Citation2014). If the spheres with coordinates based on Schurtz (Schurz et al., Citation2014) did not fit precisely into the gray matter, they were slightly shifted, which resulted in the following ROIs (). The coordinates are presented in in Supplementary Materials.
Figure 2. The localization of a priori ROIs (L – left, R – right): inferior parietal lobule (IPL), posterior temporoparietal junction (TPJp), anterior temporoparietal junction (TPJa), posterior middle temporal gyrus (pMTG), middle temporal cluster (MiddTemp), anterior temporal cluster (AntTemp), inferior frontal cluster (InfFront), ventral medial prefrontal cortex (vmPFC), dorsal medial prefrontal cortex (dmPFC), pre-supplementary motor area (preSMA), precuneus.
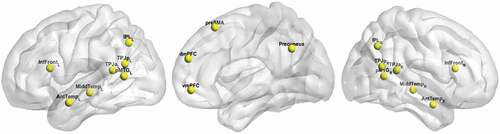
Table 1. Summary of the behavioral results.
We followed the workflow implemented in the RSA toolbox (Nili et al., Citation2014). The activity patterns representing different forms of mental state reasoning were obtained from the corresponding first-level contrast maps (see Whole-brain analysis). In this respect, for each task, the differences of beta estimates of conditions of interest were extracted from each voxel in the ROIs. Such task-related activities, otherwise called patterns, were then correlated pairwise. This procedure was repeated for all ROIs. As a result, for each subject we acquired eighteen 5 × 5 representational similarity matrices (RSMs) containing values of Spearman’s correlation coefficients between pairs of tasks. As the RSMs are symmetrical about a diagonal of ones, for the purpose of further analysis they were transformed into the lower-triangular vector format containing only the off-diagonal cells with unique pairwise similarities (see Diedrichsen & Kriegeskorte, Citation2017).
In the next step, a model RSM was specified to contrast Implicit FBT, Explicit FBT, and FB Localizer with SOPIT and HCP Soc Task (). Next, the relatedness of individual vectorized RDMs to the model was tested for each region of interest with the use of a one-sided signed-rank test across the single-subject RDM to model Spearman correlations. We report only the results which survived Bonferroni correction for multiple comparisons (p < .05; ).
To test the performance of the model for each ROI, we also computed the upper and lower bounds of the noise ceiling as implemented in the RSA toolbox (Nili et al., Citation2014). Any model that exceeded the lower bounds of the noise ceiling and had significant RDM-model correlation was considered as a reliable predictor of a representation in a given ROI.
The supplementary statistical analysis and RSA graphs were prepared using R studio (Citation2015).
Results
Behavioral results
The analysis of accuracy and reaction times suggests that, in general, participants performed well and paid attention to tasks during the fMRI examination ().
Whole-brain results
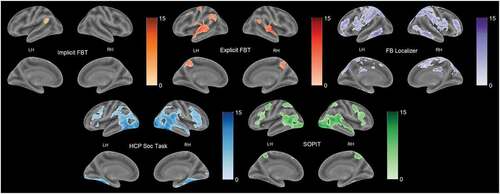
The exploratory whole-brain analysis revealed both common and differential activation patterns evoked by the tasks (). Patterns of activations for all tasks overlapped in the temporo-parietal areas bilaterally, apart from the one related to the Implicit FBT, which occurred mainly in the left hemisphere. Implicit FBT yielded activation in the bilateral frontal, left middle temporal, and left posterior superior regions. For the Explicit FBT, peaks were observed in the posterior superior and the middle temporal areas bilaterally, the temporal poles, and the precuneus. The overlap between Explicit and Implicit FBT observed only in the left temporal cortex was surprisingly small relative to what could have been expected to be revealed with the use of almost identical stimuli. Both the SOPIT and HCP Soc Task yielded similar activations in the right temporal areas along the superior temporal sulcus, and in the lateral frontal gyri. SOPIT also evoked substantial responses in the middle cingulate cortex and occipital and parietal areas. Peaks for the HCP Soc Task were found in the insula, thalamus, and cerebellum. The precentral areas were activated by the HCP Soc Task and the Explicit FBT. For the FB Localizer, large clusters were located in the precuneus, the bilateral middle temporal and the right superior temporal areas, extending to the temporal poles, similarly to the explicit FBT patterns. FB Localizer also revealed broad activation of medial prefrontal areas. Additionally, for this task large clusters of activation were observed in the lingual gyrus. Peaks related to the FB Localizer were also found in the parahippocampal gyrus and cerebellum. Finally, an overlap analysis indicated only one common cluster for all the main contrasts localized in the left middle temporal region. Tables S2 and S3 in the Supplementary Materials show all local maxima separated by more than 20 mm.
