ABSTRACT
Introduction
The clinical phenotype of severe acute asthma at the pediatric intensive care unit (PICU) is highly heterogeneous. However, current treatment is still based on a ‘one-size-fits-all approach’.
Areas covered
We aim to give a comprehensive description of the clinical characteristics of pediatric patients with severe acute asthma admitted to the PICU and available immunological biomarkers, providing the first steps toward precision medicine for this patient population. A literature search was performed using PubMed for relevant studies on severe acute (pediatric) asthma.
Expert opinion
Omics technologies should be used to investigate the relationship between cellular molecules and pathways, and their clinical phenotypes. Inflammatory phenotypes might guide bedside decisions regarding the use of corticosteroids, neutrophil modifiers and/or type of beta-agonist. A next step toward precision medicine should be inclusion of these patients in clinical trials on biologics.
1. Introduction
Severe acute asthma or status asthmaticus is referred to as an asthma exacerbation refractory to conventional therapy and patients with this condition account for up to 20% of pediatric intensive care unit (PICU) admissions. Over the past few decades, the number of PICU admissions related to severe acute asthma has shown a significant three- to fourfold increase worldwide [Citation1–3]. A simple explanation for this increase would be a simultaneous increase in the prevalence of asthma overall or an increase in severity, but this does not seem to be the case, suggesting that other factors play a role [Citation4,Citation5]. This is also reflected in an increase in pediatric asthma-related mortality despite the fact that there is a decrease in out-of-hospital deaths and stable mortality rates at the PICU [Citation1,Citation6]. Severe acute asthma is still a serious medical emergency at the PICU and can progress to respiratory insufficiency even with a fatal outcome [Citation7]. Moreover, admission at the PICU is known to have a large impact on the children and their families, not only physically but also psychologically [Citation8,Citation9]. In children, risk factors for poor cognitive and psychosocial outcomes after PICU admission were, among others, a longer length of stay, mechanical ventilation, and exposure to invasive procedures [Citation9]. Up to 21% of the children admitted to the PICU have a diagnosis of post-traumatic stress disorder (PTSD) afterward, as well as 25% of the parents of these children [Citation8,Citation9].
The clinical phenotype of severe acute asthma at the PICU is highly heterogeneous including variations in age, response to treatment and sensitivity to triggers. Respiratory viral infections are more common among preschoolers with severe acute asthma admitted to the PICU compared to their school-aged peers. In school-aged children with severe asthma, environmental smoke exposure is an important risk factor for PICU admissions [Citation1,Citation4]. It can be expected that these heterogeneous clinical phenotypes reflect a heterogeneity in underlying molecular mechanisms, the so-called endotypes. Additionally, responses to the conventional treatment of severe acute asthma at the PICU are highly variable too. While most children receive a standard treatment based on respiratory support, systemic corticosteroids and bronchodilators intravenously, not all children respond well to this ‘one-size-fits-all’ approach [Citation1,Citation4]. Looking at the heterogeneous clinical phenotype of these patients, it seems logical that we need a more personalized treatment approach. In this review, we aim to give a comprehensive description of the clinical characteristics of pediatric patients with severe acute asthma admitted to the PICU and available immunological biomarkers, providing the first steps toward precision medicine for this vulnerable patient group. For this review, we have performed a literature search using PubMed (MEDLINE database) for relevant papers on immunophenotyping of severe acute (pediatric) asthmatics published in the last 20 years (up to February 2021). This search yielded 275 articles, which were all screened. Conference abstracts, studies not conducted in humans and papers not written in English were excluded.
1.1. The challenge of defining severe acute asthma
In order to decipher the clinical and molecular heterogeneity of severe acute asthma at the PICU, we should first ask ourselves if we are merely dealing with exacerbations of severe asthma, justifying the extrapolation of data of large pediatric asthma cohorts focussing on severe asthma, or whether severe acute asthma at the PICU is a separate entity.
To answer this question, we should first define severe asthma, which in itself is rather challenging. Clinical practice shows that approximately 5–10% of all children with an asthma diagnosis remain symptomatic despite high doses of medication [Citation10,Citation11]. This is a heterogeneous patient group. A structured and extensive clinical work-up should be performed to identify comorbidities and trigger factors that might escalate the complaints and actively verify compliance to the prescribed treatment. In evaluating these patients, specific attention should be given to the question ‘why the child is not responding to what should work’, and renewed emphasis is needed on adherence and inhaler techniques, especially in children [Citation12,Citation13]. If all these factors are evaluated, if possible treated, and the asthma is still not under control, the child should be diagnosed as a patient with severe therapy resistant disease or severe refractory asthma. This process is time consuming.
In studies, it is common to define severe therapy-resistant asthma in school-aged children (>6 years) based on symptoms and medication use, for example ongoing poorly controlled asthma despite high-dose inhaled corticosteroids (ICS) and at least two other controller medications [Citation10,Citation14,Citation15]. Attempts are now being made to define severe asthma phenotypes as more than just the amount and dosage of medications that are being used [Citation14,Citation15]. Thus far, it is advised to at least measure sputum eosinophil count and fractional exhaled Nitric Oxide (FeNO) to guide therapy [Citation14,Citation15].
