ABSTRACT
Background
Functional abdominal pain disorders (FAPDs) are common among children and are associated with decreased quality of life and school attendance. Several dietary interventions have been suggested to improve symptoms of FAPDs. This systematic review assessed the efficacy and safety of dietary interventions for pediatric FAPDs.
Design and methods
Electronic databases were searched (inception–October 2021). Systematic reviews or RCTs were included if children (4–18 years) with FAPDs were treated with dietary interventions and compared to placebo, no diet or any other diet. Data extraction and assessment of quality of evidence based on GRADE system was independently performed by two review authors. Outcomes were treatment success, pain intensity and frequency, and withdrawal due to adverse events.
Results
Twelve articles were included, representing data of 819 pediatric FAPD patients. Trials investigating fibers, FODMAP diet, fructans, fructose-restricted diet, prebiotic (inulin), serum-derived bovine immunoglobulin, and vitamin D supplementation were included. We found very low-certainty evidence that the use of fibers leads to higher treatment success (NNT = 5).
Conclusion
Based on current evidence, the use of fibers can be discussed in daily practice. High-quality intervention trials are highly needed to investigate if other dietary interventions are effective in the treatment of pediatric FAPD.
KEYWORDS:
1. Background
Functional gastrointestinal disorders (FGIDs) are common among children and adolescents and are associated with impaired quality of life, functional disability, high rates of school absenteeism, and substantial increases in health-care costs [Citation1–7]. A subset of FGIDs are functional abdominal pain disorders (FAPDs) and include functional dyspepsia (FD), irritable bowel syndrome (IBS), abdominal migraine (AM), and functional abdominal pain – not otherwise specified (FAP-NOS) (Supplementary File 1) [Citation1].
To date, available treatment options are scarce. In general, standard medical treatment consists of education, reassurance and lifestyle interventions. Different dietary advices are recommended such as a lactose-free diet or increasing fiber intake [Citation8]. Furthermore, psychosocial treatment (hypnotherapy and cognitive–behavioral therapy), and different pharmacological compounds have been used successfully as well [Citation9,Citation10]. Management remains mostly symptom-based since the exact pathophysiology of pediatric FAPDs is still not completely known [Citation11].
Different mechanisms have been proposed. These derive from complex interactions of different biopsychosocial factors that influence the brain–gut axis, such as psychosocial distress, low-grade gut inflammation, intestinal dysbiosis, genetic predisposed and visceral hypersensitivity [Citation12–14]. In the last decade increasing attention has been paid to the causative role of food in FADPs. They may interfere with GI motility and sensitivity, barrier function and gut microbiota, causing an irregular modulatory mechanism in the gut, resulting in abdominal pain, diarrhea, or constipation[Citation15]. Recently, the role of diet in adults with IBS has been reviewed [Citation16,Citation17]. It has been perceived that food, such as gluten or products containing high rates of fermentable oligosaccharides, disaccharides, and monosaccharides and polyols (FODMAPs), which are present in stone fruits, beans and lentils, lactose-containing foods, nuts and (artificial) sweeteners, may precede IBS-related symptoms in about 50% of time. The majority of children with FAPDs report that their GI symptoms are food-related. A recent study identified new insights in peripheral mechanisms (i.e. IgE- and mast-cell-dependent) that underlies food-induced abdominal pain, making dietary interventions a potential fundamental treatment option of pediatric FAPDs [Citation18–23]. However, in clinical practice, it is still difficult to distinguish which specific food components trigger FAPD-symptoms, leading to an overflow of diagnostics including screening for allergies, celiac disease or performing a hydrogen breath test, and also leading to a mixture of different recommended dietary interventions, which are largely based on expert opinion [Citation24].
In general, treatment outcomes are suboptimal and children continuing to have symptoms in adulthood [Citation25]. New treatment options, including evidence-based dietary interventions, may therefore be necessary in order to improve pediatric FAPD care. To guide health-care professionals, patients, and their families in treatment decisions, the present systematic review provides an up-to-date overview concerning the efficacy and safety of dietary treatments in children with FAPDs.
2. Methods
2.1. Literature search
PubMed, MEDLINE, EMBASE, PsycINFO, and Cochrane Library (including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effect, and Cochrane Central Register of Controlled Trials (CENTRAL)) were searched from inception to October 2021. The ClinicalTrials.gov register, the WHO International Clinical Trials Registry Platform Search Portal, and the Current Controlled Trials meta-Register of Controlled Trials – active registers were searched to identify unpublished or ongoing studies. Reference lists from review articles were searched by hand to identify relevant articles missed by the search strategies. Full search strategies can be acquired upon request. The protocol was registered at The International Prospective Register of Systematic Reviews (PROSPERO 2020 CRD42020159847).
2.2. Study inclusion
After removal of duplicated records, titles and abstracts were independently reviewed by two researchers (R.R. and C.M.A.B.) using Covidence systematic review software®, Veritas Health Innovation, Melbourne, Australia. A third investigator (M.T.) was consulted in the case of inter-researcher disagreements. Inclusion and exclusion criteria are shown in . The core outcome set (COS) for FAPDs was used to identify outcome measures () [Citation26]. There were no language restrictions. All potentially relevant studies were obtained in full text. Authors of ongoing trials were contacted to ascertain that studies were still in progress.