Figure 3. Results of whole-brain analysis. The figure presents active areas for the following contrasts: implicit and explicit FBT: FB-change-of-location > NB-change-of-location; SOPIT: Interaction > scrambled motion; HCP soc task > mentalizing > random; FB localizer: belief > photo. FWEc correction at the p < .05 level of significance, cluster-forming threshold p < .001.
Representational similarity analysis results
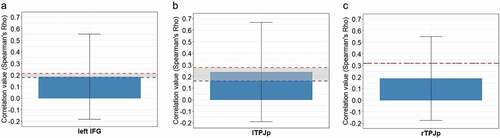
Visual inspection of the average RSMs per ROI revealed the structure of representations in the left IFG and lTPJp to be similar to the model, which assumed that the mentalizing regions represent others’ internal states differentially, depending on the form of mentalizing engaged by the task (). The model performed above chance in three regions: lTPJp (Spearman’s Rho =.25, p = .0018), rTPJp (Spearman’s Rho =.19, p = .0039) and left IFG (Spearman’s Rho =.216, p = .0072). For the results of the remaining ROIs, see Supplementary Materials, . The correlation values were in the range of the noise ceiling for the left IFG () and the lTPJp (). This means that these activity patterns can be explained well by our model and suggests that the mental states originating from belief reasoning and decoding of mental states from motion kinematics are represented distinctively in these regions. In the case of rTPJp, the correlation value did not reach the limits of the noise ceiling, suggesting that the representation in this ROI does not follow the gradient hypothesized by the model ().
Figure 4. The model and the average representational similarity matrices (RSMs) for ROIs. Panel a depicts a planned model of similarity of neural response patterns between the two groups of tasks (Implicite FBT, explicite FBT and FB localizer are grouped as belief-reasoning tasks whereas SOPIT and HPC soc task as decoding of intentions from motion kinematics). Panel B shows the average representational similarity matrices (RSMs) for the ROIs in which the model was significantly correlated with the activity patterns (Bonferroni corrected, p < .05). Scale represents degree of similarity between categories.
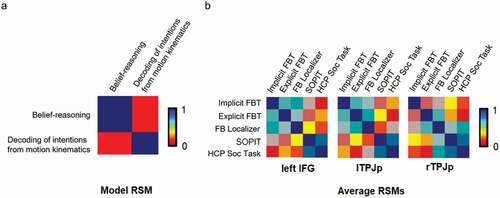
Figure 5. (a-c). Significant correlations between the model and activity patterns in the ROIs (Bonferroni corrected, p < .05). The red and blue dashed lines illustrate upper and lower bounds of the noise ceiling respectively.
In order to confirm that the results described above originated from the actual activity, we conducted additional confirmatory ROI analysis and tested whether the mean beta values for the contrasts of interest are significantly different from zero. The analysis revealed that for each ROI, at least 4 out of five tasks elicited significant responses. All results of this analysis are summarized in the Supplementary Materials.