2. Clinical phenotypes of severe acute asthma
When looking into severe acute asthma, one would expect that the severe phenotypes are the only ones seen at the PICU. Although they do succeed in numbers, those with mild-to-moderate asthma still have a risk of being admitted to a PICU (24% to 55%) [Citation5,Citation7]. A study by van den Bosch et al. showed that in 16% of all PICU-admitted children, the admission was their first asthma presentation [Citation16]. Several risk factors have been identified for admittance at the PICU, resulting in a clinical ‘high-risk phenotype’ () [Citation4,Citation16–18]. Children with a previous admission to the PICU or emergency department visit, exposure to tobacco smoke, a longer duration of symptoms or ICS use and allergic sensitization to multiple aeroallergens all seem to be at risk for severe acute asthma [Citation4,Citation16–18]. Furthermore, several studies have shown that ethnicity influences the risk of a PICU admission, with non-Caucasian and non-Hispanic Black ethnicity being a risk factor for severe asthma exacerbations and PICU admission [Citation7,Citation19]. Compared to White children with severe asthma, Black children with severe asthma were more likely to be atopic, had higher levels of IgE and were more likely to be treated by a pulmonologist. This whilst having the same drug compliance and ICS use compared to White children with severe asthma, suggesting pathobiological differences [Citation20,Citation21]. Overall, children admitted to a PICU with severe acute asthma tend to be older, although age differences vary between the different studies, and are more often boys [Citation5,Citation7]. When it comes to mortality, non-Hispanic Black ethnicity and male gender were identified as risk factors as well, but a surveillance study revealed that most pediatric asthma deaths occur in the outpatient setting with mortality rates at the PICU remaining stable [Citation19]. This could suggest that pathobiological differences influence the risk of death, but it could also mean that availability of care or recognition of severe symptoms differs in this group of patients, causing a sometimes fatal delay in seeking or receiving help.
Table 1. Studies assessing risk factors for severe acute asthma at PICU
Obesity seems to be a risk factor as well. Carroll et al. showed that despite similar severity of illness and therapeutic interventions, overweight children were more likely to be admitted to the PICU, whereas the International Study of Asthma and Allergy in Children (ISAAC) showed an increased prevalence of asthma in overweight and obese children without an association of atopy, suggesting a different pathobiological mechanism compared to school age atopic asthma [Citation22–24]. This is supported by the findings that obese children have sex-specific macrophage activation and decreased response to inhaled steroids [Citation25,Citation26].
Which of these risk factors also influence therapy response is not known. However, some of these risk factors may exert their effect by genetic heterogeneity, for example through variation in the gene encoding the β2 adrenergic receptor (ADRB2). Single nucleotide polymorphisms of this gene at position 16 and 27 have been shown to be functionally relevant influencing therapy response, length of stay at the PICU, and duration of oxygen therapy and even risk of intubation [Citation27–29]. No studies are done to assess differences in corticosteroid responses in severe acute asthma at the PICU, but it has been shown that in severe therapy-resistant asthma, responses are heterogeneous [Citation30].
3. From clinical phenotype to immunological endotype
While clinical phenotypes may help characterize different patient populations, they are often not associated with the pathobiological underlying mechanisms, which are referred to as endotypes. Within these endotypes, biomarkers need to be identified to guide treatment for the individual patient, with the biomarkers being seen as treatable traits [Citation31,Citation32]. Clinical asthma phenotypes vary with age, triggers, clinical presentation, comorbidities, response to treatment and so on, and this heterogeneity is reflected in several inflammatory pathways.
Asthma has long been seen as a TH2 disease of the lungs with eosinophilia in the sputum. However, the clinical heterogeneity as seen in variable age of onset, trigger of exacerbation, environmental risk factors and clinical presentation can also be defined by their pathophysiological endotype. Up till now, four different inflammatory endotypes have been recognized (). The Type 2 high endotype most common in children is characterized by an eosinophilia-driven inflammation, triggered by allergens. The hallmark type 2 cytokines like Interleukin(IL)-4, IL-5 IL-13, as well as alarmins, including IL-25, IL-33, thymic stromal lymphopoietin (TSLP) and IgE are major molecular drivers and the endotype has a moderate-to-good response to corticosteroids. Less common in children is the non-allergic eosinophilic asthma, triggered by pollutants, microbes, and glycolipids and driven by eosinophils, airway epithelial cells and innate lymphoid cells (ILCs). Besides IL-25, IL-33 and TSLP, prostaglandin D2 also plays a role in the inflammation and this endotype seems relatively insensitive to corticosteroids [Citation11].
Table 2. Immunological endotypes
The T2-low endotypes are characterized by steroid insensitive paucigranulocytic or neutrophilic inflammation. The paucigranulocytic endotype is triggered by environmental factors such as tobacco smoke and allergens and seems to be characterized by airway smooth muscle dysfunction and high levels of oxidative stress. The neutrophilic endotype is triggered by infections leading to the activation of TH17 lymphocytes and neutrophils and release of IL-8, IL-17, IL-21, and IL-22 [Citation11,Citation33].
Another endotype may lie in the discovery of the role of anti-inflammatory mediators in asthma. In severe and refractory asthma phenotypes, also increased levels of TNF-α have been measured [Citation34]. This cytokine seems to be under regulation of the IL-1 family, with levels of IL-33 correlating with TNF-α [Citation35]. IL-37 is an anti-inflammatory member of the IL-1 receptor family and known for its regulatory capacities in inflammatory diseases. It is involved in several aspects of allergic inflammation, from recruitment of eosinophils and neutrophils to inhibition of T1/T2/T17 inflammatory mediators [Citation36]. In asthmatic children, low levels of IL-37 were found in induced sputum, contrasting with increased levels of TNF-α, IL-1β, IL-6 and IL-17 [Citation37]. This may suggest another endotype where downregulation of anti-inflammatory mediators such as IL-37 or SCGB1A1 plays a role [Citation38,Citation39]. Up till now, the scarce literature on severe asthma at the PICU has focussed on traditional T2 biomarkers such as eosinophils, IgE and FeNO. Looking at the different endotypes, their responses to corticosteroids and newly available biomarker techniques, we need to explore whether other cytokines or inflammatory cells may be relevant in determining the phenotype and underlying endotype.