Table 1. Eligibility criteria
2.3. Quality assessment and data extraction
Data extraction was independently performed by two review authors (R.R. and C.M.A.B.). A pre-designed data extraction form was used (available upon request), containing items on study details (author, publication year, country), participants (subjects, age, gender, disease, and definition), inclusion and exclusion criteria of the study, intervention characteristics (type and length of dietary intervention), control characteristics (no intervention, placebo, or other dietary intervention), total number of patients randomized (number of patients/controls), number of dropouts/withdrawals, outcome measures, instruments, and results (type of outcome measures used, time of assessment, and length of follow-up).
The Cochrane risk of bias tool was independently used by the same authors to assess the risk of bias of all included studies [Citation27]. Bias for individual elements from five domains (selection, performance, attrition, reporting, and other) were judged as high, low, or unclear. The GRADE approach was used to assess the overall quality of evidence for each outcome (Supplementary File 2) [Citation28,Citation29]. A third investigator (M.T.) was consulted in the occasion of inter-researcher disagreements.
2.4. Data analysis
Odds ratios (ORs) or relative risks (RRs) along with 95% confidence intervals (CIs) were reported in case of dichotomous outcomes. If outcomes were continuous, mean differences (MDs) with 95% CIs were reported. Chi2 tests and the I2 statistics were used to quantify heterogeneity. First, a random effects model was performed, and then a fixed-effects analysis was performed to further test for heterogeneity. If trials used a cross-over study design, if possible only data from the first phase of the study were extracted (i.e. before the crossover occurred). Data from individual trials were combined for meta-analysis if consensus was reached on similarity of interventions, patient groups, and outcomes. Meta-analysis was performed using the Cochrane Collaboration Review Manager (RevMan) software (version 5.3).
3. Results
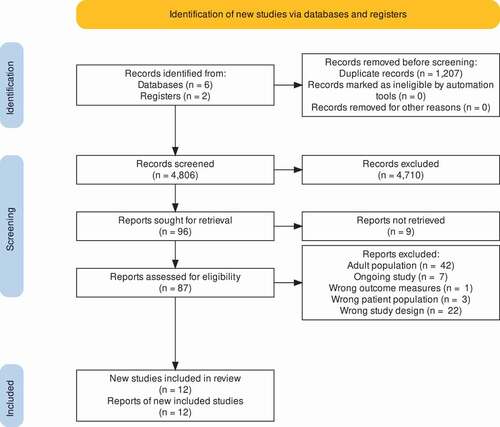
A total of 6013 potentially relevant articles and abstracts were identified ( PRISMA flow diagram). After removal of duplicates (n = 1207) and screening of title and abstract, 96 full-text articles were assessed for eligibility. Sixty-one articles did not meet the inclusion criteria and were excluded. Details of excluded studies are shown in Supplementary File 3. Sixteen systematic reviews were identified [Citation8, Citation30–43]. Search by hand from through reference lists from these systematic reviews did not reveal other relevant articles. Seven ongoing trials were identified. Authors of ongoing trials were contacted. Finally, 12 articles were included for analysis [Citation44–55].
Data of 819 FAPD patients aged 4–18 years, with the majority suffering from IBS, were included for analysis. Sample sizes varied from 20 to 116 children from Africa, Asia, Europe, and North America. Period of follow-up ranged from no follow-up after end of intervention to 4 weeks. Five trials evaluated treatment with fibers compared to placebo [Citation44,Citation45,Citation48,Citation49,Citation54], two trials investigated a diet low in FODMAPs [Citation50,Citation55]. Remaining studies determined whether fructans worsen symptoms [Citation51], studied fructose-restricted diet [Citation46], evaluated treatment with prebiotic (inulin) [Citation47], compared oral serum-derived bovine immunoglobulin (SBI) versus placebo [Citation52], and vitamin D supplementation [Citation53]. No studies were included studying additional fluid intake or histamine-free diet as dietary intervention.
Data from seven individual trials were combined for meta-analyses: 4 trials on the efficacy of fibers [Citation44,Citation45,Citation48,Citation49] and 2 trials on the safety of fibers [Citation49,Citation54]. Due to heterogeneity and lack of reporting results with absolute numbers, no further meta-analysis was possible. Primary outcomes and meta-analysis are described for trials evaluating fibers and low-FODMAP diet. Results of remaining studies are reported in Supplementary File 4. shows the characteristics of all included studies. Secondary outcomes are shown in .