Discussion
In this work we aimed to broaden the understanding of the functional architecture of the ability to mentalize by investigating the representational content of the key nodes of the mentalizing network. By conducting representational similarity analysis (RSA) we complement the results obtained with classical methods on the whole-brain level. For the majority (4 out of 5) of tasks we observed similar whole-brain patterns of response in bilateral temporo-parietal areas. However, as a more sensitive method the RSA revealed that, the multivoxel patterns of activity originating from belief and action tasks were similar in the right posterior temporo-parietal junction (rTPJp), but not in the left posterior temporo-parietal junction (lTPJp). This is in line with our hypothesis that the rTPJp supports a common mechanism of representing the mental states (Molenberghs et al., Citation2016; Schurz et al., Citation2014). However, this would also imply the division of roles between lTPJp and rTPJp and suggest that even the core of the mentalizing network might be functionally heterogeneous. What is more, while the patterns of response in the lateral frontal gyri occurred specifically for the motion kinematics tasks, substantial clusters of activation in the medial-prefrontal cortical areas (mPFC) were present on the whole-brain level only for the belief-reasoning tasks (False Belief Localizer and the Implicite FBT). Accordingly, differential patterns of activity suggestive of the separate cognitive mechanisms related to the two groups of tasks were revealed by the RSA in left inferior frontal gyrus (left IFG), but not in the mPFC. Overall, our results add to previous literature on the functional organization of the mentalizing network.
The RSA shows that the rTPJp appears to be equally sensitive to social cues derived from actions (e.g., communicative intentions encoded in motion) and reasoned based on understanding the other person’s perspective (e.g., false beliefs). In line with what has been proposed in a vast majority of previous studies on the neurocognitive mechanisms of mentalizing, this suggests that the rTPJp represents others’ internal states irrespective of what is the format and complexity of source social information (Saxe & Kanwisher, Citation2003). Therefore it supports a general mechanism of mentalizing. This would conform to the notion of rTPJp as a key node of the social brain (Santiesteban et al., Citation2012). Our data are also consistent with the nexus model proposed by Carter and Huettel (Citation2013) that assumes that the rTPJp receives and integrates abstract forms of social information derived from different regions of the social brain, such as the lTPJp. In this way, it might establish the representation of social context and thus enable successful navigation in the social world (Schuwerk et al., Citation2014; see also: Schuwerk et al., Citation2017). This result should be interpreted with caution, as our whole-brain and confirmatory ROI analyses found that only 4 out of 5 tasks (excluding the Implicit FBT) evoked significant activation in this region. However, we assume that representational similarity analysis is more sensitive to how the information is represented. For instance, a region responding more strongly to one category might also have some sensitivity to information from another category (Haxby, Citation2012; Popal et al., Citation2019).
The lTPJp has been considered a part of the core mentalizing network, alongside the rTPJp, and thus should support the general ability to reason about the mental states (intentions, desires, beliefs), irrespective of the format and complexity of the source of the social information. Nevertheless, its exact role in mentalizing has remained unclear (Molenberghs et al., Citation2016; Schurz et al., Citation2014). Our data provide new insights into the function of the lTPJp in the mentalizing network as we show that it differentiates between mental state computations derived from belief-reasoning and decoded from motion kinematics. Previous studies have reported that focal lesions in the area of the lTPJp are related to selective deficits in mentalizing, suggesting that the role of the lTPJp in mental state inference is more than subsidiary and, in fact, that it might be necessary for successful perspective taking (Biervoye et al., Citation2016; Samson et al., Citation2004). Based on an analysis of patterns of errors made by patients with lesions, Biervoye et al. speculated that the lTPJp might trigger automatic bottom-up signaling of the detection of others’ perspective and thus induce mental state inference (Biervoye et al., Citation2016). This would be supported by the fact that we observed an overlap in the lTPJp on the whole-brain level for all tasks despite differences in stimulus complexity and perceptual features. Accordingly, based on analysis of event-related potentials (McCleery et al., Citation2011) as well as dynamic causal modeling (Schuwerk et al., Citation2014), it has been proposed that the lTPJ not only signals the mere presence of others’ perspectives, but more precisely represents the discrepancy between one’s own and others’ perspectives (see also: Arora et al., Citation2015; Perner et al., Citation2006; Schurz et al., Citation2013). Our RSA results suggest that such representations are not general but they rather depend on the form of mentalizing. Whilst others’ intentions are inherent to their behavior in tasks requiring mental state inference from motion kinematics (e.g., A intends to greet B) and so must be consistent with the observer’s perspective, a significant discrepancy can occur in the case of belief reasoning when the protagonist’s perspective (e.g., A believes that the toy is in box X) does not conform to reality (the toy is in box Y) and to the observer’s knowledge (I know that the toy is in box Y). Thus one might hypothesize that the differential patterns of activity in the lTPJ might reflect the level of incompatibility between one’s own and others’ perspectives, which would be low in the case of action observation tasks and high for belief-reasoning tasks. Such nuanced information could be then elaborated by other regions of the mentalizing network, especially rTPJp, and eventually used for constructing an up-to-date model of the other minds.
