ABSTRACT
Introduction
Cross-sectional imaging techniques including MR and CT enterography and ultrasound are integral to Crohn’s disease management, accurate, responsive, and well tolerated. They assess the full thickness of the bowel wall, perienteric environment, and distant complications. As we strive toward tighter disease control, imaging’s role will expand further with transmural healing becoming an increasingly important therapeutic target.
Areas covered
MEDLINE and Web of Science were searched from 2012 to 2023 inclusive. We review the evidence for cross-sectional imaging in assessing disease activity, phenotyping, and therapeutic response assessment. Emerging novel imaging applications such as quantifying enteric motility and fibrosis, prognostication, and potential utility of artificial intelligence will be covered. Recent international consensus statements highlight the need for standardized imaging reporting and definitions of transmural healing and remission. We will discuss how recent advances may be best integrated into patient care and highlight key outstanding research questions.
Expert opinion
Cross-sectional imaging is established in Crohn’s disease management. Research emphasis should be placed on optimal integration of imaging modalities in clinical care pathways, workforce training, definitions, and evidence for use of imaging based therapeutic targets such as transmural healing, better phenotyping of stricturing disease, and developing novel techniques, including integration of artificial intelligence.
1. Introduction
There are a variety of well-established and validated methods to non-invasively image the small bowel, often negating the need for less well tolerated and costly ileocolonoscopy and/or capsule endoscopy. Cross-sectional imaging techniques are now a key component of inflammatory bowel disease (IBD) diagnosis and management pathways [Citation1,Citation2]. MR enterography (MRE) and intestinal ultrasound (IUS) are mainly utilized, with CT enterography (CTE) usually playing a lesser role outside the acute setting given its use of ionizing radiation. Imaging plays a key role in assessing disease phenotype including extent, activity, penetrating, and stricturing complications and in therapeutic response assessment. Perienteric assessment is a crucial advantage of cross-sectional imaging over endoscopic techniques, for example, evaluating fat wrapping, increasingly implicated in disease pathophysiology and prognosis [Citation3].
Cross-sectional imaging can image the small bowel beyond the reach of ileocolonoscopy and upstream of tight stricturing disease and is a less invasive option to whole bowel enteroscopy. There has been a paradigm shift to using objective markers of activity to try and achieve disease control and sustained deep remission [Citation2]. A number of histological and endoscopically validated imaging-based scores of activity have been developed which provide an objective assessment of enteric inflammation [Citation4,Citation5]. Cross-sectional imaging therefore plays an important role in this treat-to-target approach, with increasing evidence of responsiveness to therapeutic interventions, and convergence with endoscopic evaluation [Citation6]. Furthermore, novel therapeutic targets are receiving considerable attention, notably transmural healing. Although currently not yet formally adopted as primary treatment target [Citation2], early transmural healing may predict longer term response [Citation7,Citation8], and those patients who exhibit transmural healing have improved outcomes over those achieving endoscopic mucosal healing alone [Citation9,Citation10].
Crohn’s disease is a lifelong condition, and patients must undergo multiple tests in their lifetime. Patient experience is thus a vital part of optimizing clinical care pathways. Patients generally prefer minimally or noninvasive tests over repeated ileocolonoscopy with MRE and IUS found to be generally acceptable for disease monitoring [Citation11–13].
This review builds on several recent excellent reviews with an updated review of the recent literature [Citation5,Citation14,Citation15]. As part of this, MEDLINE and Web of Science were searched from 2012 to 2023 inclusive for the following terms; ‘magnetic resonance,’ ‘ultrasound,’ and ‘computerized tomography’ cross-referenced with the keywords ‘inflammatory bowel disease,’ ‘Crohn’s disease,’ ‘scores,’ ‘activity,’ ‘damage,’ ‘transmural healing,’ ‘transmural remission.’ It considers the current evidence base for cross-sectional imaging in Crohn’s disease to support optimal clinical decision-making. Emerging novel applications including assessing enteric motility, quantifying fibrosis, and potential use in prognostication are considered. We will highlight key priorities for future research, suggest how these may be best addressed, and speculate on how emerging trends in small bowel cross-sectional imaging may be integrated into patient care in the coming 5–10 years.
2. Optimizing cross-sectional imaging protocols-current controversies
MRE and IUS acquisition protocols are generally standardized although there remain areas of controversy.
2.1. Intravenous contrast
Current MRE protocols vary with respect to the use of intravenous contrast agents which may provide additional measures of disease activity and better evaluation of penetrating complications. There is a move away from routine intravenous contrast administration driven by pressures to reduce imaging time, costs, potential for allergic reactions and recent evidence and patient awareness of gadolinium deposition in the brain [Citation16]. This is particularly an issue with repeated administration in tight follow-up protocols in often young patients.
The evidence that intravenous contrast increases diagnostic accuracy in Crohn’s disease is relatively weak. Indeed, against a Crohn’s disease Endoscopic Index of Severity (CDEIS) reference standard in 98 patients, Puylaert et al. reported no significant difference in detecting active disease between protocols using intravenous contrast enhanced MRE, diffusion-weighted imaging (DWI)alone, or combinations of both sequences. There was higher interpreter diagnostic confidence with the addition of intravenous contrast, further improved with the combined DWI/contrast protocols [Citation17]. The non-inferiority of DWI to intravenous contrast enhanced MRI for identifying small bowel inflammation was also reported by Seo et al. in 50 consecutive patients also using an endoscopic reference standard [Citation18]. A sub-study of the METRIC trial utilized 27 radiologists and also found no diagnostic advantage in adding a contrast enhanced sequence to non-enhanced sequences [Citation19].
Given this, many centers now prefer to administer intravenous contrast only in patients suspected of penetrating complications. The potential utility of contrast enhanced US (CEUS) is discussed in the ‘novel cross-sectional imaging applications’ section below.
2.2. Oral contrast
To provide an accurate assessment of the bowel wall and disease complications such as stricturing, small bowel luminal distention is required prior to MRE [Citation20]. This is typically with mannitol or polyethylene glycol (PEG) with a typical preparation regime being 1.6 liters (L) of 1.67% mannitol ingested over 60 minutes. Good luminal distension using large volumes of osmotic agents must be balanced with acceptability to patients with side effects common, including abdominal cramps, bloating, and transient diarrhea. In the METRIC trial, drinking the oral bowel preparation was by far the least acceptable aspect of MRE, which overall was significantly less acceptable than IUS [Citation7].
There is therefore interest in refining current oral preparation protocols. A sub-study by the same METRIC group found no significant difference in per patient distension quality when using mannitol or PEG as distension agents, with comparable patient tolerability. There was, however, improved jejunal distension when using mannitol versus PEG, and no difference if > or <1 L of mannitol was ingested, suggesting routine protocols can use smaller volumes [Citation21]. Early unpublished results from our group also indicate that reduced volume preparation (1 L mannitol) has comparable qualitative and quantitative distension quality with an improved side effect profile versus standard preparation (1.6 L) in healthy volunteers. These parameters are, however, compromised when the mannitol volume was reduced further to 0.6 L.
One of the major advantages of IUS is that it does not require oral contrast. There has, however, been work investigating the utility of oral contrast prior to IUS. Parente et al. administered PEG solution orally in 102 Crohn’s disease patients. There was minimally increased sensitivity for the detection of endoscopically confirmed disease with oral preparation (96.1%) versus conventional IUS performed immediately prior (91.4%). There was also significantly increased sensitivity for stricture detection (89% versus 74%) and improved interobserver agreement for bowel wall thickness (BWT) measurement and disease location [Citation22]. In another group of 49 patients, this technique had good agreement and accuracy for surgical stricture detection and length, and penetrating complication detection [Citation23]. A METRIC trial sub-study investigated hydrosonography where an osmotic oral preparation was given prior to the IUS to distend the small bowel lumen in an effort to improve bowel wall characterization and was found to be well tolerated and of comparable acceptability to MRE. However, this group did not find any diagnostic advantage of hydrosonography over standard IUS [Citation13,Citation19].
Despite potential advantages of oral contrast, current practice usually does not include routine use of oral agents prior to IUS given its impact of service efficiency and patient experience.
3. Current use of cross-sectional imaging in the assessment of inflammation and disease phenotype
Targeting small bowel inflammation is a key therapeutic strategy in Crohn’s disease to control symptoms and prevent long-term bowel damage. Imaging provides contemporaneous activity assessment and then can be used for disease monitoring to guide treatment (de-) escalation, also evaluating penetrating complications. International consensus guidelines now provide a standardized, reproducible approach to reporting cross-sectional imaging for these various indications [Citation1,Citation14].
MRE and IUS are well tolerated and accurate in the subjective and objective assessment of disease activity and its severity in the small bowel. The METRIC study used an expert consensus panel reference standard encompassing all available clinical data including biochemical, endoscopic, and histopathological in 284 Crohn’s disease patients. MRE had a high sensitivity and specificity for active disease presence (96% and 83%) which was slightly superior to IUS in the small bowel, 90% and 77%, respectively [Citation24]. Using endoscopic assessment as the reference standard, IUS parameters of activity have a reported accuracy ranging between 73% and 100% [Citation24,Citation25]. The STARDUST study group found that IUS and endoscopy have a > 90% agreement in detecting the most affected small bowel segment based on baseline BWT measurement, particularly for terminal ileal disease [Citation7].
Several imaging parameters reflect disease activity () and have been validated against biochemical, endoscopic, and histopathological reference standards [Citation1,Citation26]. The best evaluated is bowel wall thickness with recent consensus panel recommendations that BWT >3 mm is reasonably sensitive for mural inflammation [Citation1]. This threshold is generally accepted in both clinical practice and in the research setting. However, BWT is a nonspecific finding and can result from a number of alternative causes and other underlying pathological processes. Inflammation and fibrosis virtually always coexist, a further confounder when considering BWT alone [Citation14].