Table 2. Study characteristics of included studies
Table 3. Secondary outcomes
3.1. Methodologic quality
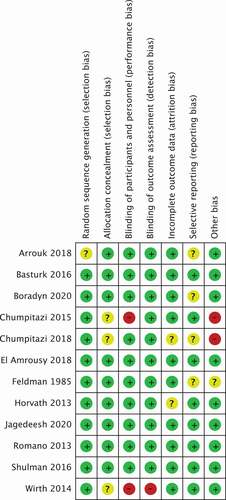
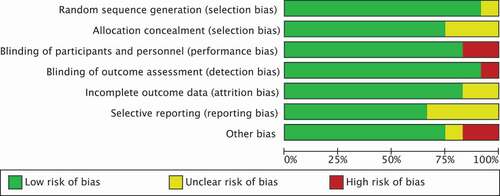
Overall, two studies were rated as having high risk of bias. In the study of Wirth et al., there was lack of blinding of participants (performance bias) and lack of blinding of outcome assessors (detection bias), because patients were not blinded for the allocated intervention (fructose-restricted-diet vs. normal eating practice) [Citation46]. In the study of Chumpitazi et al., it was not clear if the type of food or drink provided during interventions was similar (performance bias). Second, since it was a cross-over study and washout period was only 5 days, the carry over effect could not be excluded [Citation55]. Furthermore, 6 of the 12 studies (50%) were rated as having unclear risk of bias in at least one domain as a result of inadequate reporting [Citation44–46,Citation50–52]. Five studies had low risk of bias across all domains () [Citation47–49,Citation53,Citation54]. Supplementary File 5 presents detailed information about the risk of bias for the included studies.
The overall certainty of evidence based on the GRADE system was very low to low, with reasons for downgrading of certainty presented in Supplementary File 2.
3.1.1. Fiber
Five randomized placebo-controlled trials met the pre‐specified inclusion criteria (n = 385, age 4–18 years). Horvath et al. [Citation45] compared glucomannan, a water-soluble high-molecular-weight dietary fiber (HMWDF), with placebo. Romano et al. [Citation49] also investigated another soluble HMWDF and compared Partially Hydrolyze Guar Gum (PHGG) versus placebo. Jagadeesh et al. [Citation48] and Shulman et al. [Citation54] compared psyllium, a plant-based soluble fiber (high water-holding capacity) versus placebo. Feldman et al. [Citation44] randomized patients to receive either a fiber cookie (containing 5 g corn per cookie) or a placebo cookie twice a day. The predominant components of corn fiber are water-soluble cellulose and hemicellulose, which increases fecal bulk and decreases gastro-intestinal transit time [Citation56,Citation57].
3.1.1.1. Primary outcomes
3.1.1.1.1. Treatment success
Four studies reported treatment success as primary outcome. The study of Feldman [Citation44] reported an improvement in 13/26 (50%) in the fiber cookie group vs 7/26 (27%) in the placebo group (P = 0.004). Horvath et al. Citation45 concluded that 23/41 (56%) (glucomannan group) versus 20/43 (47%) (placebo group) reported treatment success (RR 1.21, 95% CI 0.79–1.83). Jagadeesh et al. [Citation48] showed that treatment success was reported in 18/41 (44%) in the psyllium group compared with 3/31 (10%) in the placebo group (P < 0.001). Romano et al. [Citation49] reported treatment success in 13/30 (43%) in the PHGG group and 2/30 (7%) in the placebo group (P = 0.025). Shulman et al. [Citation54] did not predefine treatment success.
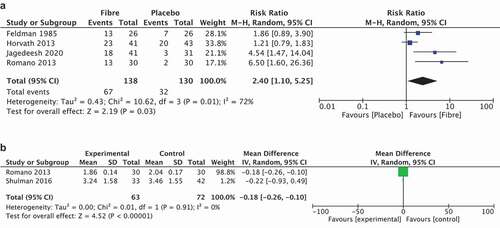
Four studies could be included in meta-analysis [Citation44,Citation45,Citation48,Citation49]. Analysis found very low-certainty, downgraded due to inconsistency and imprecision due to low numbers, evidence that there may be a difference when water-soluble fibers (including corn, glucomannan, PHGG, and psyllium) are compared with placebo in favor of fibers (RR 2.40, 95% CI 1.10–5.25; NNT = 3, 4 studies, 268 participants; I2 = 72%; random-effects model, ). When considering the heterogeneity, a visual outlier is the study of Horvath et al. When removing this study, heterogeneity drops to 44% (RR 5.24, 95% CI 2.31–11.91; NNT = 3, 3 studies, 184 participants; I2 = 44%; random-effects model).
3.1.1.1.2. Pain frequency and intensity
Jagadeesh et al. [Citation48] reported a significant reduction in pain intensity (Median (IQR): 25 (0–25) vs. 50 (25–50), P < 0.001), as well as pain frequency (10 (0–27.5) vs. 50 (30–50), P < 0.001) scores in the psyllium group compared with the placebo group. Shulman et al. [Citation54] reported that there was a significant reduction in pain episodes in the fiber group compared with the placebo group (P = 0.03), whereas no differences were seen in pain intensity between the two groups. Horvath et al. [Citation45] reported pain frequency and pain intensity, with no statistically significant differences between the two groups (RR = 2.1,95%CI: 0.87–5.07). Romano et al. [Citation49] reported that there were no differences in pain frequency in the PHGG group compared to the placebo group (23.0 ± 6.15 vs. 28.7 ± 7.54, P > 0.05). Romano et al. [Citation49] also assessed pain intensity, where mean severity of pain scores did not significantly differ between the PHGG group and the placebo group (1.63 ± 0.16 vs. 2.05 ± 0.19, P > 0.05).