We also demonstrated that the representations of the internal states resulting from different forms of social inference are distinctive in the left IFG. The IFG is regarded as important for processing the meaning of the basic social cues encoded in observed behavior as a key part of an action observation network (AON) along with the inferior parietal lobule and superior temporal sulcus (Caspers et al., Citation2010; Press et al., Citation2012). This could mean that the lateral frontal patterns of response that we found on the whole brain level specifically for the decoding of mental states from motion kinematics might reflect encoding of the semantic features of others’ behavior (Press et al., Citation2012). In this context, it seems plausible that the representations of the mental states in the left IFG derived from motion kinematics and belief reasoning differ in complexity and thus might originate from separate computational mechanisms. In the case of false belief tasks, the semantic features of behavior (A wants to use an object B) additionally have to be considered in a wider context of changing circumstances (The object B has changed its location in the absence of A), in which the meaning of behavior needs to be updated (A will look for the object B in a wrong location). For motion kinematics tasks, the semantic features are inherent to perceived actions and are therefore directly available. Interestingly, the AON has been also described as the mirror network as it responds when an individual completes an action or when they observe another complete that action (Rizzolatti & Craighero, Citation2004). Thus the left IFG might be involved in the sensorimotor loop that takes part in the preparation for the execution of an equivalent motor response based on visuospatial information about the movements of others (Molenberghs et al., Citation2012). Such visuo-spatial cues are more crucial for recognizing others’ mental states in the motion kinematics than in false belief tasks (Koul et al., Citation2018). In contrast, a successful performance in the later requires greater executive and memory control which are supported i.a. by the mPFC (Friedman & Robbins, Citation2022). Thus, the differential activation patterns observed for the two groups of tasks in the frontal area might reflect the differences in the level of complexity of cognitive functions supporting the general mentalizing processes which, in the light of our whole-brain and RSA results, are probably subserved by the rTPJp. In fact, in accordance with what has been suggested by Centelles et al. (Citation2011), we demonstrate that the mirror and mentalizing systems can be simultaneously active during motion kinematics tasks, with the former possibly aiding the latter in structuring the representations of others’ intentions. In general, our data add to the existing knowledge on the role of frontal cortices in mentalizing.
The medial prefrontal cortex (mPFC) has been considered to be responsible for higher-order, stimuli-independent computations, as a part of the core mentalizing network besides the rTPJp (Gallagher & Frith, Citation2003; Schurz et al., Citation2014; Schuwerk et al., Citation2014). In line with this hypothesis, we found that the activity patterns within medial prefrontal ROIs were not explained by our theoretical model, which assumed distinct representations for belief-reasoning and decoding of mental states from motion kinematics. However, our whole-brain data do not support this idea, as we did not register any pattern in the medial-prefrontal areas common to belief-reasoning and decoding of mental states from motion kinematics tasks, despite them both requiring mental state ascription. The multi-study investigation of Boccadoro et al. also did not find evidence for the activity of mPFC during mental state inference which might suggest that mPFC is not involved in the general process of mental state representation (Boccadoro et al., Citation2019). Others observed the mPFC activity only in the outcome phase of mentalizing tasks (Bardi et al., Citation2017). It has been hypothesized that the mPFC might be particularly engaged in self-referential processing of social cues and, as discussed above, such processes are not required to the same extent by different forms of mental state reasoning. Thus we argue that more research is needed to reveal whether and how the mPFC plays a role in mentalizing, as it might not be specific to reasoning about internal states.