Figure 1. Cross-sectional imaging signs of active disease. i) Conventional B-mode ultrasound of the terminal ileum, including normal mural thickness (≤3 mm), mural stratification and mesentery. ii) Active disease on IUS showing disruption of mural stratification, particularly at the mesenteric border which is blurred (arrowhead). There is bowel wall thickening, including of the submucosa where there is patchy hypoechogenicity. There is also mesenteric inflammation, represented by marked mesenteric expansion (double headed arrow). Linear mural defects with gas are in keeping with deep ulceration (arrow), a sign of more severe inflammation. iii) Intestinal ultrasound image showing hypervascularity of the terminal ileum manifest as increased color Doppler signal. Note the other signs of active disease; bowel wall thickening, edema (with patchy changes in the submucosa, in contrast to i) where it is uniformly echogenic), deep ulceration (arrow), blurring of the bowel mesenteric border (arrowhead) and mesenteric expansion (double headed arrow). iv) Coronal T2-weighted MRE image showing a long segment of severely active disease involving the distal jejunum and proximal ileum (arrowheads). There is bowel wall thickening and edema, in addition to perimural free fluid, also collecting in the right iliac fossa (asterisk). Additionally, there is chronic disease of the more distal ileum (arrows) which appears damaged with multiple pseudosacculations and likely fibrotic predominant strictures. v) Axial fat-saturated T2-weighted imaging better depicts mural and perimural edema. There is severe active disease in a loop of terminal and distal ileum (arrows) with high T2 signal in part reflecting marked mural and perimural edema, including linear high signal extending into the mesentery. vi) Axial post-contrast image showing mural hyperenhancement, particularly mucosal, and engorgement of the vasa recta supplying the inflamed terminal ileal loop (arrows); the comb sign.
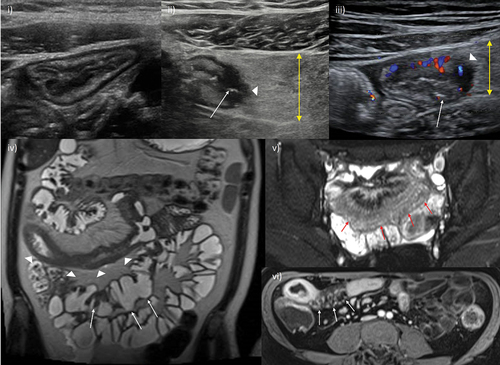
Establishing the relative burden of inflammatory activity on the background of chronic, fibrotic changes necessitate using additional markers of activity, mainly mural and extramural edema and hypervascularity. On MRE, mural edema is best appreciated on fluid-sensitive MRI sequences (T2-weighted) where the fat is suppressed, meaning that edema can be largely separated from fibrofatty proliferative changes [Citation14]. Transmural edema is manifested by perienteric fat stranding and free fluid. On IUS, mural edema is represented by disrupted mural stratification and may be associated with visible ulceration [Citation27,Citation28].
Neoangiogenesis and hypervascularity are further pathophysiological processes in Crohn’s disease and on MRE are represented by increased mural enhancement and engorgement of the vasa recta [Citation1,Citation11,Citation14,Citation29]. Intravenous contrast is not routinely administered in some centers, and fibrostenotic disease may also cause increased enhancement. Using IUS, a recent study found that combining BWT >3 mm with increased color Doppler signal (reflecting hypervascularity) and reduced mural stratification improved the diagnostic accuracy for inflammation over using BWT >3 mm alone [Citation30].
Deep ulceration may give an additional clue to severe, transmural inflammation and is detected as linear mural defects often extending into the mesenteric fat [Citation14]. A caveat is that more superficial ulceration is usually beyond the resolution of cross-sectional imaging and so cannot be depended upon in isolation to detect and grade activity.
4. Imaging based activity scores and their validation
4.1. MRE activity scores
Various scores of activity on MRE have been developed and internally and externally validated against clinical, endoscopic, and/or histopathological reference standards () [Citation4]. The range of scoring systems generally encompasses a similar group of independent prognostic parameters with varying weighting to provide quantitative, objective, and more reproducible activity assessment. Due to their relative complexity, the scores are not in widespread clinical use but particularly in the research and clinical trial setting they form an objective assessment of segmental and global disease activity [Citation31,Citation32].
Table 1. Magnetic resonance enterography-based activity scores: summary of scores including their derivation.
The earliest and best validated MRE score of activity is the MaRIA score [Citation4,Citation33]. This comprises four predictors of activity: mural thickening, mural edema, ulceration, and mural hyperenhancement with a global index formed from six individual segmental indices. A threshold score of 7 has been proposed to denote active disease. It has been validated against activity ileocolonoscopic and capsule endoscopic activity scores [Citation34]. The MaRIA score is seldom used in routine practice as it necessitates multiple, time-consuming, manual measurements of the bowel wall on more than one MRI sequence, as well as the use of intravenous contrast.
Several of these disadvantages are circumnavigated by the simplified MaRIA (sMaRIA) score, which has also been endoscopically validated and reported to be as accurate as the full MaRIA score [Citation35–38]. sMaRIA is less time-consuming, does not require intravenous contrast, and only considers diseased bowel segments. There is more limited evidence for the specificity and reproducibility of the sMaRIA score. A recent retrospective study of 275 bowel segments found an AUC of 0.908 (95% confidence interval [0.857–0.959]) for the sMaRIA to detect active inflammation as judged by the Simple Endoscopic Score for Crohn’s Disease (SES-CD) [Citation39].
The Clermont score is similar to the MaRIA score, although it uses DWI sequences instead of post-contrast enhanced images [Citation12]. It also has the disadvantage of requiring time consuming manual region of interest placement [Citation34]. Further alternative MRE-based activity scores are the London and ‘Extended’ London scores which are partially based on mural thickening and edema, applied to either individual enteric segments or globally to the entire small bowel. Both scores have been validated against histology and endoscopy (CDEIS) with good inter- and intra-reader reproducibility [Citation17,Citation40].
4.2. IUS activity scores
Although numerous IUS activity scores have been proposed, they are currently less robustly validated than MRE scores and also not usually used in routine clinical practice () [Citation25,Citation29]. However, large-scale validation studies are underway. Examples include the Simple US activity score [Citation41,Citation42], and the Bowel US score (BUSS) [Citation43]. Scores typically include parameters generally considered as reliable markers of activity, namely, BWT, increased vascularity on color Doppler, loss of mural stratification, and perimural changes, e.g. fat wrapping, edema [Citation27,Citation28,Citation30].
Table 2. Intestinal ultrasound-based activity scores: summary of more commonly used scores.
BUSS uses BWT and mural vascularity as independent predictors and has been prospectively tested and validated against ileocolonoscopy and the SES-CD. In a study of 49 patients starting treatment, a threshold score of 3.52 was proposed to stratify patients with endoscopically active and inactive Crohn’s disease, achieving a sensitivity of 83% and specificity of 85% [Citation43].
The International Bowel Ultrasound Segmental Activity Score (IBUS-SAS) has recently been developed through a Delphi consensus of 11 experts who identified four main parameters of activity; i) BWT, ii) bowel wall stratification, iii) bowel wall hyperemia on color Doppler, iv) inflammatory mesenteric fat. On blinded case reads, BWT had high reproducibility, and the IBUS-SAS had a near perfect correlation to a global assessment of disease activity [Citation44]. Wang et al. recently externally tested the IBUS-SAS and simple US activity score (SUS-CD) in a retrospective study of 140 patients. They reported excellent inter-operator reproducibility (correlation coefficient of 0.96 and 0.78 respectively). There was reasonable correlation with endoscopic scores and inflammatory biomarkers for the IBUS-SAS (0.511–0.666) which was generally weaker for the SUS-CD, 0.434–0.534 [Citation45].
As we will discuss in the ‘expert opinion’ section, a research priority is to investigate the interchangeability of IUS and MRE parameters of activity, and further validate IUS activity scores and test their responsiveness relative to endoscopy and MRE in larger, prospective, multicenter studies.
5. Imaging therapeutic targets and assessing response
Cross-sectional imaging is an attractive alternative to costly, invasive, and less well-tolerated ileocolonoscopy and nonspecific blood and stool markers of inflammation for assessing treatment response. It also facilitates assessment of the full thickness of the bowel wall and perienteric environment, potentially moving beyond simple endoscopic mucosal healing as a treatment target. However, for imaging to be a useful objective marker in any ‘treat-to-target’ paradigm [Citation2], it must be both responsive to treatment changes and reproducible.
Four categories of therapeutic response based on cross-sectional imaging criteria have been recently proposed by an international expert consensus panel review [Citation1,Citation14]; i) transmural remission (all markers of activity are normalized, both mural and perimural), ii) response (unequivocal improvement in activity severity or extent in a disease segment), iii) stable (no clear change), iv) progression (unequivocal worsening of activity measures, or new sites of disease, or development of complications). In routine clinical practice, such assessments are based on the subjective opinion of the imaging reporter using established imaging activity parameters such as BWT, submucosal T2 intensity/clarity on IUS, and mesenteric changes.
Responsiveness of activity scores has mainly been investigated for MRE. Using paired MRE images from 41 patients pre- and 12 to 14 weeks post-treatment, Hanžel et al. tested the responsiveness of 4 MRE activity scores against a global assessment of response captured using a 100 mm visual analogue scale. All four scores tested exhibited moderate to large responsiveness. The sMaRIA was significantly more responsive than the London score although not the full MaRIA or extended London scores [Citation31].
The response criteria for individual imaging parameters are less clear, as is the optimal time window for reevaluation. Individual imaging parameters of activity vary in their responsiveness and specificity, and response rate is influenced by disease location and phenotype. BWT is an attractive parameter for disease response assessment, being relatively quick and simple to measure. However, as yet no agreed optimal time point for response evaluation has been defined, and when used in isolation and at non-standardized time points, simple changes in BWT may not give an accurate reflection of true response status. In 2022, the International Bowel Ultrasound (IBUS) group provided an expert consensus based on a systematic review, attempting to bring clarity to some of these issues for both adult and pediatric populations [Citation28]. They suggest that, on IUS, treatment response can be defined as BWT reduction of >25% or >2 mm, or >1 mm if there is associated reduced color Doppler signal. They suggested that IUS should be performed at baseline and response assessed at 14 ± 2 weeks (but may be beneficial in some cases at 4–8 weeks) and also 26–52 weeks. Inter-modality response comparisons, for example, between MRE and IUS remain challenging.