Two studies could be included in meta-analysis [Citation49,Citation54]. Analysis found no evidence that there may be a difference when water-soluble fibers (including PHGG and psyllium) are compared with placebo (SMD – 0.63, 95% CI −1.61 to 0.35; 2 studies, 135 participants; I2 = 87%; random-effects model, ).
3.1.1.1.3. Withdrawal due to adverse events
Feldman et al. [Citation44] reported that the number of children who experienced gas or diarrhea as side effects was small in both groups and insignificant. All other studies reported that there were no adverse events.
3.1.2. FODMAP
Two RCTs (n = 79, age 5–17 years) were included [Citation50,Citation55]. Boradyn et al. [Citation50] investigated FODMAP diet versus diet based on NICE-guidelines (National Institute for health and Care Exellence, Poland). Chumpitazi et al. [Citation55] performed a double-blind, cross-over trial, in which patients received either a FODMAP diet (containing 0.15 g/kg/day of FODMAPs, maximum 9 g/day) or a typical American childhood diet (TACD, contained 0.7 g/kg/day, maximum 50 g/day). No separate results reported for the first and second phase of the study (i.e. before and after cross-over) were described. Patients followed a washout period of 5 days.
3.1.2.1. Primary outcomes
3.1.2.1.1. Treatment success
Only the study by Chumpitazi et al. [Citation55] predefined the primary outcome ‘treatment success.’ The study reported 8 out of 16 (50%) responders in the FODMAP group and 10/17 (59%) in the TACD group (P > 0.05). Boradyn et al. [Citation50] did not predefine treatment success.
3.1.2.1.2. Pain frequency and intensity
Pain frequency and pain intensity were reported in both studies [Citation50,Citation55]. Chumpitazi et al. [Citation55] reported significant less abdominal pain during the low FODMAP diet versus TACD (1.1 ± 0.2 (SEM) episodes/day vs 1.7 ± 0.4, P < 0.05). As compared to baseline, median pain severity decreased significantly in both the FODMAP and TACD group, with no significant differences between the two diets. Boradyn et al. [Citation50] reported no statistically significant differences between the two diets.
3.1.2.1.3. Withdrawal due to adverse events
Both studies reported that there were no adverse events [Citation50,Citation55].
4. Discussion
We systematically reviewed 12 articles to determine the efficacy and safety of dietary interventions in children with FAPDs and demonstrated the lack of high-quality intervention trials. Based on the current evidence we found some beneficial effects for the use of water-soluble fibers (including corn, guar gum, glucomannan, and psyllium) with a slight preference for the use of psyllium, which can therefore be discussed in daily practice. Whilst certainty is very low, impacted by the limited sample size in particular, the magnitude of treatment effect was substantial, with an NNT of 3. Given the low cost, easy availability and observed safety, in spite of these limitations, such therapies appear to have a role in treatment packages. When abdominal pain intensity (assessed with a dairy or validated questionnaire as described in ) was used as primary endpoint, fructose-restricted diet, SBI, and vitamin D supplementation might be potential effective treatments in some children. However, well-designed intervention studies are needed before these conclusions can be firmly drawn, since quality of current evidence based on GRADE system was low to very low. There was no evidence that any other dietary intervention has a significant role in the treatment of pediatric FAPDs. All included interventions (i.e. fibers, FODMAP diet, fructans, fructose-restricted diet, prebiotic (inulin), SBI, and vitamin D supplementation) appear to be safe treatment options, whereas no serious adverse effects were reported. No studies were included on treatment with extra fluid intake or histamine-free diet [Citation58].
A recently published Cochrane review assessed the efficacy and safety of probiotics and synbiotics for the management of FAPD in children [Citation59]. Therefore, probiotics and synbiotics were outside the scope of this review and not included. In 2017, a Cochrane review on dietary interventions for recurrent abdominal pain in childhood was published [Citation8]. These findings however are not in line with our results. When excluding probiotic intervention studies, the current review included 12 studies compared to only 6 studies in the previous SR, and this review is therefore an improvement on existing literature on this topic. In contrast to the outcome of our study, they found that children treated with fibers were not more likely to experience treatment success. An explanation for this could be that the number of studies included in the previous meta-analysis was limited to two intervention trials and only included a small patient group (136 children).