It seems important to point out that although we selected five different social cognitive tasks for our study, we were still unable to consider mentalizing in all its complexity. Other forms of mental state inference might be engaged in tasks requiring, inter alia, reading the mind in the eyes, trait judgments, strategic games, rational actions, and moral reasoning (Schurz et al., Citation2020). This might be the reason that our model only explained the activity patterns in two out of eighteen regions highlighted by the previous neuroimaging studies as the key nodes of the social brain engaged in mentalizing (Schurz et al., Citation2014). The remaining structures might be sensitive to aspects of the mentalizing cues not considered in the current paper; this can be addressed by future studies. However, we believe that by focusing on the discrepancies between belief-reasoning and decoding of mental states from action kinematics, we were able to look into the differences between high and low-level constituent processes of the mentalizing ability and therefore explore the functional organization of the mentalizing network.
Limitations
A potential limitation of this study might be a fixed order of the tasks, especially considering relatively long scanning time. However, a post-hoc comparison of the average accuracy levels has shown that the scanning time did not significantly affect subjects’ performance in the second part of the experiment as overall there was no significant downward trend in accuracy (see Supplementary Materials). The differences between tasks might rather result from varied levels of difficulty, with the FB Localizer as the most demanding one. Additionally, the accuracy levels were well above chance (min. 82.6% for the FB Localizer task) confirming that the subjects were engaged in tasks till the end of the experiment.
Moreover, in the current study we used canonical tasks, widely used in research on mentalizing, albeit based on relatively complex stimuli and a limited number of items per stimulus category. This results in certain constraints being placed on the use of the full potential of representational similarity analysis (see for example: Dimsdale-Zucker & Ranganath, Citation2019). Our results should be the starting point for future studies that could benefit from unifying the methodology and analyzing the representational similarity of various forms of mental state inference at the level of single items.
Conclusions
Our study informs the ongoing debate concerning the neurocognitive basis of mental state inference and provides new insights into the representational content of the mentalizing network. We provide evidence that the structure of such representations in lTPJ and left IFG depends on the form of mental state inference, as exemplified by the patterns related to belief-reasoning and decoding of intentions from motion kinematics, while the rTPJp seems to support a general mechanism of representing the mental states. In the light of these results, we propose that the rTPJp subserves the functional core of mentalizing. With the involvement of low (e.g., visuo-spatial processing, mirror system) and high-level (e.g., working memory, executive functions) cognitive functions in varying proportions, depending on the type of available social cues, it creates a high-level stimulus-independent representations of social context, in which others’ mental states are the important constituents.
SNS-RP_30.22_Supplementary_Material_Clean.docx
Download MS Word (64.8 KB)Acknowledgements
We would like to thank all the participants who agreed to take part in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated for this study are available upon request from the corresponding author.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17470919.2022.2138536.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abell, F., Happé, F., & Frith, U. (2000). Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cognitive Development, 15(1), 1–16.
- Andersson, J. L. R., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage, 20(2), 870–888. https://doi.org/10.1016/S1053-8119(03)00336-7
- Arora, A., Weiss, B., Schurz, M., Aichhorn, M., Wieshofer, R. C., & Perner, J. (2015). Left inferior-parietal lobe activity in perspective tasks: Identity statements. Frontiers in Human Neuroscience, 9, 360.
- Barch, D. M., Burgess, G. C., Harms, M. P., Petersen, S. E., Schlaggar, B. L., Corbetta, M., Glasser, M. F., Curtiss, S., Dixit, S., Feldt, C., Nolan, D., Bryant, E., Hartley, T., Footer, O., Bjork, J. M., Poldrack, R., Smith, S., Johansen-Berg, H., Snyder, A. Z., & WU-Minn HCP Consortium. (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage, 80, 169–189.
- Bardi, L., Desmet, C., Nijhof, A., Wiersema, J. R., & Brass, M. (2017). Brain activation for spontaneous and explicit false belief tasks overlaps: New fMRI evidence on belief processing and violation of expectation. Social Cognitive and Affective Neuroscience, 12(3), 391–400.
- Biervoye, A., Dricot, L., Ivanoiu, A., & Samson, D. (2016). Impaired spontaneous belief inference following acquired damage to the left posterior temporoparietal junction. Social Cognitive and Affective Neuroscience, 11(10), 1513–1520.
- Boccadoro, S., Cracco, E., Hudson, A. R., Bardi, L., Nijhof, A. D., Wiersema, J. R., Brass, M., & Mueller, S. C. (2019). Defining the neural correlates of spontaneous theory of mind (ToM): An fMRI multi-study investigation. Neuroimage, 203, 116193.