5.1. Transmural healing
Transmural healing is receiving considerable attention as a potentially superior treatment target to mucosal healing alone (). Transmural healing can only be assessed by cross-sectional imaging given its ability to evaluate the full thickness of the bowel wall and perienteric changes. Although there is good agreement between transmural healing and endoscopic mucosal healing based on both IUS and MRE (k = 0.63 and 0.64 respectively) [Citation51], there is evidence that transmural healing is associated with improved disease course in terms of reduced progressive bowel damage, better long-term patient outcomes, and more predictive than endoscopic mucosal healing alone [Citation9,Citation10,Citation46–48,Citation52,Citation53]. In an observational study of 80 patients on anti-TNF agents, ‘complete’ responders on IUS (improvement in all diseased segments) had an improved long-term disease trajectory, less need for surgery, steroids, and surgery, compared to partial or non-responders [Citation46]. A recent multicentre study of 404 patients replicated these findings over 5 years of follow-up in the cohort with transmural remission on MRE and ileocolonoscopy [Citation48]. Transmural response on MRE at only 12 weeks could predict steroid-free remission at 1 year after initiating anti-TNF agents [Citation8].
Table 3. Transmural healing: cross-sectional imaging criteria used in recent studies, rates of transmural healing achieved and clinical outcomes.
Although the recent STRIDE (Selecting Therapeutic Targets in Inflammatory Bowel Disease) II consensus statement does not formally list transmural healing as a treatment target, it recognizes that it may represent a deeper level of healing and should be used as an adjunct to endoscopic remission [Citation2].
Transmural healing can be assessed by both MRE and IUS. However, the definition of full imaging remission and transmural healing remain contentious [Citation6]. Strict criteria for transmural healing comprise normalization of mural thickening (≤3 mm), normal perfusion, no mural stratification disruption, perienteric fat stranding, and on MRE, no evidence of edema () [Citation1,Citation2,Citation28,Citation54]. Applying a strict definition of transmural healing means a minority of patients on currently available therapeutics actually achieve this endpoint. For example, the recent STARDUST RCT found that 24% (13/54) of patients on ustekinumab achieved transmural healing on IUS [defined as normalization of all IUS parameters] at 48 weeks [Citation7]. In another cohort on varying forms of biologic therapy, 28% (43/156) achieved transmural healing at 1 year [Citation49]. The VERSIFY trial of 101 patients treated with vedolizumab found a radiological remission rate based on a MaRIA score <7 of 22% at 26 weeks and 38% at 52 weeks, although a MaRIA <7 permits some minor residual bowel wall abnormalities which may explain this higher rate of response compared to trials requiring full normalization of the bowel wall [Citation50]. The recent IBUS group expert consensus statement suggested that transmural response is best assessed at 26–52 weeks [Citation28].
Figure 2. Transmural healing. A 15-year-old male patient with newly diagnosed Crohn’s disease. At baseline, MRE showed moderately active disease involving the terminal and distal ileum (i) axial fat-saturated T2-weighted MRI image showing terminal ileal mural thickening and increased mural T2 signal (arrow), and mesenteric free fluid mainly in the right iliac fossa, ii) corresponding high signal on diffusion weighted images with enlarged reactive mesenteric nodes (arrows). iii) Baseline ileocolonoscopy documented a simple endoscopic score (SES-CD) of 15 iv) after 6 months of infliximab and methotrexate therapy ultrasound fulfilled the criteria for true transmural healing with normal mural thickness (≤3 mm) and stratification, and complete resolution of mesenteric changes. Small bowel motility was subjectively normal. Axial fat-saturated MRE image confirmed transmural healing of the terminal ileum (arrow) was maintained at 12 months (v), and at again at 18 months on IUS (vi). MRI: Magnetic resonance imaging; MRE: MR enterography; IUS: intestinal ultrasound.
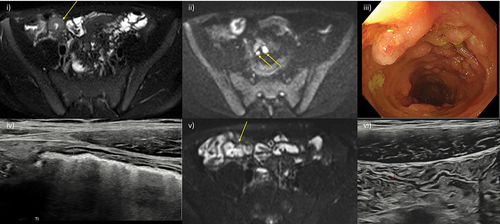
A more pragmatic and less stringent definition of transmural healing may ultimately be more appropriate. A recent study of 40 patients starting anti-TNFα therapy reported a threshold BWT of 3.2 mm best predicted endoscopic remission (SES-CD = 0) at 12–34 weeks. The same study found that those destined to reach endoscopic remission had significantly lower BWT as early as 4–8 weeks after starting therapy compared to those not achieving remission [Citation55]. Similarly, the STARDUST study group found that when response criteria were relaxed to BWT >3 mm, 32% of patients were now deemed to be in sonographic remission (versus 24% using complete bowel wall normalization). This less stringent definition would accommodate the often multiple, subtle residual changes on imaging, particularly in the context of a long disease course and progressive bowel damage. The prognostic significance of ‘full’ bowel wall normalization versus ‘near full normalization’ remains to be determined.
5.2. Cumulative bowel damage
Irreversible bowel damage can result from repeated cycles of inflammation and healing long term. The Lémann index measures this cumulative, irreversible bowel damage including the longitudinal extent and severity of stricturing and penetrating disease, and surgical resection on MRI, CT, and/or endoscopy [Citation56,Citation57]. The index has been validated and updated by the original authors. It may evaluate treatment efficacy beyond simply the burden of disease activity, potentially capturing irreversible damage at an early stage, and also in disease modification trials. The prospective METRIC-EF study (ISRCTN76899103) will report on the predictive ability of the Lémann index, in addition to other disease scores, for disabling disease at 5 years in newly diagnosed Crohn’s disease patients [Citation58].
6. Clinical integration of cross-sectional imaging modalities
The current ECCO-ESGAR guidelines suggest either MRE, IUS, or capsule endoscopy are appropriate for diagnosis and follow-up in Crohn’s disease [Citation1]. Deciding which test is most appropriate for imaging the small bowel is dependent on patient factors (e.g. body mass index, claustrophobia, individual preferences), disease factors (e.g. penetrating disease), and the specific clinical question and access (e.g. availability of imaging platforms and a trained workforce).
A robust IBD service should ideally have access to all imaging modalities which requires investment in technology and training. In the UK for example, IUS is underused in some centers, often due to difficulty in accessing training for radiologists and/or gastroenterologists, and because referral pathways traditionally utilize MRE and CT. Indeed, a national UK survey reported that clinicians were less confident in basing their treatment decisions on IUS findings than MRE [Citation59].
IUS, especially at the point of care, has the advantage of enabling real-time clinical decisions and immediate patient feedback and seems cost-effective [Citation24]. No requirement for oral preparation also contributes to it being an excellent option for regular disease monitoring. The largest study to date directly comparing IUS and MRE in new diagnosis and suspected relapse Crohn’s disease cohorts was the multicenter, prospective METRIC trial, and included 288 patients [Citation24]. Although both IUS and MRE were well tolerated, IUS was generally reported as being more acceptable to patients than MRE, mostly due to the oral bowel required for the latter [Citation7]; 99% patients were willing to repeat the test versus 91% for MRE [Citation13,Citation24]. Despite this, patients generally considered diagnostic accuracy as the most important factor and so this should always be considered when counseling patients in joint decision-making.
MRE and IUS are both accurate in the initial diagnosis and assessment of Crohn’s disease, and there is good agreement between these two modalities [Citation24,Citation60,Citation61]. The METRIC study group reported that the sensitivity of MRE was slightly greater than IUS for detecting active small bowel disease, 97% and 92%, respectively. Their respective specificities were 96% and 84% [Citation24]. Furthermore, there is high concordance between IUS and MRE in disease longitudinal length assessment [Citation62]. However, in the METRIC trial, MRE was superior in assessingthe full extent of disease in the small bowel [Citation1,Citation20,Citation55]; the sensitivity and specificity for small bowel disease extent was 80% and 95% for MRE, significantly greater than IUS (70% and 81% respectively). Of note, compared to MRE, IUS had higher sensitivity for colonic disease in newly diagnosed patients.
A meta-analysis comparing capsule endoscopy, MRE and IUS found a comparable diagnostic accuracy for the three modalities in detecting small bowel disease in both suspected and established Crohn’s disease [Citation63], although it was published just before the METRIC trial. Capsule endoscopy was superior to MRE in detecting proximal small bowel disease; portions of the jejunum are often sub optimally distended using traditional MRE oral preparation protocols. No significant difference was observed in detecting severe disease on resected surgical specimens between IUS and contrast-enhanced IUS, compared to MRE [Citation62].
The METRIC study findings were replicated in a recent retrospective study of 115 patients. MRE had a greater diagnostic accuracy for detecting small bowel disease versus IUS and CTE. However, there was no significant difference between the accuracy for active disease between MaRIA and IBUS-SAS against an endoscopic SES-CD reference standard [Citation64].
MRE and IUS have both been shown to have a large impact on the decision-making process.
In one study by Allocca et al., 60 patients with ileocolonic disease were assessed with MRE, IUS, and ileocolonoscopy. The resultant management changes were highly concordant between these IUS and MRE (0.8) [Citation60]. A sub-study of the METRIC trial also found similar impact of MRE and IUS on clinical decision-making [Citation19]. MRE alone was found to be sufficient to make a management decision in 80% of 100 Crohn’s disease patients versus 34% using ileocolonoscopy alone. In another study, 100 patients with ileocolonic disease underwent both MRE and ileocolonoscopy within 1 week of each other. The addition of MRE information to endoscopic findings led to therapeutic change in 28% of cases and increased the clinicians’ level of confidence. Interestingly, surgery and anti-tumor necrosis factor (TNF) therapy were indicated more often when MRE was performed before endoscopy [Citation65].
Ultimately, MRE and IUS both play an important role in the management of Crohn’s disease and local clinical pathways should maximize their relative advantages. We await robust data on the interchangeability of MRE and IUS markers and scores of activity, a common conundrum when different modalities are used in the follow-up schedule.
7. Novel cross-sectional imaging applications
7.1. Imaging fibrostenotic disease
Invariably both fibrosis, muscle hypertrophy, and inflammation co-exist in strictures due to progressive bowel damage accumulated over time. A challenging aspect in Crohn’s disease management is the assessment of fibrosis relative to the inflammatory burden, particularly as this may be distributed heterogeneously even within an individual stricture. Each of these components is targeted in different ways depending on the predominant phenotype, conventionally medical therapy for inflammation and endoscopic balloon dilation and/or surgical, e.g. stricturoplasty, for fibrosis predominance. New antifibrotic therapies are being developed which may impact on current therapeutic paradigms. An advantage of cross-sectional imaging is the transmural assessment of a stricture, in contrast to the limited mucosal interrogation during endoscopy, even if the stricture is endoscopically accessible [Citation66].