4. What to measure, where to measure?
4.1. Bronchoalveolar lavage
Several studies performing bronchoalveolar lavages in children with severe therapy resistant asthma have shown that the compartment in which inflammatory cells are being measured greatly influences the outcome. Andersson et al. measured eosinophils and neutrophils in bronchoalveolar lavage fluid, and submucosal and intra-epithelial biopsies in children with severe therapy resistant asthma [Citation33]. Although levels of eosinophils were increased in bronchoalveolar lavage fluid and the submucosa, intra-epithelial eosinophils could not be demonstrated. Instead, high levels of intraepithelial neutrophils were detected. These NeutrophilHigh patients had higher Asthma Control Test (ACT) scores, higher FEV1% than predicted and lower doses of maintenance inhaled corticosteroids. However, there were no correlations with other clinical factors such as age, body mass index, parental smoking or infections [Citation33]. Teague et al. demonstrated four different granulocyte categories in bronchoalveolar lavage of children with severe therapy resistant asthma and linked these to clinical features [Citation40]. The most prevalent category was pauci-granulocytic. These children were significantly older, had better lung function tests and were less likely to be treated with maintenance corticosteroids. Furthermore, there was a significantly lower prevalence of infection with enterovirus/human rhinovirus. The second category was mixed granulocytic, found in children with lower lung function test outcomes, a higher prevalence of maintenance corticosteroid treatment and the highest prevalence of enterovirus/human rhinovirus transcripts. Children with isolated neutrophilia in their bronchoalveolar lavage (16%) tended to be younger and have a nonwhite ethnicity but had greater FEV1% compared to those with eosinophilia. In this category, detection of a microbe or virus was most prevalent (Haemophilus influenza and Rhinovirus) [Citation40,Citation41]. Only 9.5% of the children had isolated eosinophilia in their bronchoalveolar lavage, but this category had the highest prevalence of non-White children, previous hospitalizations in the past year and PICU admissions. When they correlated inflammatory markers in blood with bronchoalveolar lavage results, sensitivity and specificity of blood eosinophil count for bronchoalveolar lavage count was poor, but children in the neutrophilia category did have significantly lower blood eosinophil counts and percentages, and lower blood IgE levels [Citation40]. Unfortunately what these studies also show is that cell counts from peripheral blood poorly correlate and other markers should be used. The same group also looked into cytokine patterns in the fluid of the bronchoalveolar lavage of the patients [Citation41]. They divided the group into neutrophilic and non-neutrophilic and found that cytokines responsible for TH17 (IL-6, IL-17, G-CSF) and TH1 differentiation and expansion (IL-12, TNF-α, IFN-γ) were elevated in the neutrophilic patients. Associations with chemokines responsible for neutrophil chemotaxis (CXCL8 and CXCL10) were identified as well [Citation41]. These data were comparable with previous studies of cytokine levels in bronchoalveolar lavage fluid of severe pediatric asthmatics [Citation42]. When children with severe asthma are divided into a neutrophilhigh versus a neutrophillow group based on cell counts in their bronchoalveolar lavage fluid, a greater amount of dysfunctional neutrophils was seen, characterized by the release of pro-inflammatory mediators, greater phagocytic capacity and neutrophil extracellular trap formation but more impaired respiratory burst [Citation43]. Clinical features in the neutrophilhigh group displayed more boys, more emergency room visits and hospitalizations in the previous year and higher levels of FeNO. Twice as many patients needed mechanical ventilation ever in life (13.2% in the neutrophilhigh vs. 6.9% in the neutrophillow group), but due to the small sample size, the difference was not statistically significant. Whereas lack of eosinophils might be influenced by inhaled corticosteroid treatment, neutrophils are relatively insensitive to corticosteroids and indeed, neutrophil numbers were not correlated to inhaled corticosteroid treatment [Citation43–45].
Whether these cytokine and neutrophil levels are also reflected in increased levels in peripheral blood remains unknown unfortunately, but levels of neutrophils in blood were not discriminative between severe therapy-resistant asthma, difficult-to-treat asthma or patients with chronic inflammation such as cystic fibrosis [Citation46]. Increased levels of blood eosinophils did correlate with increased odds of hospitalization, but another study showed no correlation between sputum and blood eosinophil levels [Citation46,Citation47]. Since only a small proportion of children admitted to the PICU with severe asthma needs mechanical ventilation, bronchoalveolar lavage during the admission is not available nor desirable in the acute phase so these markers cannot be used in that phase. However, these data show that there are indeed clinical features giving direction to what immunological endotypes could be looked at during a PICU admission.