In the management of pediatric FAPDs, the low FODMAP diet has been of great interest over the last decade. FODMAPs are small molecules containing different carbohydrates and can be found in several daily food products (i.e. common fruits and vegetables, honey, milk, and sweeteners) [Citation60]. It is hypothesized that FODMAPs’ mechanism of action is linked to the intestinal osmolarity and is nutritious for the intestinal microbiota [Citation60]. Fermentation of these FODMAPs increases hydrogen, methane, and carbon dioxide production and subsequently leads to increased luminal distension, provoking abdominal pain, bloating, flatulence, and alterations in bowel habits [Citation61]. Reducing the intake of FODMAPs will reduce intestinal osmolarity and gas production and therefore potentially reduces FAPD symptoms. In addition, microbiota composition and functioning will alter, however, the impact of microbiota changes and functional consequences are not completely understood [Citation62]. The low FODMAP diet includes three phases: (1) elimination; (2) reintroduction; and (3) personalization phase [Citation63]. Clinical guidelines for adult IBS patients recommend that the duration of the elimination phase solely should be 2–6 weeks, whereas the duration of the included FODMAP diets in this review were only 48 h and 4 weeks [Citation50,Citation55,Citation63]. Therefore, the results of these included studies should be interpreted with caution. Adult studies on the efficacy of the FODMAP diet showed promising results [Citation64]. Meta-analysis containing 12 studies concluded that a low FODMAP diet in adult patients with IBS reduces GI symptoms and improves quality of life. For this reason, the FODMAP diet can be considered as a symptomatic treatment, especially in children with excessive gas formation. However, evidence to support the use of low FODMAP diet in daily practice in children is still lacking [Citation50,Citation55]. When prescribing the FODMAP diet in clinical practice, it is preferable to involve a dietician to better explain the diet and improve adherence. Adherence to the FODMAP diet is crucial to the overall success of the diet. Adult studies estimated rates of adherence to the FODMAP diet between 75% and 80% [Citation65]. Rates were lower in studies with a duration of diets more than 6 weeks and in studies not providing the participants of foods [Citation66]. Furthermore, when a dietician is not involved, rates will decrease even more [Citation61]. It may be hypothesized that due to the duration of the diet and food restrictions, adherence to the strict FODMAP diet in pediatric population can be challenging. Noteworthy, in both FODMAP studies included in this review, adherence to the strict diet was not assessed and data regarding this issue in pediatric population are lacking [Citation50,Citation55].
As described above, the use of probiotics and synbiotics was not included in this review. However, there is a growing body of evidence for the use of probiotics and synbiotics in the management of pediatric FAPDs. A recently published Cochrane review [Citation59][] on the effectiveness of probiotics and synbiotics concluded that the use of probiotics (i.e. Lactobacillus rhamnosus GG and Lactobacillus reuteri) provide better symptom relief and can reduce the frequency of pain episodes. Therefore, Lactobacillus rhamnosus GG and Lactobacillus reuteri may be considered in clinical management in children with FAPDs. Evidence on the effectiveness of synbiotics is sparse and inconclusive and revealed little to no beneficial effects. Also in adult patients with FAPDs, new dietary interventions have been explored. Some preliminary data suggested that low histamine diets [Citation67] and green kiwifruit [Citation68] improve symptoms of abdominal pain. However, evidence on the beneficial effect of these dietary interventions in the pediatric population with FAPDs is lacking. Currently, a double-blind placebo controlled RCT on oral butyrate is ongoing in children with IBS (NCT04566679).
The last decade, there is great interest in the role of gluten sensitivity as a potential trigger of gastrointestinal symptoms in adults with IBS. In a double-blind randomized placebo-controlled trial, adult IBS patients with self-reported gluten intolerance (in whom celiac disease was excluded) received either gluten or placebo. Results showed that adequate symptom control differed significantly between two groups (gluten-exposed: 32% vs placebo: 60%) [Citation69]. These results suggest that IBS patients may react to gluten, despite the lack of a celiac disease diagnosis. This clinical condition is known as non-celiac gluten sensitivity (NCGS) and may also be present in children, contributing to their IBS symptoms [Citation70]. Nevertheless, the association between NCGS and FAPDs in the pediatric population is not yet revealed. Future studies are needed before gluten avoidance can be recommended for children with FAPDs. Currently, a trial to evaluate the prevalence of gluten sensitivity in pediatric IBS patients is ongoing (NCT02431585).
The systematic methodology applied in this review is in line with the high-quality standards of the Cochrane Collaboration, which is a major strength of this review. First, in consultation with an Information Specialist from the Cochrane group, the search strategy was developed. Second, two independent reviewers performed the screening process. In case of indistinctness of the study design or need for additional data, authors of included studies were contacted. Furthermore, the Cochrane Risk of Bias tool and GRADE were used to assess the strength of evidence, increasing the transparency of this review and therefore support readers in interpreting the results. Finally, the number of eligible studies has increased significantly since this topic was last systematically evaluated.
Although there is no direct relationship with our review process, the limitations of this study are mostly associated to low- to very-low-quality evidence that is available nowadays. First, evidence was downgraded due to significant imprecision from extremely sparse data. Because of small sample sizes, no subgroup analyses were possible, in particular for patient characteristics such as gender or by specific pain disorder. However, this is a considerable issue in pediatric literature and future intervention studies should take this into account. Second, two studies used a crossover design without reporting separate results for the first and second phase of the study (i.e. before the cross-over occurred). The combination of selective reporting and the use of such design raises concerns regarding the external validity of the results. Third, there was evidence of heterogeneity in our meta-analyses, including differences in type and length of dietary intervention and the choice of outcome measures. To address this issue, the ROME foundation developed recommendations for designing clinical trials in pediatric FAPDs [Citation71]. This committee recommends considering a treatment period of at least 4 weeks (preferably 6 weeks or more), whereas they highlight that treatment periods shorter than 4 weeks are disrecommended due to the variable course of the disease. Also, the pediatric FAPD COS was recently created [Citation26]. This COS generated a standardized minimum set of outcome measures. If future clinical trials will use these outcome measures, and associated measurement instruments, heterogeneity will decrease and consequently the comparability of study results will increase. This may improve overall quality of available information, GRADE certainty of evidence and finally clinical decision-making [Citation72]. Final, significant changes in the ROME definition have taken place with time. Only one study used the latest ROME IV, the bulk of studies used the ROME III or any other criteria. This should be considered when interpreting the findings.