If fibrosis becomes a therapeutic target, it is crucial to establish a standardized, reproducible, and validated definition of a stricture to triage patients into clinical trials and then assess response. To date, heterogeneous definitions have been applied in the literature. The STAR consortium [Citation15] and the CONSTRICT expert consensus panel [Citation67] comprising gastroenterologists and radiologists define a stricture on MRE as i) luminal narrowing >50%, ii) BWT > 25% relative to adjacent normal bowel, and iii) pre-stenotic bowel dilation >3 cm. Requiring all three criteria maximizes specificity, likely at the cost of reduced sensitivity, although this may be the most robust approach for initial trials of new therapeutics.
Many of the imaging parameters used to assess disease activity are influenced by fibrosis, particularly BWT and mural hyperenhancement. A recent systematic review found that the sensitivity and specificity of MRE for histopathologically confirmed fibrosis was 75–100% and 91–96%, respectively, and that of IUS was 80–100% and 63–75%, although stricture definitions varied or were not stated [Citation15].
Active inflammation tends to produce mucosal enhancement, ulceration, and blurring of mural stratification and margins. Conversely, delayed progressive enhancement at 7 minutes has been reported to correlate with underlying fibrosis [Citation60]. Another promising MRI technique is the magnetization transfer ratio (MTR, ). As collagen is deposited in the bowel wall, this along with other macromolecules can be estimated. Several mainly single-center studies have reported that MTR is related to histopathologically quantified fibrosis [Citation68]. However, in a recent prospective multicenter study of 60 patients, neither delayed contrast enhancement nor MTR reliably differentiated between grades of fibrosis [Citation69], emphasizing the translation gap between single and multisite research.
Figure 3. Imaging and quantifying fibrosis. i) Axial T2-weighted image of the pelvis demonstrating a long stricture of the terminal ileum (arrow). ii) Corresponding magnetization transfer imaging of the stricture alongside a quantitative color scale shows decreased magnetization transfer ratio (MTR) in the wall of this segment with a range of 20 to 60.
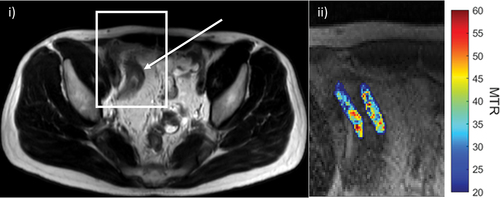
Other promising MRI techniques include T1 mapping, which has been used in other organs, most notably the heart, and the DWI perfusion fraction (as a surrogate for tissue blood flow) [Citation70], although the research in the area is relatively immature.
IUS elastography can quantify tissue stiffness, either through strain or shear-wave elastography, acting as a surrogate for fibrosis. A recent systematic review of 275 patients reported an overall moderate to good accuracy for IUS elastography to detect histologically confirmed fibrosis (in 10 out of 12 studies, using varied measures) [Citation71], and in patients on anti-TNF therapy, elevated strain ratios were predictive of future surgery, with lower ratios present in patients with transmural healing [Citation72]. Currently, IUS elastography remains mainly a research tool with multi-center validation studies still pending.
It is also possible to measure bowel wall stiffness on MRI using shear waves produced by external hardware. Again, research is in its infancy but a recent prospective study of 69 patients found a threshold of 3.57 kPa was predictive of adverse clinical events over a median follow-up of 450 days [Citation73].
Functional nuclear medicine techniques also show promise in fibrosis quantification, particularly when combined with conventional MRI sequences. Gallium-68 labeled fibroblast activation protein inhibitor positron emission tomography MR (68Ga-FAPI PET/MR) enterography is of particular current interest. Scharitzer et al. used histopathological FAPI expression in the bowel wall as a reference standard to show that 68Ga-FAPI uptake was significantly greater in fibrotic segments across all bowel layers (mean maximum standardized uptake value [SUVmax] of 7.6 versus 2.0 without fibrosis). Further, it was able to distinguish between mild, moderate, and severe grades of histological fibrosis [Citation74]. Using a cut-off SUVmax value of 3.2, the AUC for fibrosis prediction was 0.94. New total body PET technology will significantly reduce the radiation exposure for PET scanning techniques [Citation75].
7.2. Fat wrapping
There is increasing recognition of the role of adipose tissue in several physiological and disease pathways, including the inflammatory disease [Citation76]. In the context of Crohn’s disease, mesenteric fat hypertrophy or fat wrapping is not simply a bystander of chronic inflammation but may in part be driving the underlying pathophysiology.
Fat wrapping is linked to transmural inflammation, fibrosis, and stricturing on histopathology [Citation77]. Mesenteric fat dysfunction may play an active role in the inflammatory process as adipocytes have been shown to be a source of pro-inflammatory and pro-fibrotic cytokines in Crohn’s disease [Citation78]. Further indicators for this role come from the observation of reduced disease recurrence following small bowel resections which include the mesentery [Citation79]. In surgical resection specimens, translocated bacteria cause immune cell activation and production of pro-adipogenic and pro-fibrotic factors [Citation80]. Therefore, as our knowledge of Crohn’s disease pathophysiology grows, we can start to consider fat wrapping as a potential novel therapeutic target and predictor of disease course.
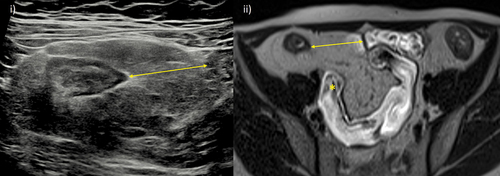
Mesenteric fat wrapping may be observed on MRE, usually manifesting as loop and mesenteric vessel separation. Often, it is more readily delineated on IUS with fat ‘creeping’ around >180° of the small bowel circumference (). Fat wrapping as measured by MRI has been shown to reduce with successful anti-TNF therapy [Citation81]. Fat wrap may also act as a further noninvasive marker of intestinal fibrosis. A mesenteric creeping fat index formed by measurements on CTE has been proposed. This index was moderately accurate for distinguishing mild and moderate-severe fibrostenotic disease in surgical specimens [Citation82].
Figure 4. Mesenteric fat wrapping. A 29-year-old female Crohn’s disease patient with signs of extensive fat wrapping of the terminal ileum. i) IUS with mesenteric fat wrapping involving most of the small bowel circumference and displacing a loop of ileum (arrow). ii) Comparable axial T2-weighted MRE image, also showing resultant separation of the terminal ileum from other loops of pelvic ileum (arrow). MRE also demonstrates terminal ileal stricturing disease with reduced luminal caliber and mild pre-stenotic dilation (asterisk).
Body fat distribution and muscle quality (sarcopenia) can be accurately measured using both CT and MRI. An increased ratio of visceral to subcutaneous fat in Crohn’s disease is linked to disease activity, e.g. biochemical and clinically based disease activity scores [Citation83] and following therapy, the prognosis for patients who exhibit sarcopenia and high visceral to subcutaneous fat ratio may be worse [Citation84].
Cross-sectional imaging assessment of fat and muscle may play an increasing role in disease activity assessment and prognostication.
7.3. Motility
Inflamed small bowel exhibits reduced peristaltic motility from a variety of underlying, often coexistent, processes. Motility can now be assessed using cine-MRI techniques where multiple images of the same bowel loop are taken in either a 2- or 3-dimensional plane. IUS enables clinically useful real-time, subjective assessment of enteric motility, although validated, reproducible quantitative parameters of IUS motility are as yet lacking.
Software (e.g. using image registration techniques [Citation85]) can now quantify both segmental and global bowel motility on MRI (). Inflamed small bowel segments demonstrate reduced motility which is correlated with endoscopic and pathological inflammation, i.e. motility on MRI is a biomarker of activity [Citation72,Citation73]. A multicenter, prospective study noted a negative correlation of terminal ileal motility with the CDEIS and a histologic inflammatory score and highly sensitivity (93% and 92% respectively), outperforming the MaRIA score (78% and 75%) [Citation86]. Motility is also responsive to changes in therapy [Citation87]. Patients clinically responding to biological treatment have significantly improved motility from baseline compared to non-responders, as early as 12 weeks [Citation88]. The MOTILITY trial (ISRCTN14481560) will shortly report on the ability of segmental small bowel motility measurements to improve prediction of therapeutic response at 1 year in patients starting biologic therapy for small bowel Crohn’s disease in comparison to plasma C-reactive protein (CRP).
Figure 5. Software assisted quantification of motility. i) Pre-treatment motility map of active terminal ileal disease (region of interest drawn based on a reference image) with an average motility value of 170. ii) After 8 weeks on treatment, disease in the terminal ileum responded clinically and based on MRE parameters of activity. This included a measurable improvement in motility; average of 354.
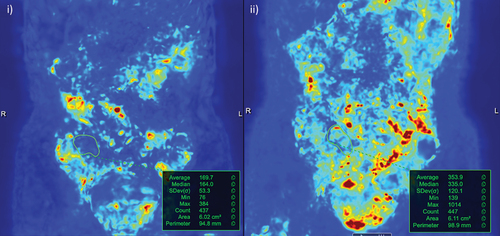
Abnormal patterns of motility in small bowel remote from diseased segments have been associated with the severity of abdominal symptoms and may have mechanistic insights into abdominal pain in patients with Crohn’s disease [Citation89].
7.4. Diffusion-weighted imaging
DWI is an additional MRI method which provides information on tissue composition and histology. The differential Brownian motion of water molecules within tissues is captured. When this random motion is impeded, e.g. hypercellular tissue, there is a corresponding higher value on DWI (diffusion restriction).
DWI is reported to accurately separate active inflammation and inactive disease, reported 80–100% sensitivity against a range of reference standards of differing quality [Citation90]. However, fibrosis also restricts diffusion and so in itself DWI is not a ‘magic bullet’ for the quantification of enteric inflammation. Indeed, it has relatively low specificity for inflammation with a false-positive rate as high as 40% [Citation90]. Nonetheless, it plays a useful role in clinical practice.