4.2. Sputum
One of the methods used to take a closer look at what is happening in the airways is induced sputum. It is a minimally invasive and safe but labor-intensive technique [Citation48]. Patients are asked to inhale nebulized 3% saline for a certain amount of time, and requested to spit sputum in a cup. This sample can then be worked up to retrieve a cell pellet reflecting the cell types circulating the airways [Citation49,Citation50]. From the early eighties on it has been recognized that sputum eosinophil numbers can distinguish between eosinophilic and neutrophilic asthma and reflect the response to steroids during an acute exacerbation [Citation51,Citation52]. One of the type of cells found by this method in the airways of children with severe therapy-resistant asthma and drivers of one of the major immunological endotypes are innate lymphoid cells (ILCs). ILCs are important early regulators of immune responses at barrier surfaces and are involved in the pathophysiology of multiple airway diseases [Citation46,Citation53,Citation54]. ILCs are capable of adapting to changing local environmental cues by acquiring cytokine-producing capacities associated with plasticity toward other ILC subsets, commonly observed in pathologies [Citation53]. Dysregulated type 2 ILCs (ILC2s) and other subtypes are increased in inflamed tissues and contribute to the disease by amplifying the effects of pathogenic T cells [Citation55]. Little data are available on the role of ILCs in the pathology of pediatric asthma. ILC2s are activated by IL-33 and TSLP, and elevated levels of IL-33 were found in the lower airways of severe pediatric asthma patients [Citation56]. Increased numbers of ILC2s were found in the blood of preschool children with acute wheeze as compared with the recovery phase, although both numbers were lower than in healthy controls [Citation57]. Other studies demonstrated increased ILC2s in the sputum and peripheral blood of children with severe therapy-resistant asthma. At the same time, the frequencies of IL-17-producing ILCs, which could be either bona fide type 3 ILCs (ILC3s) or transdifferentiated ILC2s, were similar between severe asthma, difficult-to-treat asthma and healthy controls. Upon administration of systemic steroids, ILC2 frequencies in the airways were reduced, while cultured ILC2s produced lower amounts of IL-5 and IL-13 when co-stimulated with steroids. In contrast, co-exposure to steroids did not affect IL-17 production by ILCs in vitro [Citation50,Citation56]. Whether the endotype of asthma where ILCs play a role is reflected in certain recognizable clinical factors usable for the PICU is not clear yet, but these data do indicate a role for ILCs. In adults with severe asthma, other markers such as RANTES (CCL-5), IL-8, GM-CSF, fibroblast-growth factor-2 (FGF-2), periostin and TGF-β were measured in induced sputum, with only periostin and TGF-β being discriminative for a subgroup of patients with persistent airway obstruction [Citation58].
Despite the increasing evidence supporting the measurement of inflammatory markers in the induced sputum, this technique might not always be feasible provided the technical difficulties and considerable poor success rates [Citation59,Citation60].
4.3. Exhaled breath
Various compounds can be measured in exhaled breath. The measurement of volatile organic compounds (VOCs) in exhaled breath have been proposed as a feasible alternative for diagnostic and prognostic biomarkers for the pathologies of the lower airways. This is because they reflect the status of various metabolic pathways taking place in the local environment of the lungs, as well as systemic metabolic processes, which can be associated with inflammatory or oxidative activity [Citation61]. VOCs can either be exogeneous and inhaled by the patient, or endogeneous, formed by metabolic processes or resident bacteria [Citation11]. Exhaled breath can be analyzed using different approaches, such as gas chromatography-mass spectrometry to identify individual VOCs or electric nose (e-Nose) technologies to assess patterns of VOCs. Studies have demonstrated a potential role for the VOC analysis in prediction models for asthma exacerbations and breath profiles could distinguish severe therapy-resistant asthma from stable asthma in children [Citation62–64]. A study in ventilated ICU-patients demonstrated that breath profiles could recognize patients with a hospital acquired bacterial pneumonia, but it remains unclear whether breath profiles can distinguish immunological endotypes at the PICU [Citation65]. However, the noninvasiveness and practical applicability in children from three years old onwards makes this technique one of the promising new methods [Citation62].
4.4. Nasal compartment
Especially in pediatric airway diseases, the search for proper biomarkers outside the lung is crucial in finding minimally invasive approaches to guide clinical decision-making. The nose seems to be a good alternative, especially when considering the relatively new hypothesis of the united airway diseases [Citation66]. In this concept, both the lower and upper airways are lined with respiratory epithelium playing an important role in the immune response against pathogens, allergens and other insults [Citation66]. In adults with allergic asthma, cytokines measured in the nasal lining fluids, including cytokines related to type 2 immune responses, such as IL-5, IL-13, CCL-26 and especially IL-24 reflected the cytokines composition of matched sputum. This observation suggests that nasal biomarker analytes mirror the inflammatory state of the tissues in the lower airways affected by pathologies [Citation67]. Quite some studies have focused on the nasal compartment in children, for instance regarding the epigenome. It has been well accepted that asthma is a disease with both genetic susceptibility as well as being influenced by environmental factors. At the intersection of these two lies the epigenome, and previous studies have suggested that nasal cells could reveal epigenetic biomarkers representing lower airway disease [Citation68,Citation69]. In nasal swabs of children with asthma, epigenome-wide associations were found related to asthma, allergy and lung function with multiple differentially methylated regions in genes observed for FeNO and IgE, possibly altering structure and function of nasal epithelial cells [Citation68]. Another study revealed that three differential methylated positions are discriminative between severe and non-severe asthma in a cohort of Black children [Citation70]. This suggests that nasal epithelium (epi)genetic fingerprint may allow to discriminate between clinical phenotypes and, because of the minimal burden for the sample collection and tissue accessibility, it could potentially become a noninvasive tool for the investigation whether asthma endotypes reflect epigenetics.
The recent development of novel molecular biology techniques provides a promising tool for a better prediction of the validity of molecule or genome alterations as a potential biomarker. CRISPR-Cas9 technology facilitates gene editing through a direct modification of specific genome sequences [Citation71]. With this tool, we are now able to verify functional gene polymorphisms or single nucleotide polymorphisms (SNPs) that have been associated with asthma or reduced responsiveness to medications by recreating these mutations in established cell lines or primary cells obtained from healthy controls. Upcoming results from the PERMEABLE (personalized medicine approach for asthma and allergy biologics selection) consortium may shed some light on pediatric phenotypes and biomarkers related to the control of severe childhood asthma.