In clinical practice, management of pediatric FAPDs can be challenging. The first step in treatment consists of reassurance and education[Citation11]. Subsequently, non-pharmacological and pharmacological and interventions could be considered. Non-pharmacological interventions, such as cognitive behavior therapy, hypnotherapy, and dietary interventions, as described in this review, have showed their effectiveness and safety in the pediatric FAPD population [Citation32,Citation73,Citation74]. The following pharmacological agents have been examined in pediatric FAPDs patients: antispasmodics, antidepressants, antibiotics, antihistaminic, anti-emetic, H2-receptor-antagonist, 5-HT4-receptor-agonist, melatonin and buspirone. Based on the current evidence it is not possible to recommend any specific agent, but antispasmodics and antidepressants may be discussed due to their favorable treatment outcomes and lack of important side effects [Citation9]. However, the optimal therapeutic algorithm is undetermined since the pathogenesis of FAPDs in children is not yet fully understood. Therefore, a tailor-made approach for each patient, based on the concomitant symptoms such as nausea, acid, diarrhea, or constipation is proposed to date, where both non-pharmacological and pharmacological interventions should be discussed to encourage shared decision-making during consultations.
5. Conclusion
In summary, based on the findings of this systematic review and low to very low quality of the included studies, it is not possible to recommend any specific dietary intervention for the treatment of pediatric FAPDs. However, evidence demonstrates that the use of fibers can be discussed in daily practice due to their favorable treatment outcomes and reported lack of side effects. These new findings should be considered by international guideline committees. High-quality RCTs on dietary interventions are needed to provide adequate clinical management, since a substantial proportion of children still experience abdominal complaints in adulthood. In future, we recommend to adhere to the guidelines established by the ROME committee and to use homogenous outcome measures and instruments, as recommend by the pediatric FAPD COS.
Abbreviations
Data availability
The datasets generated and analyzed during the current study are available upon request.
Trial registration
PROSPERO, registered 28/04/2020, CRD42020159847
Declaration of interests
Since August 2016, M Gordon has received travel fees to attend international scientific and training meetings from Pharma companies. These grants included no honoraria, inducement, advisory role or any other relationship and were restricted to the travel and meeting-related costs of attending such meetings. These include DDW May 2017, World Congress of Gastroenterology October 2017, DDW May 2018, Advances in IBD December 2018, DDW May 2019. None of these companies have had any involvement in any works completed by M Gordon and he has never had any payments for any other activities for them. M Benninga is a consultant for Shire, Norgine, Coloplast, Danone, Takeda, Allergan, Shire, FrieslandCampina, United Pharamceuticals, HiPP. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (141.7 KB)Acknowledgments
We acknowledge the great work and contributions by Yuhong Yuan. She developed the search strategies (Information Specialist, Cochrane Gut Group). We are grateful to the authors who kindly responded to our request for clarification of the protocol and additional data on the trials in which they were involved.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Hyams JS, Di Lorenzo C, Saps M, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2016;150(6):1456–1468e2.
- Korterink JJ, Diederen K, Benninga MA, et al. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One. 2015;10(5):e0126982.
- Youssef NN, Murphy TG, Langseder AL, et al. Quality of life for children with functional abdominal pain: a comparison study of patients’ and parents’ perceptions. Pediatrics. 2006;117(1):54–59.
- Hoekman DR, Rutten JMTM, Vlieger AM, et al. Annual costs of care for pediatric irritable bowel syndrome, functional abdominal pain, and functional abdominal pain syndrome. J Pediatr. 2015;167(5):1103–1108.e2.
- Lewis ML, Palsson OS, Whitehead WE, et al. Prevalence of functional gastrointestinal disorders in children and adolescents. J Pediatr. 2016;177:39–43.e3.
- Assa A, Ish-Tov A, Rinawi F, et al. School attendance in children with functional abdominal pain and inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. 2015;61(5):553–557.
- Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr. 2010;51(5):579–583.
- Newlove-Delgado T, Martin AE, Abbott RA, et al. Dietary interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;276–278. DOI:https://doi.org/10.1093/pch/pxz132.
- Rexwinkel R, de Bruijn CMA, Gordon M, et al. Pharmacologic treatment in functional abdominal pain disorders in children: a systematic review. Pediatrics. 2021;147(6):e2020042101. DOI:https://doi.org/10.1542/peds.2020-042101.