One of the potential uses of DWI is to negate the need for intravenous contrast. A modified sMaRIA score using DWI had a comparable diagnostic performance for active inflammation as the original sMaRIA using intravenous contrast, and both protocols were moderately correlated with the SES-CD [Citation39]. This finding was replicated in a group of 61 patients, where DWI was non-inferior to intravenous contrast enhanced MRI sequences for identifying small bowel inflammation using an endoscopic reference standard [Citation17,Citation18].
ADC maps are calculated from DWI and provide quantitative information on the diffusion of water molecules in tissues. A recent meta-analysis of 21 studies found a pooled correlation coefficient of −0.8 between the ADC value and endoscopic inflammation (CDAI) and −0.66 for both SES-CD and MaRIA score [Citation91]. There is some evidence that low ADC values are linked to histologically confirmed fibrosis, including from a prospective, multicenter study of Crohn’s disease patients with fibrostenotic disease prior to surgery [Citation69,Citation92]. However, as noted, the overlap of inflammation and fibrosis effects on DWI and ADC means reliable separation between both process is currently not possible.
7.5. Contrast enhanced ultrasound
CEUS is an adjunct to conventional B-mode IUS and has been used widely to image blood flow in the macro- and micro-circulation in a variety of organs. It uses intravenous contrast agents comprising microbubbles or nanobubbles of gas which oscillate in response to applied sonographic waves. The need for an intravenous injection of a contrast agent means compared to conventional IUS, CEUS is a more timely, costly, and potentially less acceptable option for patients.
The additional value of CEUS to assess for activity remains controversial and is infrequently used in practice and currently mainly reserved for the research setting. CEUS and conventional IUS have comparably high levels of sensitivity and specificity to detect activity [Citation43,Citation62]. CEUS can provide a more quantitative assessment of mural and extramural vascularity, e.g. time intensity curve analysis derived parameters; however, to date, there is little evidence that this adds to accuracy for disease activity or responsiveness to changes [Citation62,Citation93].
There are preliminary data in small cohorts suggesting CEUS may help separate fibrosis from inflammation in stricturing disease, but utility for this indication remains to be fully validated [Citation93].
7.6. Dual energy CT
Given its use of ionizing radiation, CT is mainly used in the acute setting. However, new techniques such as iterative reconstruction are reducing radiation doses from CT. Dual energy CT (DECT) simultaneously gathers data from two different energy levels enabling creation of distinct datasets such as iodine maps of tissue iodine accumulation. These maps can act as a surrogate marker of perfusion. This technique facilitates improved detection of differences in bowel wall characteristics between adjacent loops as well as extramural findings [Citation94].
Iodine levels are greater with bowel inflammation and a recent systematic review reported reasonable accuracy for active disease (optimal iodine cutoff values of 2–3.7 mg/ml based on endoscopic reference standards), although there was insufficient evidence to show any superiority of DECT relative to conventional CT [Citation95]. A normalized measure of iodine concentration was accurate in differentiating endoscopic mucosal healing from non-mucosal healing in 94 patients post-infliximab therapy (AUC 0.929), further improved with the addition of fecal calprotectin levels (AUC 0.964) [Citation96].
The utility of DECT awaits multicenter clinical trial validation.
7.7. Harnessing artificial intelligence
Artificial intelligence (AI) has several applications to augment our ability to assess the small bowel and potentially streamline clinical practice. One key emerging trend is semi-automated bowel segmentation to identify and quantify inflammation in a reproducible manner, which is less subjective than individual reporter interpretation alone [Citation97,Citation98]. These segmentation tools more objectively extract small bowel features like BWT, luminal diameter, and length of disease. 3D segmentation models are likely to emerge in the near future, validated against endoscopy and assessed for inter-reader agreement, key to any potential clinical use.
There has been a surge in interest in radiomics where software can extract a large number of candidate quantitative imaging-based features which are assessed for prognostic value. A few radiomics-based models pertaining to Crohn’s disease have appeared in the literature. One such developed radiomics model uses features on MRE and CTE to identify moderate-to-severe fibrosis with a similar accuracy as the interpreting radiologists [Citation31]. A recent mouse model of intestinal fibrosis found that the bowel wall magnetization transfer ratio and textural analysis on T2-weighted MRI can non-invasively distinguish histological fibrosis. The MRI textural feature of entropy was superior in inflammation detection with coexistent fibrosis and able to assess response to antifibrotic therapy [Citation99]. Furthermore, there are models which can automatically extract the most discriminatory imaging features without prior human interaction, deep-learning models. One such model has been found to exceed the ability of radiologists to identify fibrosis on CTE [Citation100].
The precise nature of how these promising AI solutions may be implemented in practice is unclear, but it is a rapidly evolving field with potential to improve automated analysis, diagnostic accuracy, radiology workflow, and reduce interobserver variation. A key limitation is that models are generally poorly externally evaluated, partly due to suboptimal reporting of the parameters required to validate the index model, and difficulties accessing the large number of external datasets need for validation. It is also crucial that radiomics models incorporate clinical variables given their already proven utility. Ultimately, such techniques must be shown to improve patient outcomes if they are to be widely adopted.
8. Predicting disease trajectory and response to treatment
Cross-sectional imaging may have a role in predicting clinical outcomes and progressive bowel damage [Citation101,Citation102]. Early, noninvasive biomarkers of therapeutic response and disease course are a crucial research interest in IBD. The use of transmural healing as a treatment target has been discussed above, but imaging features at baseline may also have prognostic utility.
8.1. IUS predictors
BWT just prior to starting treatment may predict response on IUS. In a prospective cohort of 188 patients starting varying biological therapies, a greater BWT at baseline IUS independently predicted reduced rates of transmural healing at 3 and 12 months in [Citation49]. Contrast enhanced IUS washout rate and absence of color Doppler signal improved accuracy of a predictive model for early endoscopic response at 4–8 weeks versus reduced BWT alone in patients commencing anti-TNF therapy [Citation55].
In a large prospective study of ileocolonic Crohn’s disease including 49 patients, baseline BUSS predicted endoscopic response with an accuracy of 80%, and remission with 78% accuracy. At 12 months, BUSS also predicted adverse disease outcomes, and a baseline complication, e.g. penetrating or stricturing disease, was predictive of future surgery [Citation43].
8.2. MRE predictors
A recent longitudinal cohort study of 72 patients with at least 5 years of follow-up identified MRE parameters predicting long-term bowel damage. The baseline predictors were ileal disease location, stricturing, and/or fistulating disease phenotype. Disease duration >10 years was also predictive [Citation103]. In a multicenter study of 142 Crohn’s disease patients, 39.4% had signs of bowel damage on cross-sectional imaging at diagnosis. Using multivariable analysis, bowel damage independently predicted future surgery and hospitalization (hazard ratios of 3.21 and 1.88 respectively) [Citation104]. Other reported potential MRE predictors for future surgical intervention include increased disease length, perienteric inflammation, diffusion restriction, complex fistulation, mesenteric fat wrapping, and stricturing with pre-stenotic dilation [Citation105], although such data require prospective external validation.
There are emerging data that residual cross-sectional imaging changes in the neoterminal ileum following surgery may better predict recurrence than mucosal assessment with endoscopy. Specifically in a retrospective analysis of 216 patients undergoing endoscopy and either CTE or MRE after resection, those negative on endoscopic assessment but with positive imaging for active disease (41.7%, 90/216) had greater future endoscopic and surgical recurrence (hazard ratio 4.16) [Citation106].
The ongoing prospective METRIC-EF study (ISRCTN76899103) will report on the predictive ability of disease activity and severity scoring systems, including MaRIA and sMaRIA scores, for disabling disease at 5 years in newly diagnosed Crohn’s disease patients [Citation58].
9. Conclusion
MR enterography and ultrasound are accurate, objective techniques for diagnosis, disease phenotyping and response assessment in Crohn’s disease. These qualities can be harnessed to update therapeutic targets to sustained, deep remission, and transmural healing as part of a ‘treat-to-clear’ paradigm. The value added by cross-sectional imaging is ever-increasing, with objective validated scores of disease activity and severity, quantitation of motility, evolving techniques to measure fibrosis, harnessing artificial intelligence, and identifying predictors of disease trajectory and response.
Making full use of the technology available to non-invasively image the small bowel in a standardized manner can improve timely clinical decision-making, lead to better patient outcomes and experience, contribute to a more personalized management approach, and accelerate pharmacological development.
10. Expert opinion
Cross sectional imaging is established in the management of small bowel Crohn’s disease. We know its accuracy for diagnosis and staging, activity assessment and increasingly for treatment response evaluation. We also know the importance of training, the range of interobserver variability, and the strengths and limitations of the various imaging modalities. However, there is much still to be done.
Much imaging research suffers from being single center with relatively small patient cohorts. In recent years, there have been several pivotal prospective multicenter trials of cross-sectional imaging in IBD, but more are needed. MRE, IUS, and CT all play a role in managing Crohn’s disease patients. Radiologists need to work closely with their clinical colleagues to establish care pathways which define how the various imaging modalities are best employed, considering availability, cost-effectiveness, and patient preference. We need to understand the interchangeability of MRE and IUS biomarkers of activity, fibrostenotic disease, and other complications. We currently do not fully understand which modality (if any) is superior in assessing treatment response, but it is clear all have utility. Multicenter, multi-arm prospective trials need to assess if they can be used interchangeably, and their respective concordance and accuracy for categorizing response. Such data would inform training and aid service planning.
It is crucial that imaging is integrated with other clinical information, including blood and stool markers and endoscopy. The clinical and cost-effectiveness of a combined clinical-radiological pathways needs investigation. Suggested endpoints for disease modification trials by the SPIRIT-IOIBD consensus group comprise patient quality of life outcomes, medium-term complications, e.g. accumulated bowel damage, surgery, hospitalization, permanent stoma, and long-term complications [Citation107], and are an example of a synergistic approach.
MRE disease activity scores are generally better validated than IUS scores, but larger scale trials of the latter are underway which will provide important data to justify wider use. Imaging activity scores are currently not accepted as primary endpoints in clinical trials of new therapeutics but are an attractive alternative to nonspecific patient symptom scores and invasive colonoscopy. It is crucial that we collate multicenter trial data to assess the responsiveness and reproducibility of these scores in the ‘real world,’ their association with currently accepted primary trial endpoints, and ultimately on patient outcomes.