5. Five-year view
Following treatment in adults with severe asthma, targeted treatment methods such as human monoclonal antibodies (biologics) are now becoming increasingly available for children. The European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force recently published a recommendation which stated that four biologics are now approved in children with severe asthma; omalizumab and mepolizumab in children over 6 years of age, and dupilumab and benralizumab in children over 12 years of age [Citation72]. These biologics might be useful in treating children with severe asthma and might be guided by specific biomarkers, although these are not available up till now [Citation12]. Omalizumab, which binds to the high-affinity IgE receptor, is suggested to be indicated in severe non-allergic asthma with total IgE levels between 76 and 1500 [Citation12]. No other biomarker data for children are available, but especially the IL-33 and TSLP pathways and activation of ILCs seem interesting in this phenotype [Citation72], possibly indicating a role for sputum or nasal compartment analyses in guidance. Especially in children with severe viral induced exacerbations, omalizumab might be of interest since treatment with this biologic boosted peripheral blood mononuclear cell IFN-α responses to rhinovirus, correlating with clinical responses [Citation73]. Mepolizumab, which blocks the activity of IL-5, is recommended in children with uncontrolled severe eosinophilic asthma, but inclusion criteria are extrapolated from data in adults since only very small cohorts were studied in children, and basically exists of blood eosinophilia ≥300/µl. It seems logical that mepolizumab should be used in children with a diagnosed T2 high endotype of asthma only. Dupilumab inhibits IL-4 and IL-13 signaling and is also indicated for severe eosinophilic asthma with raised blood eosinophils (>150) and/or raised FeNO >20 ppb [Citation72]. For this biologic, we should also be looking for children with a T2 high endotype, as is the case for benralizumab, which blocks the IL-5 receptor. Thus far, data on biologics are scant, inclusion criteria are based on data from studies in adults and the only biologicals available for children are all directed at the T2 high endotype, except for the interesting effects of omalizumab. Furthermore, the long-term effects of biologics on the immune system in children are still unclear and targeted therapy must be even further personalized when treating children, since cutoffs for biomarkers may not suffice for this heterogeneous group in terms of age and personal health [Citation74,Citation75]. Even though biologics might not be able to directly treat children with severe acute asthma at the PICU in the acute phase, improving control of severe asthma with biologics may help decrease the incidence of new events of severe acute asthma for the time being, which is certainly useful.
In 2016, the new concept of ‘treatable traits’ has been proposed for the management of chronic airway diseases. Treatable traits are clinically measurable ‘treatable’ features found by the phenotyping of individual patients [Citation31,Citation74]. A better understanding of these traits in pediatric severe acute asthma and their underlying endotypes, the ‘treatable mechanisms’ is needed to optimize treatment for the individual patient [Citation74]. Exploration of these treatable traits and linking them to measurable biomarkers could help to identify subgroups of children with severe acute asthma. Although large cohorts of children with various types of asthma have been or are being investigated, severe acute asthma (especially at the PICU) seems to be a missing link [Citation31,Citation74].
At the bedside, biomarkers could help treatment decisions for example by focusing on fighting the inflammation through systemic steroids, or introducing azithromycin in the case of a more neutrophilic high endotype [Citation76,Citation77], or rather choosing for aminophylline when the genotype indicates less susceptibility for the salbutamol [Citation27–29]. Furthermore, being able to discriminate the endotypes at the baseline will improve the research of novel treatment options because of proper patient selection.
The Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED) consortium has been a forefront in using advanced ‘omics’ technologies ((epi)genomics, transcriptomics, metabolomics, proteomics, breathomics) to understand the mechanisms underlying adult and pediatric severe asthma [Citation78]. New collaborations (e.g. SPACE and PERMEABLE) focusing on pediatric biologics users are currently ongoing [Citation78]. Omics technologies might be able to help investigate the causal relationship between cellular molecules and pathways, and their clinical phenotypes: high rates of atopy, young age of asthma diagnosis, low airway conductance, and a high asthma burden were found [Citation79]. There is an urgency for such a large-scale project for children with severe acute asthma at the PICU as well. Omics analyses have not yet led to a breakthrough in asthma clinical care because of some limitations, such as natural variations of certain biomarkers [Citation80,Citation81]. Still, with new noninvasive technologies improving it should be able to contribute knowledge in this specific patient group of children with severe acute asthma at the PICU and help to better understand their clinical high-risk phenotypes and their relationship with their underlying immunological endotypes.
6. Expert opinion
Severe acute asthma at the PICU has often been regarded as pediatric asthma with a severe exacerbation-prone phenotype. However, based on the latest scientific insights, severe acute asthma at the PICU is likely to be a separate – yet heterogeneous – clinical asthma entity. The increase in PICU admissions of children with severe acute asthma (while the overall asthma prevalence is not increasing), as well as the observation that not all children with asthma at the PICU have severe therapy-resistant asthma, or even ever had a diagnosis of asthma, forces us to reconsider how we should see severe acute asthma. There is a need to understand the molecular mechanisms underlying this heterogeneous clinical phenotype. The distinct clinical features of children with asthma at the PICU can be a first guidance, combined with the currently known biomarkers to distinguish distinct immunological endotypes.
In pediatric asthma research, we stand at a crossroad. Promising novel omics techniques make it possible to discover biomarkers at a multisystem level with minimally to noninvasive techniques. This grants us the opportunity to dive deeper in the underlying pathophysiological mechanisms of childhood asthma and discover treatable mechanisms. Various European initiatives and consortia have been established to study pediatric endotypes of severe disease. A joint registry, in which clinical characteristics and biomarker data are available, could be a promising next step to lead the way for precision medicine. However, since severe acute asthma is likely a distinct clinical entity, it is pivotal to also specifically include children who are admitted at the PICU with asthma, and study common as well as unique profiles compared to the non-PICU severe asthma population.