- de Bruijn CMA, Rexwinkel R, Gordon M, et al. Antidepressants for functional abdominal pain disorders in children and adolescents. Cochrane Database Syst Rev. 2021;2(2):CD008013. DOI:https://doi.org/10.1002/14651858.CD008013.pub3.
- Korterink J, Devanarayana NM, Rajindrajith S, et al. Childhood functional abdominal pain: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2015;12(3):159–171.
- Chumpitazi BP, Shulman RJ. Underlying molecular and cellular mechanisms in childhood irritable bowel syndrome. Mol Cell Pediatr. 2016;3(1). DOI:https://doi.org/10.1186/s40348-016-0036-8
- Drossman DA, Tack J, Ford AC, et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut−brain interaction): a Rome foundation working team report. Gastroenterology. 2018;154(4):1140–1171.e1.
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology. 2016;150(6):1262–1279e2.
- Oświęcimska J, Szymlak A, Roczniak W, et al. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62(1):17–30.
- Camilleri M. Diagnosis and treatment of irritable bowel syndrome: a review. JAMA - J Am Med Assoc. 2021;325(9):865–877.
- Ford A, Lacy B, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376(26):66–78.
- Carlson MJ, Moore CE, Tsai CM, et al. Child and parent perceived food-induced gastrointestinal symptoms and quality of life in children with functional gastrointestinal disorders. J Acad Nutr Diet. 2014;114(3):403–413.
- Chumpitazi BP, Weidler EM, Lu DY, et al. Self-Perceived Food Intolerances Are Common and Associated with Clinical Severity in Childhood Irritable Bowel Syndrome. J Acad Nutr Diet. 2016;116(9):1458–1464.
- Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915–1920.
- Eswaran S, Tack J, Chey WD. Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40(1):141–162.
- Hayes P, Corish C, O’Mahony E, et al. A dietary survey of patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27:36–47.
- Aguilera-Lizarraga J, Florens MV, Viola MF, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature. 2021;590(7844):151–156.
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107(5):657–666.
- Horst S, Shelby G, Anderson J, et al. Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin Gastroenterol Hepatol. 2014;12(12):2026–2032.
- Zeevenhooven J, Rexwinkel R, Van Berge Henegouwen VWA, et al. A core outcome set for clinical trials in pediatric functional abdominal pain disorders. J Pediatr. 2020;221:115–122.e5.
- Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):1–9.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Chinese J Evidence-Based Med. 2009;9:8–11.
- Schünemann HJ, Vist GE, Higgins JPT, et al. Chapter 12: interpreting results and drawing conclusions. Cochrane Handb Syst Rev Interv. 2019;403–431. DOI:https://doi.org/10.1002/9781119536604.ch15.
- Abbott RA, Martin AE, Newlove-Delgado TV, et al. Recurrent abdominal pain in children: summary evidence from 3 systematic reviews of treatment effectiveness. J Pediatr Gastroenterol Nutr. 2018;67(1):23–33.
- Pourmand H, Esmaillzadeh A. Consumption of a low fermentable oligo-, di-, mono-saccharides, and polyols diet and irritable bowel syndrome: a systematic review. Int J Prev Med. 2017;8. DOI:https://doi.org/10.4103/ijpvm.IJPVM_175_17
- Rutten JMTM, Korterink JJ, Venmans L, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135(3):522–535.
- Scarpato E, Auricchio R, Penagini F, et al. Efficacy of the gluten free diet in the management of functional gastrointestinal disorders: a systematic review on behalf of the Italian Society of Paediatrics. Ital J Pediatr. 2019;45(1):1–11.
- Turco R, Salvatore S, Miele E, et al. Does a low FODMAPs diet reduce symptoms of functional abdominal pain disorders? A systematic review in adult and paediatric population, on behalf of Italian Society of Pediatrics. Ital J Pediatr. 2018;44(1):1–14.
- Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics. 2003;111(1):e1–e11.
- Anheyer D, Frawley J, Koch AK, et al. Herbal medicines for gastrointestinal disorders in children and adolescents: a systematic review. Pediatrics. 2017;139(6). DOI:https://doi.org/10.1542/peds.2017-0062.
- Axelrod CH, Saps M. The role of fiber in the treatment of functional gastrointestinal disorders in children. Nutrients. 2018;10(11):1650.
- Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4(3):293–304.
- Chumpitazi BP, Shulman RJ. Dietary carbohydrates and childhood functional abdominal pain. Ann Nutr Metab. 2016;68(Suppl. 1):8–17.
- Duncanson KR, Talley NJ, Walker MM, et al. Food and functional dyspepsia: a systematic review. J Hum Nutr Diet. 2018;31(3):390–407.
- Horvath A, Szajewska H. Probiotics, prebiotics, and dietary fiber in the management of functional gastrointestinal disorders. World Rev Nutr Diet. 2013;108:40–48.
- Huertas-Ceballos A, Logan S, Bennett C, et al. Pharmacological interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev. 2008. DOI:https://doi.org/10.1002/14651858.CD003017.pub2
- Paul SP, Basude D. Non-pharmacological management of abdominal pain-related functional gastrointestinal disorders in children. World J Pediatr. 2016;12(4):389–398.