- Carter, R. M., & Huettel, S. A. (2013). A nexus model of the temporal-parietal junction. Trends in Cognitive Sciences, 17(7), 328–336.
- Caspers, S., Zilles, K., Laird, A. R., & Eickhoff, S. B. (2010). ALE meta-analysis of action observation and imitation in the human brain. Neuroimage, 50(3), 1148–1167.
- Castelli, F., Frith, C., Happé, F., & Frith, U. (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain: A Journal of Neurology, 125(Pt 8), 1839–1849.
- Castelli, F., Happé, F., Frith, U., & Frith, C. (2000). Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage, 12(3), 314–325.
- Centelles, L., Assaiante, C., Etchegoyhen, K., Bouvard, M., & Schmitz, C. (2013). From action to interaction: Exploring the contribution of body motion cues to social understanding in typical development and in autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(5), 1140–1150.
- Centelles, L., Assaiante, C., Nazarian, B., Anton, J.-L., & Schmitz, C. (2011). Recruitment of both the mirror and the mentalizing networks when observing social interactions depicted by point-lights: A neuroimaging study. PLoS One, 6(1), e15749.
- Csibra, G., Bíró, S., Koós, O., & Gergely, G. (2003). One-year-old infants use teleological representations of actions productively. Cognitive Science, 27(1), 111–133.
- Dennett, D. (1978). Beliefs about beliefs [P&W, SR&B]. The Behavioral and Brain Sciences, 1(4), 568–570.
- Diedrichsen, J., & Kriegeskorte, N. (2017). Representational models: A common framework for understanding encoding, pattern-component, and representational-similarity analysis. PLoS Computational Biology, 13(4), e1005508.
- Dimsdale-Zucker, H. R., & Ranganath, C. (2019). Representational similarity analyses. In Manahan-Vaughan, Denise (Ed), Handbook of in vivo neural plasticity techniques (Vol. 28, pp. 509–525). Elsevier.
- Dodell-Feder, D., Felix, S., Yung, M. G., & Hooker, C. I. (2016). Theory-of-mind-related neural activity for one’s romantic partner predicts partner well-being. Social Cognitive and Affective Neuroscience, 11(4), 593–603.
- Dodell-Feder, D., Koster-Hale, J., Bedny, M., & Saxe, R. (2011). fMRI item analysis in a theory of mind task. Neuroimage, 55(2), 705–712.
- Fehlbaum, L. V., Borbás, R., Paul, K., Eickhoff, S. B., & Raschle, N. M. (2022). Early and late neural correlates of mentalizing: ALE meta-analyses in adults, children and adolescents. Social Cognitive and Affective Neuroscience, 17(4), 351–366.
- Flynn, E. (2007). The role of inhibitory control in false belief understanding. Infant and Child Development, 16(1), 53–69.
- Friedman, N. P., & Robbins, T. W. (2022). The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology, 47(1), 72–89.
- Frith, C. D., & Frith, U. (2008). Implicit and explicit processes in social cognition. Neuron, 60(3), 503–510.
- Gallagher, H. L., & Frith, C. D. (2003). Functional imaging of “theory of mind. Trends in Cognitive Sciences, 7(2), 77–83.
- Gobbini, M. I., Koralek, A. C., Bryan, R. E., Montgomery, K. J., & Haxby, J. V. (2007). Two takes on the social brain: A comparison of theory of mind tasks. Journal of Cognitive Neuroscience, 19(11), 1803–1814.
- Happé, F., Cook, J. L., & Bird, G. (2017). The structure of social cognition: In(ter)dependence of sociocognitive processes. Annual Review of Psychology, 68, 243–267.
- Haxby, J. V. (2012). Multivariate pattern analysis of fMRI: The early beginnings. Neuroimage, 62(2), 852–855.
- Jacoby, N., Bruneau, E., Koster-Hale, J., & Saxe, R. (2016). Localizing pain matrix and theory of mind networks with both verbal and non-verbal stimuli. Neuroimage, 126, 39–48.