In addition, there is a strong need to improve the treatment of children with severe acute asthma at the PICU. Over the past years, treatment protocols for severe acute asthma at the PICU have not changed and treatment options are still extremely limited. The mainstay is to give steroids systemically and a bronchodilator intravenously, despite the age of the child, the trigger causing the exacerbation, or whether the child has been diagnosed with asthma prior to admission. We need to improve treatment of these children in three ways. First, we should adapt a treatable trait approach and individualize treatment based on type of asthma, trigger, age, comorbidities, and immunological endotype. There is a need to identify novel biomarkers that are abundant, can be easily and quickly measured (preferably point of care), and accurately reflect the dynamic nature of the disease. This requires longitudinal assessment of biomarkers in asthmatic children admitted to the PICU, not only during the acute phase, but also during recovery. Markers in exhaled breath could be suitable candidates to monitor disease and identify distinct types of exacerbations. At the same time, already existing biomarkers (e.g. T2 endotyping based on biomarkers in peripheral blood, nasal samples or sputum) need to be implemented in clinical care in order to guide treatment upon recovery to prevent new exacerbations (). Being able to distinguish these differences will give us new opportunities to (re)discover treatment options beyond steroids and bronchodilators. With this precision medicine approach we can hopefully shorten the length of stay at the PICU, decreasing the trauma it has on the child, as well as its caregivers and family. Second, clinical trials are needed to assess the safety and efficacy of biologics in this specific population. In general, asthmatic children have been underrepresented in the clinical trials on biologics. Asthmatic children with a previous PICU admission have not been studied separately. Focusing on biologics response in pediatric patients with different underlying immunological phenotypes might provide valuable information on which child might benefit from these treatments and how long these treatments should be continued in children with acute asthma. Lastly, monitoring strategies need to be improved. One of the most stable risk factors for PICU admission is a previous PICU admission or hospital admission in the previous year. How can we recognize these children before they need to be admitted to the PICU in the first place, or prevent that a new PICU admission is needed? This requires identification of biomarkers or (modifiable) characteristics that predict deterioration, a better understanding of fluctuations of these factors and new insights on successful monitoring strategies to prevent (new) admissions. Severe acute asthma at the PICU is a distinct type of childhood asthma. Let us start looking at it like that.
Figure 1. Potential precision medicine strategies for severe acute asthma at the PICU. Immunophenotyping might help to monitor disease, diagnose type of asthma and/or guide treatement. Eo, eosinophil; FENO, fraction of exhaled nitric oxide; IL, interleukin, ILC2, type 2 innate lymphoid cells; Th2, T helper 2 cell.
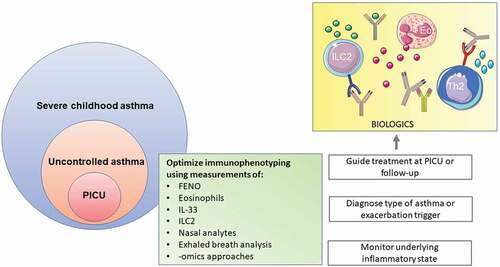
Some of the drawn objects were adapted from smart.servier.com, which are used under Creative Commons Attribution 3.0 Unported License; https://creativecommons.org/licenses/by/3.0/.
Article highlights
Severe acute asthma at the PICU is a distinct type of childhood asthma
The clinical phenotype of severe acute asthma at the PICU is highly heterogeneous; including variations in age, response to treatment and sensitivity to triggers
Risk factors for severe acute asthma at the PICU include an admission to the PICU or the hospital for exacerbations in the previous year, active or passive smoking, non-White ethnicity, obesity, atopy
A better understanding of treatable traits and treatable mechanisms in pediatric severe acute asthma is needed to optimize treatment for the individual patient
Biomarkers could help treatment decisions for severe acute asthma at the PICU, for example deciding which patients might benefit from systemic steroids or aminophylline.
There is a need for in-depth omics analyses of children with severe acute asthma to investigate the relationship between cellular molecules and pathways, and their clinical phenotypes
Abbreviations
ATS – American Thoracic Society
BAL – Bronchoalveolar lavage
ERS – European Respiratory Society
FeNO – Fractional exhaled nitric oxide
GCMS – Gas chromatography with mass spectrometry
ICS – Inhaled corticosteroids
IFN – Interferon
IgE – Immunoglobulin E
IL – Interleukin
iILCs – Inflammatory innate lymphoid cells
ILCs – Innate lymphoid cells
ISAAC – International Study of Asthma and Allergy in Children
PERMEABLE – Personalized medicine approach for asthma and allergy biologics selection (consortium)
PICU – Pediatric intensive care unit
PTSD – Post-traumatic stress disorder
SNP – Single nucleotide polymorphism
Th cells – T helper cells
VOCs – Volatile organic compounds
U-BIOPRED – Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (consortium)
References
- Boeschoten SA, Buysse CMP, Merkus P, et al. Children with severe acute asthma admitted to Dutch PICUs: a changing landscape. Pediatr Pulmonol. 2018;53:857–865.
- Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in admissions for pediatric status asthmaticus in New Jersey over a 15-year period. Pediatrics. 2010;126:e904–911.
- Al-Eyadhy AA, Temsah MH, Alhaboob AA, et al. Asthma changes at a pediatric intensive care unit after 10 years: observational study. Ann Thorac Med. 2015;10:243–248.
- Boeschoten SA, Boehmer AL, Merkus PJ, et al. Risk factors for intensive care admission in children with severe acute asthma in the Netherlands: a prospective multicentre study. ERJ Open Res. 2020;6:00126–2020.
- Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. 2012;3:1–58.
- Szefler SJ, Zeiger RS, Haselkorn T, et al. Economic burden of impairment in children with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2011;107:110–119 e111.
- Grunwell JR, Travers C, Fitzpatrick AM. Inflammatory and comorbid features of children admitted to a PICU for status asthmaticus. Pediatr Crit Care Med. 2018;19:e585–e594.
- Boeschoten SA, Dulfer K, Boehmer ALM, et al., Dutch collaborative PrnAclornpipita. Quality of life and psychosocial outcomes in children with severe acute asthma and their parents. Pediatr Pulmonol. 2020;55:2883–2892.
- Rees G, Gledhill J, Garralda ME, et al. Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med. 2004;30:1607–1614.