- Feldman W. The use of dietary fiber in the management of simple, childhood, idiopathic, recurrent, abdominal pain. Am J Dis Child. 1985;139(12):1216.
- Horvath A, Dziechciarz P, Szajewska H. Glucomannan for abdominal pain-related functional gastrointestinal disorders in children: a randomized trial. World J Gastroenterol. 2013;19(20):3062–3068.
- Wirth S, Klodt C, Wintermeyer P, et al. Positive or negative fructose breath test results do not predict response to fructose restricted diet in children with recurrent abdominal pain: results from a prospective randomized trial. Klin Padiatr. 2014;226(5):268–273.
- Başturk A, Artan R, Yilmaz A. Efficacy of synbiotic, probiotic, and prebiotic treatments for irritable bowel syndrome in children: a randomized controlled trial. Turkish J Gastroenterol. 2016;27(5):439–443.
- Jagadeesh MVR, Thapa BR, Lal SB, et al. Efficacy of oral psyllium versus placebo in pediatric irritable bowel syndrome: a double blind randomized control trial.
- Romano C, Comito D, Famiani A, et al. Partially hydrolyzed guar gum in pediatric functional abdominal pain. World J Gastroenterol. 2013;19(2):235–240.
- Boradyn KM, Przybyłowicz KE, Jarocka-Cyrta E. Low FODMAP diet is not effective in children with functional abdominal pain: a randomized controlled trial. Ann Nutr Metab. 2021;76(5):334–344.
- Chumpitazi BP, McMeans AR, Vaughan A, et al. Fructans exacerbate symptoms in a subset of children with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2018;16(2):219–225.e1.
- Arrouk R, Herdes RE, Karpinski AC, et al. Serum-derived bovine immunoglobulin for children with diarrhea-predominant irritable bowel syndrome. Pediatr Heal Med Ther. 2018;9:129–133.
- El Amrousy D, Hassan S, El Ashry H, et al. Vitamin D supplementation in adolescents with irritable bowel syndrome: is it useful? A randomized controlled trial. Saudi J Gastroenterol. 2018;24(2):109–114.
- Shulman RJ, Hollister EB, Cain K, et al. Psyllium fiber reduces abdominal pain in children with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol. 2017;15(5):712–719.e4.
- Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated. Aliment Pharmacol Ther. 2015;42(4):418–427.
- Ai Y, Jane J-L. Macronutrients in corn and human nutrition. Compr Rev Food Sci Food Saf. 2016;15(3):581–598.
- Korczak R, Kamil A, Fleige L, et al. Dietary fiber and digestive health in children. Nutr Rev. 2017;75(4):241–259.
- Kline RM, Kline JJ, Di Palma J, et al. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138(1):125–128.
- Gordon M, Farrell M, Thomas AG, et al. Probiotics for management of functional abdominal pain disorders in children. Cochrane Database Syst Rev. 2017;2017(11):CD012849.
- Bellini M, Rossi A. Is a low FODMAP diet dangerous? Tech Coloproctol. 2018;22(8):569–571.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25(2):252–258.
- Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66(8): 1517 LP – 1527.
- Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol. 2021;116(1):17–44.
- van Lanen AS, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. Eur J Nutr. 2021. DOI:https://doi.org/10.1007/s00394-020-02473-0
- Rej A, Shaw CC, Buckle RL, et al. The low FODMAP diet for IBS; A multicentre UK study assessing long term follow up. Dig liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2021. DOI:https://doi.org/10.1016/j.dld.2021.05.004.
- Alfaro-Cruz L, Heitkemper M, Chumpitazi BP, et al. Literature review: dietary intervention adherence and adherence barriers in functional gastrointestinal disorder studies. J Clin Gastroenterol. 2020;54:203–211.
- Lackner S, Malcher V, Enko D, et al. Histamine-reduced diet and increase of serum diamine oxidase correlating to diet compliance in histamine intolerance. Eur J Clin Nutr. 2019;73(1):102–104.
- Cremon C, Wrona D, Barbaro MR, et al. The effect of Zespri green kiwifruit on digestive functions in constipated patients: a randomized, controlled, single-blind, cross over study. Neurogastroenterol Motil. 2019;31. DOI:https://doi.org/10.1111/nmo.13627.
- Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Off J Am Coll Gastroenterol | ACG. 2011;106(3):508–514.
- Barbaro MR, Cremon C, Wrona D, et al. Non-celiac gluten sensitivity in the context of functional gastrointestinal disorders. Nutrients. 2020;12(12):3735.
- Saps M, van Tilburg MAL, Lavigne JV, et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterol Motil. 2016;28(11):1619–1631.
- Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: version 1.0. Trials. 2017;18(S3):280.
- Martin AE, Newlove-Delgado TV, Abbott RA RA, et al. Pharmacological interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;3(3):CD010973.
- Santucci NR, Saps M, van Tilburg MA. New advances in the treatment of paediatric functional abdominal pain disorders. Lancet Gastroenterol Hepatol. 2020;5(3):316–328.