Going further, transmural healing is associated with better long-term patient outcomes but is hard to achieve with current therapeutics and as not yet a primary treatment target. An internationally agreed definition of transmural is required, with arguments both for complete normalization of the bowel wall, or for allowing minor persisting changes. Data suggest that in many patients with reassuring colonoscopy, the presence of residual imaging abnormalities infers a worse prognosis, but a strict definition requiring bowel wall normalization (‘treat-to-clear’) may not be pragmatic in a real world setting given how difficult it is to achieve currently.
With new anti-fibrotic drugs on the horizon, there is a need for imaging to be better able to quantify fibrosis and muscle hypertrophy. Significant advances have been made by the STAR and CONSTRICT consortia to unify stricture definitions and response criteria in anticipation of clinical trials [Citation15,Citation67]. These definitions currently use morphological observations such as bowel wall thickening and upstream bowel dilation. Imaging techniques that directly probe stricture composition such a delayed contrast enhanced enhancement and MTR for MRI and elastography for IUS are still undoubtedly worthy of research, but early multicenter trials have not been promising to date. Lessons must be learned from such trials for the next iterations. Research into new imaging techniques including functional tracers, for example 68 Ga-FAPI, are a priority. In future years, radiation dose exposure will fall significantly with the development of total body PET technology, removing a significant barrier to wider use of nuclear medicine techniques.
The role of imaging in prognostication both at diagnosis and as part of treatment pathways is another area requiring further investigation. Initial data suggest that those with more advanced imaging activity parameters may have worse longer-term outcomes, but this needs to be replicated in large prospective studies. The utility, if any, of imaging in the decision to stop biologics is also unknown.
As a community, we should not neglect training the next generation. Radiologists and clinicians most work together to develop robust training infrastructure informed by consensus guidelines and put aside any ‘turf wars’ for the ultimate benefit of patients.
Looking further forward, it is clear AI will play a major role in modern IBD management. Imaging analysis will likely become (semi)automated and although very much in its infancy, machine learning and radiomics techniques may extract useful data from medical image beyond the resolution of the human eye.
Looking forward over the next 5 years or so, we speculate that multidisciplinary collaborative efforts between key stakeholders including patients, radiologists, gastroenterologists, endoscopists, histopathologists, and surgeons will translate into better defined patient care pathways and integration of cross-sectional imaging. Such efforts will lead to improved training pathways and resource planning.
Cross-sectional imaging disease response assessment criteria will become standardized and supported by large-scale trial data. The large number of cross-sectional imaging-based activity scores will be further validated and play an increasing role in therapeutic trials, particularly those targeting the small bowel.
Transmural healing will be better defined and will become formally accepted as a primary treatment target and potentially included as an end point for clinical trials. Trials of new antifibrotic agents will have commenced with imaging at the forefront of patient selection and response assessment. Such trials will allow testing of novel imaging techniques to better define stricture composition and quantify the relative fibrotic and inflammatory burden. Falling radiation doses from PET scanning will facilitate wider use of novel tracers. We envisage that extraction of data from cross-sectional imaging will become increasingly automated and new metrics such as motility will be more widely used. Baseline predictors of disease course will be invaluable to provide a more personalized patient management pathway and improved transparency in clinical decision-making. AI technology will be implemented to better monitor patients’ progress and facilitate early investigations and treatment.
Article highlights
Cross-sectional imaging facilitates noninvasive evaluation of the full thickness of small bowel wall and perienteric environment, and accordingly, is now central in Crohn’s disease management.
Techniques such as magnetic resonance enterography, intestinal ultrasound, and CT enterography are accurate in identifying and grading disease activity, defining morphological disease phenotype, and are responsive to therapeutic interventions.
Transmural healing is emerging as a therapeutic endpoint with improved long-term outcomes compared with those achieving endoscopic mucosal healing alone.
Novel imaging applications can now non-invasively quantify motility, which may have utility as a biomarker of activity and therapeutic response. There is increasing recognition of the importance of extramural disease manifestations such as mesenteric fat wrapping. Accurate and robust quantification of stricture fibrosis currently remains elusive.
Standardized and validated imaging disease activity scores together with morphological observations in stricturing disease will likely play an increasing role in evaluating the small bowel in pharmacological trials.
Research priorities include optimizing use of the various imaging modalities in patient care pathways, definitions of, and evidence for imaging-based therapeutic targets such as transmural healing, better phenotyping of stricturing disease, and developing novel techniques, including integration of artificial intelligence.
Abbreviations
| AI | = | Artificial Intelligence |
| AUC | = | Area Under the receiver operating characteristic Curve |
| BUSS | = | Bowel US Score |
| BWT | = | Bowel Wall Thickness |
| CDEIS | = | Crohn’s Disease Endoscopic Index of Severity |
| CEUS | = | Contrast Enhanced Ultrasound |
| CDAI | = | Crohn’s disease Activity Index |
| CRP | = | C-reactive protein |
| DECT | = | Dual Energy Computed Tomography |
| DWI | = | Diffusion Weighted Imaging |
| ECCO | = | European Crohn’s and Colitis Organisation |
| ESGAR | = | European Society of Gastrointestinal and Abdominal Radiology |
| 68Ga-FAPI PET | = | Gallium-68 labeled fibroblast activation protein inhibitor positron emission tomography |
| IBD | = | Inflammatory bowel disease |
| IBUS-SAS | = | International Bowel Ultrasound Segmental Activity Score |
| IUS | = | Intestinal Ultrasound |
| MaRIA | = | Magnetic Resonance Index of Activity |
| MRI | = | Magnetic Resonance Imaging |
| MRE | = | Magnetic Resonance Enterography |
| MTR | = | Magnetization Transfer Ratio |
| POCUS | = | Point of Care Ultrasound |
| PEG | = | polyethylene glycol |
| SES-CD | = | Simple Endoscopic Score for Crohn’s Disease |
| sMaRIA | = | simplified Magnetic Resonance Index of Activity |
| STRIDE | = | Selecting Therapeutic Targets in Inflammatory Bowel Disease |
| SUS-CD | = | Simple US activity score |
| SUVmax | = | maximum standardized uptake value |
| TNF | = | Tumor Necrosis Factor |
| Treat-to-Target | = | treat to target approach in inflammatory bowel disease. |
Declaration of interest
S Taylor is a Motilent shareholder, has grant support from Takeda, and is a consultant for AstraZeneca. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgement
We would like to acknowledge Beth Fisher, Motilent for , and Dr Caroline Hoad, University of Nottingham for .
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Kucharzik T, Tielbeek J, Carter D, et al. ECCO-ESGAR topical review on optimizing reporting for cross-sectional imaging in inflammatory bowel disease. J Crohns Colitis. 2022;16:523–543. doi: 10.1093/ecco-jcc/jjab180
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting therapeutic targets in inflammatory bowel disease [STRIDE] initiative of the international organization for the study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031
- Wilkens R, Novak KL, Maaser C, et al. Relevance of monitoring transmural disease activity in patients with Crohn’s disease: current status and future perspectives. Ther Adv Gastroenterol. 2021;14:17562848211006672. doi: 10.1177/17562848211006672
- Grand DJ, Deepak P, Rimola J. MRE evaluation of intestinal inflammation: qualitative and quantitative assessment. Top Magn Reson Imaging. 2021;30(1):13–22. doi: 10.1097/RMR.0000000000000270
- Rimola J, Torres J, Kumar S, et al. Recent advances in clinical practice: advances in cross-sectional imaging in inflammatory bowel disease. Gut. 2022;71:2587–2597. doi: 10.1136/gutjnl-2021-326562
- Geyl S, Guillo L, Laurent V, et al. Transmural healing as a therapeutic goal in Crohn’s disease: a systematic review. Lancet Gastroenterol Hepatol. Lancet Gastroenterol Hepatol. 2021;6(8):659–667. doi: 10.1016/S2468-1253[21]00096-0
- Kucharzik T, Wilkens R, D’Agostino M-A, et al. Early ultrasound response and progressive transmural remission after treatment with ustekinumab in Crohn’s disease. Clin Gastroenterol Hepatol. 2023;21(1):153–163.e12. doi: 10.1016/j.cgh.2022.05.055
- Messadeg L, Hordonneau C, Bouguen G, et al. Early transmural response assessed using Magnetic resonance imaging could predict sustained clinical remission and prevent bowel damage in patients with Crohn’s disease treated with anti-tumour necrosis factor therapy. J Crohns Colitis. 2020;14:1524–1534. doi: 10.1093/ecco-jcc/jjaa098
- Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn’s disease. Clin Gastroenterol Hepatol. 2018;16(7):1089–1097.e4. doi: 10.1016/j.cgh.2018.01.024
- Ma L, Li W, Zhuang N, et al. Comparison of transmural healing and mucosal healing as predictors of positive long-term outcomes in Crohn’s disease. Ther Adv Gastroenterol. 2021;14:175628482110162. doi: 10.1177/17562848211016259
- Goodsall TM, Noy R, Nguyen TM, et al. Systematic review: patient perceptions of monitoring tools in inflammatory bowel disease. J Can Assoc Gastroenterol. 2021;4:e31–e41. doi: 10.1093/jcag/gwaa001
- Buisson A, Gonzalez F, Poullenot F, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1425–1433. doi: 10.1097/MIB.0000000000001140
- METRIC investigators, Miles A, Bhatnagar G, et al. Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn’s disease: patient acceptability and perceived burden. Eur Radiol. 2019;29(3):1083–1093.