- Fitzpatrick AM, Moore WC. Severe asthma phenotypes - How should they guide evaluation and treatment? J Allergy Clin Immunol Pract. 2017;5:901–908.
- Licari A, Manti S, Castagnoli R, et al. Measuring inflammation in paediatric severe asthma: biomarkers in clinical practice. Breathe (Sheff). 2020;16:190301.
- Bush A. Which child with asthma is a candidate for biological therapies? J Clin Med. 2020 9 ;1237.
- Goodwin R, Chander T, Shah N, et al. Inhaler counselling, the real deal or just fresh air? Arch Dis Child. 2016;101:e2.
- Bush A, Saglani S, Fleming L. Severe asthma: looking beyond the amount of medication. Lancet Respir Med. 2017;5:844–846.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373.
- van Den Bosch GE, Merkus PJ, Buysse CM, et al. Risk factors for pediatric intensive care admission in children with acute asthma. Respir Care. 2012;57:1391–1397.
- Belessis Y, Dixon S, Thomsen A, et al. Risk factors for an intensive care unit admission in children with asthma. Pediatr Pulmonol. 2004;37:201–209.
- McDowell KM, Kercsmar CM, Huang B, et al. Medical and social determinants of health associated with intensive care admission for asthma in children. Ann Am Thorac Soc. 2016;13:1081–1088.
- Arroyo AJC, Chee CP, Camargo CA Jr., et al. Where do children die from asthma? National data from 2003 to 2015. J Allergy Clin Immunol Pract. 2018;6:1034–1036.
- Guilbert T, Zeiger RS, Haselkorn T, et al. Racial disparities in asthma-related health outcomes in children with severe/difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2019;7:568–577.
- Puranik S, Forno E, Bush A, et al. Predicting severe asthma exacerbations in children. Am J Respir Crit Care Med. 2017;195:854–859.
- Carroll CL, Stoltz P, Raykov N, et al. Childhood overweight increases hospital admission rates for asthma. Pediatrics. 2007;120:734–740.
- Weinmayr G, Forastiere F, Buchele G, et al. Overweight/obesity and respiratory and allergic disease in children: international study of asthma and allergies in childhood (ISAAC) phase two. PLoS One. 2014;9:e113996.
- Bush A, Fleming L, Saglani S. Severe asthma in children. Respirology. 2017;22:886–897.
- Forno E, Lescher R, Strunk R, et al. Childhood Asthma Management Program Research Group. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749.
- Periyalil HA, Wood LG, Scott HA, et al. Macrophage activation, age and sex effects of immunometabolism in obese asthma. Eur Respir J. 2015;45:388–395.
- Carroll CL, Sala KA, Zucker AR, et al. beta2-adrenergic receptor haplotype linked to intubation and mechanical ventilation in children with asthma. J Asthma. 2012;49:563–568.
- Carroll CL, Sala KA, Zucker AR, et al. Beta-adrenergic receptor polymorphisms associated with length of ICU stay in pediatric status asthmaticus. Pediatr Pulmonol. 2012;47:233–239.
- Carroll CL, Stoltz P, Schramm CM, et al. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest. 2009;135:1186–1192.
- Bossley CJ, Fleming L, Ullmann N, et al. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. J Allergy Clin Immunol. 2016;138:413–420 e416.
- Chung KF, Adcock IM. Precision medicine for the discovery of treatable mechanisms in severe asthma. Allergy. 2019;74:1649–1659.
- Cazzola M, Ora J, Cavalli F, et al. Treatable mechanisms in asthma. Mol Diagn Ther. 2021;25:111–121.
- Andersson CK, Adams A, Nagakumar P, et al. Intraepithelial neutrophils in pediatric severe asthma are associated with better lung function. J Allergy Clin Immunol. 2017;139:1819–1829 e1811.
- Bousquet J. Stratification of patients with severe asthma. Lancet Respir Med. 2015;3:330–331.
- Hamzaoui A, Berraies A, Kaabachi W, et al. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013;50:803–809.
- Zhang L, Zhang J, Gao P. The potential of interleukin-37 as an effective therapeutic agent in asthma. Respir Res. 2017;18:192.
- Charrad R, Berraies A, Hamdi B, et al. Anti-inflammatory activity of IL-37 in asthmatic children: correlation with inflammatory cytokines TNF-alpha, IL-beta, IL-6 and IL-17A. Immunobiology. 2016;221:182–187.
- Zissler UM, Jakwerth CA, Guerth F, et al. Allergen-specific immunotherapy induces the suppressive secretoglobin 1A1 in cells of the lower airways. Allergy. 2021;76:2461–2474.
- Zhu L, An L, Ran D, et al. The club cell marker SCGB1A1 downstream of FOXA2 is reduced in asthma. Am J Respir Cell Mol Biol. 2019;60:695–704.
- Teague WG, Lawrence MG, Shirley DT, et al. Lung lavage granulocyte patterns and clinical phenotypes in children with severe, therapy-resistant asthma. J Allergy Clin Immunol Pract. 2019;7:1803–1812 e1810.
- Steinke JW, Lawrence MG, Teague WG, et al. Bronchoalveolar lavage cytokine patterns in children with severe neutrophilic and paucigranulocytic asthma. J Allergy Clin Immunol. 2021;147:686–693 e683.
- Fitzpatrick AM, Higgins M, Holguin F, et al.; National Institutes of Health/National Heart L, Blood Institute’s Severe Asthma Research P. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–857 e818.
- Grunwell JR, Stephenson ST, Tirouvanziam R, et al. Children with neutrophil-predominant severe asthma have proinflammatory neutrophils with enhanced survival and impaired clearance. J Allergy Clin Immunol Pract. 2019;7:516–525 e516.
- Belvisi MG. Regulation of inflammatory cell function by corticosteroids. Proc Am Thorac Soc. 2004;1:207–214.