- Johansson, G. (1973). Visual perception of biological motion and a model for its analysis. Perception & Psychophysics, 14(2), 201–211.
- Koster-Hale, J., Richardson, H., Velez, N., Asaba, M., Young, L., & Saxe, R. (2017). Mentalizing regions represent distributed, continuous, and abstract dimensions of others’ beliefs. Neuroimage, 161, 9–18.
- Koul, A., Cavallo, A., Cauda, F., Costa, T., Diano, M., Pontil, M., & Becchio, C. (2018). Action observation areas represent intentions from subtle kinematic features. Cerebral Cortex, 28(7), 2647–2654.
- Kovács, Á. M., Téglás, E., & Endress, A. D. (2010). The social sense: Susceptibility to others’ beliefs in human infants and adults. Science, 330(6012), 1830–1834.
- Kriegeskorte, N., Mur, M., & Bandettini, P. (2008). Representational similarity analysis - connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience, 2, 4.
- McCleery, J. P., Surtees, A. D. R., Graham, K. A., Richards, J. E., & Apperly, I. A. (2011). The neural and cognitive time course of theory of mind. The Journal of Neuroscience, 31(36), 12849–12854.
- Moessnang, C., Baumeister, S., Tillmann, J., Goyard, D., Charman, T., Ambrosino, S., Baron-Cohen, S., Beckmann, C., Bölte, S., Bours, C., Crawley, D., Dell’Acqua, F., Durston, S., Ecker, C., Frouin, V., Hayward, H., Holt, R., Johnson, M., Jones, E., & EU-AIMS LEAP group. (2020). Social brain activation during mentalizing in a large autism cohort: The longitudinal European Autism project. Molecular Autism, 11(1), 17.
- Molenberghs, P., Hayward, L., Mattingley, J. B., & Cunnington, R. (2012). Activation patterns during action observation are modulated by context in mirror system areas. Neuroimage, 59(1), 608–615.
- Molenberghs, P., Johnson, H., Henry, J. D., & Mattingley, J. B. (2016). Understanding the minds of others: A neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 65, 276–291.
- Mur, M., Bandettini, P. A., & Kriegeskorte, N. (2009). Revealing representational content with pattern-information fMri–an introductory guide. Social Cognitive and Affective Neuroscience, 4(1), 101–109.
- Nili, H., Wingfield, C., Walther, A., Su, L., Marslen-Wilson, W., & Kriegeskorte, N. (2014). A toolbox for representational similarity analysis. PLoS Computational Biology, 10(4), e1003553.
- Ogawa, A., & Kameda, T. (2019). Dissociable roles of left and right temporoparietal junction in strategic competitive interaction. Social Cognitive and Affective Neuroscience, 14(10), 1037–1048.
- Okruszek, Ł. (2018). It is not just in faces! Processing of emotion and intention from biological motion in psychiatric disorders. Frontiers in Human Neuroscience, 12, 48.
- Okruszek, Ł., & Chrustowicz, M. (2020). Social perception and interaction database-A novel tool to study social cognitive processes with point-light displays. Frontiers in Psychiatry, 11, 123.
- Okruszek, Ł., Haman, M., Kalinowski, K., Talarowska, M., Becchio, C., & Manera, V. (2015). Impaired recognition of communicative interactions from biological motion in schizophrenia. PLoS One, 10(2), e0116793.
- Perner, J., Aichhorn, M., Kronbichler, M., Staffen, W., & Ladurner, G. (2006). Thinking of mental and other representations: The roles of left and right temporo-parietal junction. Social Neuroscience, 1(3–4), 245–258.
- Perner, J., & Roessler, J. (2012). From infants’ to children’s appreciation of belief. Trends in Cognitive Sciences, 16(10), 519–525.
- Phillips, A. T., & Wellman, H. M. (2005). Infants’ understanding of object-directed action. Cognition, 98(2), 137–155.
- Popal, H., Wang, Y., & Olson, I. R. (2019). A guide to representational similarity analysis for social neuroscience. Social Cognitive and Affective Neuroscience, 14(11), 1243–1253.
- Premack, D., & Woodruff, G. (1978). Does the chimpanzee have a theory of mind? The Behavioral and Brain Sciences, 1(4), 515–526.