- Bruining DH, Zimmermann EM, Loftus EV, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and Magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology. 2018;286:776–799. doi: 10.1148/radiol.2018171737
- Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019;68:1115–1126. doi: 10.1136/gutjnl-2018-318081
- Semelka RC, Ramalho M. Gadolinium deposition disease: Current state of knowledge and expert opinion. 2023. [cited 2023 Apr 16]. InternetPublish Ahead of Print Invest Radiol. 58(8):523–529. doi: 10.1097/RLI.0000000000000977
- Puylaert CAJ, Nolthenius CJT, Tielbeek JAW, et al. Comparison of MRI activity scoring systems and features for the terminal ileum in patients with Crohn disease. Am J Roentgenol. 2019;212:W25–W31. doi: 10.2214/AJR.18.19876
- Seo N, Park SH, Kim K-J, et al. MR Enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology. 2016;278:762–772. doi: 10.1148/radiol.2015150809
- Taylor SA, Mallett S, Bhatnagar G, et al. Magnetic resonance enterography compared with ultrasonography in newly diagnosed and relapsing Crohn’s disease patients: the METRIC diagnostic accuracy study. Health Technol Assess. 2019;23(66):1–270. doi: 10.3310/hta23420
- Jesuratnam-Nielsen K, Løgager VB, Munkholm P, et al. Diagnostic accuracy of three different MRI protocols in patients with inflammatory bowel disease. Acta Radiol Open. 2015;4(6):205846011558809. doi: 10.1177/2058460115588099
- Bhatnagar G, Mallett S, Quinn L, et al. Influence of oral contrast type and volume on patient experience and quality of luminal distension at MR Enterography in Crohn’s disease: an observational study of patients recruited to the METRIC trial. Eur Radiol. 2022;32:5075–5085. doi: 10.1007/s00330-022-08614-9
- Parente F. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut. 2004;53:1652–1657. doi: 10.1136/gut.2004.041038
- Pallotta N, Vincoli G, Montesani C, et al. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in crohnʼs disease: a prospective comparative study versus intraoperative findings. Inflamm Bowel Dis. 2012;18(1):74–84. doi: 10.1002/ibd.21678
- Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease [METRIC]: a multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(8):548–558. doi: 10.1016/S2468-1253[18]30161-4
- Goodsall TM, Nguyen TM, Parker CE, et al. Systematic review: gastrointestinal ultrasound scoring indices for inflammatory bowel disease. J Crohns Colitis. 2021;15:125–142. doi: 10.1093/ecco-jcc/jjaa129
- Rao N, Kumar S, Taylor S, et al. Diagnostic pathways in Crohn’s disease. Clin Radiol. 2019;74:578–591. doi: 10.1016/j.crad.2019.03.013
- Goodsall TM, Jairath V, Feagan BG, et al. Standardisation of intestinal ultrasound scoring in clinical trials for luminal Crohn’s disease. Aliment Pharmacol Ther. 2021;53:873–886. doi: 10.1111/apt.16288
- Ilvemark JFKF, Hansen T, Goodsall TM, et al. Defining Transabdominal intestinal ultrasound treatment response and remission in inflammatory bowel disease: systematic review and expert consensus statement. J Crohns Colitis. 2022;16(4):554–580. doi: 10.1093/ecco-jcc/jjab173
- Bots S, Nylund K, Löwenberg M, et al. Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. J Crohns Colitis. 2018;12:920–929. doi: 10.1093/ecco-jcc/jjy048
- Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(5):908–921.e6. doi: 10.1016/j.cgh.2020.07.067
- Hanžel J, Jairath V, Ma C, et al. Responsiveness of Magnetic resonance enterography indices for evaluation of luminal disease activity in Crohn’s disease. Clin Gastroenterol Hepatol. 2022;20(11):2598–2606. doi: 10.1016/j.cgh.2022.01.055
- Schulberg JD, Wright EK, Holt BA, et al. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn’s disease strictures [STRIDENT]: an open-label, single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7(4):318–331. doi: 10.1016/S2468-1253[21]00393-9
- Gower-Rousseau C, Sarter H, Savoye G, et al. Validation of the inflammatory bowel disease Disability index in a population-based cohort. Gut. 2017;66:588–596. doi: 10.1136/gutjnl-2015-310151
- Rimola J, Alvarez-Cofiño A, Pérez-Jeldres T, et al. Comparison of three magnetic resonance enterography indices for grading activity in Crohn’s disease. J Gastroenterol. 2017;52:585–593. doi: 10.1007/s00535-016-1253-6
- Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified Magnetic resonance index of activity for Crohn’s disease. Gastroenterology. 2019;157(2):432–439.e1. doi: 10.1053/j.gastro.2019.03.051
- Roseira J, Ventosa AR, Sousa HT, et al. The new simplified MARIA score applies beyond clinical trials: a suitable clinical practice tool for Crohn’s disease that parallels a simple endoscopic index and fecal calprotectin. United Eur Gastroenterol J. 2020;8:1208–1216. doi: 10.1177/2050640620943089
- Capozzi N, Ordás I, Fernandez-Clotet A, et al. Validation of the simplified Magnetic resonance index of activity [sMARIA] without gadolinium-enhanced sequences for Crohn’s disease. J Crohns Colitis. 2020;14:1074–1081. doi: 10.1093/ecco-jcc/jjaa030
- Tao Y, Li H, Xu H, et al. Can the simplified magnetic resonance index of activity be used to evaluate the degree of activity in Crohn’s disease? BMC Gastroenterol. 2021;21:409. doi: 10.1186/s12876-021-01987-z
- Bae H, Seo N, Kang EA, et al. Validation of the simplified magnetic resonance index of activity by using DWI without gadolinium enhancement to evaluate bowel inflammation in Crohn’s disease. Eur Radiol. Internet. [cited 2023 Apr 16] 2023;33(5):3266–3275. doi: 10.1007/s00330-023-09501-7
- Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81:2080–2088. doi: 10.1016/j.ejrad.2011.07.013
- Novak KL, Kaplan GG, Panaccione R, et al. A simple ultrasound score for the accurate detection of inflammatory activity in Crohnʼs disease: Inflamm Bowel Dis. Inflamm Bowel Dis. 2017;23(11):2001–2010. doi: 10.1097/MIB.0000000000001174
- Sævik F, Eriksen R, Eide GE, et al. Development and validation of a simple ultrasound activity score for Crohn’s disease. J Crohns Colitis. 2021;15:115–124. doi: 10.1093/ecco-jcc/jjaa112
- Allocca M, Craviotto V, Dell’avalle C, et al. Bowel ultrasound score is accurate in assessing response to therapy in patients with Crohn’s disease. Aliment Pharmacol Ther. 2022;55:446–454. doi: 10.1111/apt.16700
- Novak KL, Nylund K, Maaser C, et al. Expert consensus on optimal acquisition and Development of the International bowel ultrasound segmental activity score [IBUS-SAS]: a reliability and inter-rater variability study on intestinal ultrasonography in Crohn’s disease. J Crohns Colitis. 2021;15:609–616. doi: 10.1093/ecco-jcc/jjaa216
- Wang L, Xu C, Zhang Y, et al. External validation and comparison of simple ultrasound activity score and international bowel ultrasound segmental activity score for Crohn’s disease. Scand J Gastroenterol. 2023;8:883–889.
- Zorzi F, Ghosh S, Chiaramonte C, et al. Response assessed by Ultrasonography as target of biological treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2020;18:2030–2037. doi: 10.1016/j.cgh.2019.10.042
- Lafeuille P, Hordonneau C, Vignette J, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn’s disease. Aliment Pharmacol Ther Cited: in: PMID: 33368525. 2021;53:577–586. doi: 10.1111/apt.16232
- Fernandes SR, Serrazina J, Botto IA, et al. Transmural remission improves clinical outcomes up to 5 years in Crohn’s disease. United Eur Gastroenterol J. 2023;11(1):51–59. doi: 10.1002/ueg2.12356
- Calabrese E, Rispo A, Zorzi F, et al. Ultrasonography tight control and monitoring in Crohn’s disease during different biological therapies: a multicenter study. Clin Gastroenterol Hepatol. 2022;20:e711–e722. doi: 10.1016/j.cgh.2021.03.030
- Danese S, Sandborn WJ, Colombel J-F, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology. 2019;157(4):1007–1018.e7. doi: 10.1053/j.gastro.2019.06.038
- Castiglione F, Mainenti P, Testa A, et al. Cross-sectional evaluation of transmural healing in patients with Crohn’s disease on maintenance treatment with anti-TNF alpha agents. Dig Liver Dis. 2017;49:484–489. doi: 10.1016/j.dld.2017.02.014
- Serban ED. Treat-to-target in Crohn’s disease: will transmural healing become a therapeutic endpoint? World J Clin Cases. 2018;6:501–513. doi: 10.12998/wjcc.v6.i12.501
- Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohnʼs disease: Inflamm Bowel Dis. Inflamm Bowel Dis. 2017;23(8):1403–1409. doi: 10.1097/MIB.0000000000001143
- Zorzi F, Rubin DT, Cleveland NK, et al. Ultrasonographic transmural healing in Crohn’s disease. Am J Gastroenterol. InternetPublish Ahead of Print. [cited 2023 Apr 11] 2023;118(6):961–969. doi: 10.14309/ajg.0000000000002265
- de Voogd F, Bots S, Gecse K, et al. Intestinal ultrasound early on in treatment follow-up Predicts endoscopic response to anti-TNFα treatment in Crohn’s disease. J Crohns Colitis. 2022;16(10):1598–1608. doi: 10.1093/ecco-jcc/jjac072
- Pariente B, Mary J-Y, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015;148:52–63.e3. doi: 10.1053/j.gastro.2014.09.015
- Pariente B, Torres J, Burisch J, et al. Validation and update of the Lémann index to measure cumulative structural bowel damage in Crohn’s disease. Gastroenterology. 2021;161(3):853–864.e13. doi: 10.1053/j.gastro.2021.05.049
- Kumar S, Plumb A, Mallett S, et al. METRIC-EF: magnetic resonance enterography to predict disabling disease in newly diagnosed Crohn’s disease—protocol for a multicentre, non-randomised, single-arm, prospective study. BMJ Open. 2022;12:e067265. doi: 10.1136/bmjopen-2022-067265
- Radford SJ, Taylor S, Moran G. Ultrasound use to assess Crohn’s disease in the UK: a survey of British Society of Gastroenterology Inflammatory Bowel Disease Group members. Frontline Gastroenterol. 2022;13:471–476. doi: 10.1136/flgastro-2021-102065
- Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus Magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J Crohns Colitis. 2018;12:1280–1287. doi: 10.1093/ecco-jcc/jjy093
- Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohnʼs disease. A review with recommendations of an International panel of experts: Inflamm bowel Dis. Inflamm Bowel Dis. 2016;22(5):1168–1183. doi: 10.1097/MIB.0000000000000706
- Servais L, Boschetti G, Meunier C, et al. Intestinal conventional ultrasonography, Contrast-Enhanced ultrasonography and Magnetic resonance enterography in assessment of Crohn’s disease activity: a comparison with surgical histopathology analysis. Dig Dis Sci. 2022;67:2492–2502. doi: 10.1007/s10620-021-07074-3
- Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: systematic review and meta-analysis. Dig Liver Dis. 2017;49:854–863. doi: 10.1016/j.dld.2017.04.013
- Xu C, Li L, Zhang Y, et al. Diagnostic accuracy of different cross-sectional imaging techniques for disease location and activity in Crohn’s disease and external validation and comparison of MARIAs and IBUS-SAS. Abdom Radiol. Internet. [cited 2023 Apr 17]. 2022. doi: 10.1007/s00261-022-03751-7
- García-Bosch O, Ordás I, Aceituno M, et al. Comparison of diagnostic accuracy and impact of Magnetic resonance imaging and colonoscopy for the management of Crohn’s disease. J Crohns Colitis. 2016;10:663–669. doi: 10.1093/ecco-jcc/jjw015
- Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation Topical review on prediction, diagnosis and management of fibrostenosing Crohn’s disease. J Crohns Colitis. 2016;10:873–885. doi: 10.1093/ecco-jcc/jjw055
- Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther. 2018;48(3):347–357. doi: 10.1111/apt.14853
- Li X, Mao R, Huang S, et al. Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR Imaging. Radiology. 2018;287:494–503. doi: 10.1148/radiol.2017171221
- Coimbra A, Rimola J, Cuatrecasas M, et al. Magnetic resonance enterography and histology in patients with fibrostenotic Crohn’s disease: a multicenter study. Clin Transl Gastroenterol. 2022;13:e00505. doi: 10.14309/ctg.0000000000000505
- Caron B, Laurent V, Odille F, et al. New magnetic resonance imaging sequences for fibrosis assessment in Crohn’s disease: a pilot study. Scand J Gastroenterol. 2022;57:1450–1453. doi: 10.1080/00365521.2022.2094727
- Dal Buono A, Faita F, Peyrin-Biroulet L, et al. Ultrasound elastography in inflammatory bowel diseases: a systematic review of accuracy compared with histopathological assessment. J Crohns Colitis. 2022;16:1637–1646. doi: 10.1093/ecco-jcc/jjac082
- Orlando S, Fraquelli M, Coletta M, et al. Ultrasound elasticity imaging predicts therapeutic outcomes of patients with Crohn’s disease treated with anti-tumour necrosis factor antibodies. J Crohns Colitis. 2018;12:63–70. doi: 10.1093/ecco-jcc/jjx116
- Avila F, Caron B, Hossu G, et al. Magnetic resonance elastography for assessing fibrosis in patients with Crohn’s disease: a Pilot study. Dig Dis Sci. 2022;67:4518–4524. doi: 10.1007/s10620-021-07311-9
- Scharitzer M, Macher-Beer A, Mang T, et al. Evaluation of intestinal fibrosis with 68 Ga-FAPI PET/MR enterography in Crohn disease. Radiology. 2023;307(3):222389. doi: 10.1148/radiol.222389
- QK-T N, Triumbari EKA, Omidvari N, et al. Total-body PET/CT – first clinical experiences and future perspectives. Semin Nucl Med. Cited: in: PMID: 35272853. 2022;52(3):330–339. doi: 10.1053/j.semnuclmed.2022.01.002
- Huh JY, Park YJ, Ham M, et al. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37:365–371. doi: 10.14348/molcells.2014.0074
- Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925
- Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation – Visceral obesity and creeping fat. Front Immunol. Internet. 2014. [cited 2023 Apr 14];5 . doi: 10.3389/fimmu.2014.00462
- Coffey CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis. 2018;12:1139–1150. doi: 10.1093/ecco-jcc/jjx187
- CWY H, Martin A, Sepich-Poore GD, et al. Translocation of viable Gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell. 2020;183(3):666–683.e17. doi: 10.1016/j.cell.2020.09.009
- Boronat-Toscano A, Monfort-Ferré D, Menacho M, et al. Anti-TNF therapies suppress adipose tissue inflammation in Crohn’s disease. Int J Mol Sci Cited: in: PMID: 36232469. 2022;23:11170. doi: 10.3390/ijms231911170
- Li X-H, Feng S-T, Cao Q-H, et al. Degree of creeping fat assessed by computed tomography enterography is associated with intestinal fibrotic stricture in patients with Crohn’s disease: a potentially novel mesenteric creeping fat index. J Crohns Colitis. 2021;15:1161–1173. doi: 10.1093/ecco-jcc/jjab005
- Zhou Z, Xiong Z, Shen Y, et al. Magnetic resonance imaging-based body composition is associated with nutritional and inflammatory status: a longitudinal study in patients with Crohn’s disease. Insights Imaging. 2021;12:178. doi: 10.1186/s13244-021-01121-3
- Ando K, Uehara K, Sugiyama Y, et al. Correlation among body composition parameters and long-term outcomes in Crohn’s disease after anti-TNF therapy. Front Nutr Cited: in: PMID: 35433773. 2022;9:765209. doi: 10.3389/fnut.2022.765209
- Odille F, Menys A, Ahmed A, et al. Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn Reson Med. 2012;68:783–793. doi: 10.1002/mrm.23298
- Menys A, Puylaert C, Tutein Nolthenius CE, et al. Quantified terminal ileal motility during MR Enterography as a biomarker of Crohn disease activity: prospective multi-institution study. Radiology. 2018;289:428–435. doi: 10.1148/radiol.2018180100
- de Jonge CS, Smout AJPM, Nederveen AJ, et al. Evaluation of gastrointestinal motility with MRI: advances, challenges and opportunities. Neurogastroenterol Motil. 2018;30(1):e13257. doi: 10.1111/nmo.13257
- Plumb AA, Menys A, Russo E, et al. Magnetic resonance imaging-quantified small bowel motility is a sensitive marker of response to medical therapy in Crohn’s disease. Aliment Pharmacol Ther. 2015;42:343–355. doi: 10.1111/apt.13275
- Gollifer RM, Menys A, Plumb A, et al. Automated versus subjective assessment of spatial and temporal MRI small bowel motility in Crohn’s disease. Clin Radiol. 2019;74:.e814.9–.e814.19. doi: 10.1016/j.crad.2019.06.016
- Dohan A, Taylor S, Hoeffel C, et al. Diffusion-weighted MRI in Crohn’s disease: Current status and recommendations. J Magn Reson Imaging. 2016;44:1381–1396. doi: 10.1002/jmri.25325
- Thormann M, Melekh B, Bär C, et al. Apparent diffusion coefficient for assessing Crohn’s disease activity: a meta-analysis. Eur Radiol. 2023;33(3):1677–1686. doi: 10.1007/s00330-022-09149-9
- Tielbeek JAW, Ziech MLW, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24:619–629. doi: 10.1007/s00330-013-3015-7
- Ripollés T, Martínez-Pérez MJ, Paredes JM, et al. The role of intravenous contrast agent in the sonographic assessment of Crohn’s disease activity: is contrast agent injection necessary? J Crohns Colitis. 2019;13:585–592. doi: 10.1093/ecco-jcc/jjy204
- Fulwadhva UP, Wortman JR, Sodickson AD. Use of Dual-energy CT and iodine maps in evaluation of bowel disease. Radiographics. 2016;36:393–406. doi: 10.1148/rg.2016150151
- Dua A, Sharma V, Gupta P. Dual energy computed tomography in Crohn’s disease: a targeted review. Expert Rev Gastroenterol Hepatol. 2022;16:699–705. doi: 10.1080/17474124.2022.2105203
- Zhu C, Hu J, Rong C, et al. Mucosal healing assessment in Crohn’s disease with normalized iodine concentration from dual-energy CT enterography: comparison with endoscopy. Insights Imaging. 2023;14:63. doi: 10.1186/s13244-023-01397-7
- Stidham RW, Enchakalody B, Waljee AK, et al. Assessing small bowel stricturing and morphology in Crohn’s disease using semi-automated image analysis. Inflamm Bowel Dis. 2020;26:734–742. doi: 10.1093/ibd/izz196
- Puylaert CAJ, Schüffler PJ, Naziroglu RE, et al. Semiautomatic assessment of the terminal ileum and colon in patients with Crohn disease using MRI [the VIGOR++ project]. Acad Radiol. 2018;25(8):1038–1045. doi: 10.1016/j.acra.2017.12.024
- De Kock I, Bos S, Delrue L, et al. MRI texture analysis of T2-weighted images is preferred over magnetization transfer imaging for readily longitudinal quantification of gut fibrosis. Eur Radiol. Internet. [cited 2023 Apr 23] 2023;33(9):5943–5952. doi: 10.1007/s00330-023-09624-x
- Meng J, Luo Z, Chen Z, et al. Intestinal fibrosis classification in patients with Crohn’s disease using CT enterography–based deep learning: comparisons with radiomics and radiologists. Eur Radiol. 2022;32:8692–8705. doi: 10.1007/s00330-022-08842-z
- Jauregui-Amezaga A, Rimola J, Ordás I, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut. 2015;64:1397–1402. doi: 10.1136/gutjnl-2014-308101
- Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49:1026–1039. doi: 10.1111/apt.15190
- Fernández-Clotet A, Panés J, Ricart E, et al. Predictors of bowel damage in the long-term progression of Crohn’s disease. World J Clin Cases. 2022;10:12208–12220. doi: 10.12998/wjcc.v10.i33.12208
- Fiorino G, Morin M, Bonovas S, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J Crohns Colitis. 2016;jjwR̥185. doi: 10.1093/ecco-jcc/jjw185
- Dane B, Qian K, Krieger R, et al. Correlation between imaging findings on outpatient MR enterography (MRE) in adult patients with Crohn disease and progression to surgery within 5 years. Abdom Radiol. 2022;47(10):3424–3435. doi: 10.1007/s00261-022-03624-z
- Bachour SP, Shah RS, Lyu R, et al. Test characteristics of cross-sectional imaging and concordance with endoscopy in postoperative Crohn’s disease. Clin Gastroenterol Hepatol. 2022;20(10):2327–2336.e4. doi: 10.1016/j.cgh.2021.12.033
- Le Berre C, Peyrin-Biroulet L, Sandborn WJ, et al. Selecting end points for disease-modification trials in inflammatory bowel disease: the SPIRIT consensus from the IOIBD. Gastroenterology. 2021;160(5):1452–1460.e21. doi: 10.1053/j.gastro.2020.10.065