- Schleimer RP. Effects of glucocorticosteroids on inflammatory cells relevant to their therapeutic applications in asthma. Am Rev Respir Dis. 1990;141:S59–69.
- Nagakumar P, Puttur F, and Gregory LG, et al. Pulmonary type-2 innate lymphoid cells in paediatric severe asthma: phenotype and response to steroids. Eur Respir J. 2019 54 2 ;1801809.
- Shah SP, Grunwell J, Shih J, et al. Exploring the utility of noninvasive type 2 inflammatory markers for prediction of severe asthma exacerbations in children and adolescents. J Allergy Clin Immunol Pract. 2019;7:2624–2633 e2622.
- Baumann R, Untersmayr E, Zissler UM, et al. Noninvasive and minimally invasive techniques for the diagnosis and management of allergic diseases. Allergy. 2021;76:1010–1023.
- Peters MC, Mekonnen ZK, Yuan S, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014;133(388–394):388–394.e5.
- Nagakumar P, Denney L, Fleming L, et al. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016;137:624–626 e626.
- Baigelman W, Chodosh S, Pizzuto D, et al. Sputum and blood eosinophils during corticosteroid treatment of acute exacerbations of asthma. Am J Med. 1983;75:929–936.
- Gibson PG, Grootendor DC, Henry RL, et al. Sputum induction in children. Eur Respir J Suppl. 2002;37:44s–46s.
- Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020;20::552–565.
- Golebski K, Layhadi JA, Sahiner U, et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54:291–307 e297.
- Sonnenberg GF, Hepworth MR. Functional interactions between innate lymphoid cells and adaptive immunity. Nat Rev Immunol. 2019;19:599–613.
- Castanhinha S, Sherburn R, Walker S, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol. 2015;136:312–322 e317.
- Hussain SMA, Sen A, Barraclough BC, et al. Blood levels of type-2 innate lymphoid cells in preschool children with acute wheeze. Eur Respir J. 2017;50. PA1335.
- Cianchetti S, Cardini C, Puxeddu I, et al. Distinct profile of inflammatory and remodelling biomarkers in sputum of severe asthmatic patients with or without persistent airway obstruction. World Allergy Organ J. 2019;12:100078.
- Teague WG, Phillips BR, Fahy JV, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6:545–554 e544.
- Wilson NM, Bridge P, Spanevello A, et al. Induced sputum in children: feasibility, repeatability, and relation of findings to asthma severity. Thorax. 2000;55:768–774.
- van de Kant KD, van der Sande LJ, Jobsis Q, et al. Clinical use of exhaled volatile organic compounds in pulmonary diseases: a systematic review. Respir Res. 2012;13:117.
- Neerincx AH, Vijverberg SJH, Bos LDJ, et al. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr Pulmonol. 2017;52:1616–1627.
- van Vliet D, Smolinska A, Jobsis Q, et al. Can exhaled volatile organic compounds predict asthma exacerbations in children? J Breath Res. 2017;11:016016.
- Robroeks CM, van Berkel JJ, Jobsis Q, et al. Exhaled volatile organic compounds predict exacerbations of childhood asthma in a 1-year prospective study. Eur Respir J. 2013;42:98–106.
- van Oort PM, de Bruin S, and Weda H, et al., On Behalf Of The Mars C. Exhaled breath metabolomics for the diagnosis of pneumonia in intubated and mechanically-ventilated Intensive Care Unit (ICU)-patients. Int J Mol Sci. 2017 18 2 ;449.
- Hong H, Liao S, Chen F, et al. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy. 2020;75::2794–2804.
- Zissler UM, Ulrich M, Jakwerth CA, et al. Biomatrix for upper and lower airway biomarkers in patients with allergic asthma. J Allergy Clin Immunol. 2018;142:1980–1983.
- Cardenas A, Sordillo JE, Rifas-Shiman SL, et al. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat Commun. 2019;10:3095.
- Bergougnoux A, Claustres M, De Sario A. Nasal epithelial cells: a tool to study DNA methylation in airway diseases. Epigenomics. 2015;7:119–126.
- Zhu T, Zhang X, Chen X, et al. Nasal DNA methylation differentiates severe from non-severe asthma in African-American children. Allergy. 2021;76:1836–1845.
- Goodman MA, Moradi Manesh D, Malik P, et al. CRISPR/Cas9 in allergic and immunologic diseases. Expert Rev Clin Immunol. 2017;13:5–9.
- Agache I, Akdis CA, Akdis M, et al. EAACI biologicals guidelines-recommendations for severe asthma. Allergy. 2021;76:14–44.
- Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–1485.
- Vijverberg SJH, Brinkman P, Rutjes NWP, et al. Precision medicine in severe pediatric asthma: opportunities and challenges. Curr Opin Pulm Med. 2020;26:77–83.
- Israel E, Mb H. Personalizing precision medicine. J Allergy Clin Immunol Pract. 2020;8:1614–1615.
- Jang YJ, Kwon HJ, Lee BJ. Effect of clarithromycin on rhinovirus-16 infection in A549 cells. Eur Respir J. 2006;27:12–19.
- Richeldi L, Ferrara G, and Fabbri LM, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2005 3 ;CD002997.
- Golebski K, Kabesch M, Melen E, et al. Childhood asthma in the new omics era: challenges and perspectives. Curr Opin Allergy Clin Immunol. 2020;20:155–161.
- Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J. 2015;46:1322–1333.
- Ivanova O, Richards LB, Vijverberg SJ, et al. What did we learn from multiple omics studies in asthma? Allergy. 2019;74:2129–2145.
- Roberts J, Bratton SL, Brogan TV. Acute severe asthma: differences in therapies and outcomes among pediatric intensive care units. Crit Care Med. 2002;30:581–585.