- Press, C., Weiskopf, N., & Kilner, J. M. (2012). Dissociable roles of human inferior frontal gyrus during action execution and observation. Neuroimage, 60(3), 1671–1677.
- Quesque, F., & Rossetti, Y. (2020). What do theory-of-mind tasks actually measure? Theory and practice. Perspectives on Psychological Science, 15(2), 384–396.
- Rizzolatti, G., & Craighero, L. (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192.
- RStudio Team. (2015). RStudio: Integrated development environment for R. http://www.rstudio.com/
- Samson, D., Apperly, I. A., Chiavarino, C., & Humphreys, G. W. (2004). Left temporoparietal junction is necessary for representing someone else’s belief. Nature Neuroscience, 7(5), 499–500.
- Santiesteban, I., Banissy, M. J., Catmur, C., & Bird, G. (2012). Enhancing social ability by stimulating right temporoparietal junction. Current Biology, 22(23), 2274–2277.
- Saxe, R. (2006). Why and how to study theory of mind with fMRI. Brain Research, 1079(1), 57–65.
- Saxe, R., & Kanwisher, N. (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage, 19(4), 1835–1842.
- Schaafsma, S. M., Pfaff, D. W., Spunt, R. P., & Adolphs, R. (2015). Deconstructing and reconstructing theory of mind. Trends in Cognitive Sciences, 19(2), 65–72.
- Schurz, M., Aichhorn, M., Martin, A., & Perner, J. (2013). Common brain areas engaged in false belief reasoning and visual perspective taking: A meta-analysis of functional brain imaging studies. Frontiers in Human Neuroscience, 7, 712.
- Schurz, M., Maliske, L., & Kanske, P. (2020). Cross-network interactions in social cognition: A review of findings on task related brain activation and connectivity. Cortex, 130, 142–157.
- Schurz, M., Radua, J., Aichhorn, M., Richlan, F., & Perner, J. (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34.
- Schuwerk, T., Döhnel, K., Sodian, B., Keck, I. R., Rupprecht, R., & Sommer, M. (2014). Functional activity and effective connectivity of the posterior medial prefrontal cortex during processing of incongruent mental states. Human Brain Mapping, 35(7), 2950–2965.
- Schuwerk, T., Schurz, M., Müller, F., Rupprecht, R., & Sommer, M. (2017). The rTpj’s overarching cognitive function in networks for attention and theory of mind. Social Cognitive and Affective Neuroscience, 12(1), 157–168.
- Spunt, R. P., & Lieberman, M. D. (2014). Automaticity, control, and the social brain. In J. W. Sherman, B. Gawronski, & Y. Trope (Eds.), Dual-process theories of the social mind (pp. 279–296). The Guilford Press.
- Tamir, D. I., Thornton, M. A., Contreras, J. M., & Mitchell, J. P. (2016). Neural evidence that three dimensions organize mental state representation: Rationality, social impact, and valence. Proceedings of the National Academy of Sciences of the United States of America, 113(1), 194–199.
- Thornton, M. A., & Mitchell, J. P. (2018). Theories of person perception predict patterns of neural activity during mentalizing. Cerebral Cortex, 28(10), 3505–3520.
- Thye, M. D., Ammons, C. J., Murdaugh, D. L., & Kana, R. K. (2018). Differential recruitment of theory of mind brain network across three tasks: An independent component analysis. Behavioural Brain Research, 347, 385–393.
- Van Overwalle, F., & Baetens, K. (2009). Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage, 48(3), 564–584.
- Warnell, K. R., & Redcay, E. (2019). Minimal coherence among varied theory of mind measures in childhood and adulthood. Cognition, 191, 103997.
- Wellman, H. M. (2018). Theory of mind: The state of the art. The European Journal of Developmental Psychology, 15(6), 728–755.
- White, S. J., Coniston, D., Rogers, R., & Frith, U. (2011). Developing the Frith-Happé animations: A quick and objective test of theory of mind for adults with autism. Autism Research : Official Journal of the International Society for Autism Research, 4(2), 149–154.
- Wimmer, H., & Perner, J. (1983). Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition, 13(1), 103–128.