ABSTRACT
Introduction
Pleural diseases encompass a broad range of conditions with diverse and heterogenous etiologies. Diagnostics in pleural diseases thus represents a challenging field with a wide array of available testing to distinguish between the numerous causes of pleural disease. Nonetheless, deploying best practice diagnostics in this area is essential in reducing both duration o the investigation pathway and symptom burden.
Areas covered
This article critically appraises the optimal diagnostic strategies and pathway in patients with pleural disease, reviewing the latest evidence and key practice points in achieving a treatable diagnosis in patients with pleural disease. We also cover future and novel directions that are likely to influence pleural diagnostics in the near future. PubMed was searched for articles related to pleural diagnostics (search terms below), with the date ranges including June 2012 to June 2022.
Expert opinion
No single test will ever be sufficient to provide a diagnosis in pleural conditions. The key to reducing procedure burden and duration to diagnosis lies in personalizing the investigation pathway to patients and deploying tests with the highest diagnostic yield early (such as pleural biopsy in infection and malignancy). Novel biomarkers may also allow earlier diagnostic precision in the near future.
KEYWORDS:
1. Introduction
Pleural diseases represent a wide range of conditions with significant impacts on patients and burden to healthcare systems. The most common presentation of pleural disease is with pleural effusion resulting in symptoms of breathlessness, cough, or fatigue, or as an incidental finding on imaging. There are approximately 1.5 million new pleural effusions identified per year in the United States, and the costs to healthcare systems are significant, with recent US data showing the cost of hospitalization with pleural disease to be over $10 billion per year and readmissions costing over $1 billion annually[Citation1,Citation2]. The need for effective diagnostics in this vulnerable population of patients cannot therefore be understated, with key priorities for emergency and pleural physicians revolving around providing effective, specialized care earlier to minimize the need for hospital admission and repeated pleural procedures. Ascertaining a rapid, accurate diagnosis however is challenging in itself, with over 50 known conditions resulting in pleural disease, and clinical presentation that is often non-specific[Citation3].
Despite these significant challenges, there is reason to be optimistic in the appraisal of the current state of care for patients suffering from pleural disease, with an ever-increasing evidence based on optimal diagnostic strategies and practice changing large-scale clinical studies taking place over the past decade providing robust data to guide best practice. In addition, the increasing adoption of pleural specialist services to improve patient care is becoming widespread, forming the basis for increased ambulatory capacity and allowing more efficient care[Citation4,Citation5].
This article focuses on the typical investigation pathway and evidence base in the diagnosis of new pleural effusion, which forms the most significant diagnostic challenge, while discussing pneumothorax in the imaging section. This review will appraise the recent updates, challenges, and future directions in the field of pleural diagnostics, covering imaging techniques, pleural fluid analysis, pleural biopsy techniques, and novel diagnostic strategies. PubMed was searched for articles related to pleural diagnostics, with the date ranges including June 2012 to June 2022. Search terms used included: pleural aspiration, pleural fluid, pleural intervention, thoracocentesis, thoracoscopy, pleural biopsy, and pleural diagnostics. Older citations have been included which are relevant to current practice.
2. Imaging
Imaging of the thorax typically provides the earliest objective evidence of pleural disease. While symptoms of breathlessness, cough, or chest pain may alert a clinician to the presence of pleural effusion or pneumothorax, these are non-specific, as is the clinical examination in these conditions. Thus, imaging is required to move the diagnostic process forward and allow management of the underlying condition. Selection of the most appropriate imaging technique is crucial to diagnose and therefore appropriately manage patients.
2.1. Chest radiograph
Chest Radiograph (CXR) is amongst the most widely available radiological investigation. It is likely to provide first confirmation of the presence of fluid or air within the pleural space, as well as excluding differentials of breathlessness or chest pain. A postero-anterior (PA) CXR will detect around 200 mL of fluid within the pleural space and should remain the first radiological investigation in most cases[Citation6]. Supine films are less useful for obvious reasons. CXR can also point toward causes of effusion, for example the presence of a lung mass or lymphadenopathy in the presence of a unilateral effusion which may indicate a malignant diagnosis. CXR is also useful following therapeutic thoracentesis to determine non-expansile lung in malignant pleural effusion. The presence of a hydropneumothorax with a clear air fluid level not respecting the lobar anatomy of the lung would sway one toward indwelling pleural catheter insertion rather than attempting pleurodesis as the definitive fluid control strategy.
2.2. A note on pneumothorax
Pneumothorax can be identified on CXR as a visceral line between lung and chest wall and the absence of lung markings beyond. Pneumothorax may be missed in high-risk acutely unwell patients, for example in those who can only tolerate a supine or semi-erect CXR. The deep sulcus sign on supine CXR describes the phenomenon of pleural air collecting in the anterior costophrenic sulcus, with resultant hyperlucency[Citation7]. It is estimated that additional subtle information on CXR, such as the deep sulcus sign, may identify pneumothorax on 24% of previously missed occult pneumothoraces[Citation8].
Thoracic ultrasound (TUS) is therefore increasingly used to identify or exclude pneumothorax in the acutely unwell patient. Ultrasound for pneumothorax has a high sensitivity and specificity, reaching 0.88 and 0.99, respectively, in a large meta-analysis [Citation9]. The presence of a ‘lung point’, lung sliding replacing absent lung sliding, is 100% specific, but 66% sensitive for pneumothorax[Citation10]. The absence of lung sliding, the presence of A-lines, and the absence of B-lines are also suggestive of pneumothorax, but clinicians must be aware of diagnoses such as COPD or bullae which can mimic this appearance on TUS[Citation11]. In this high-risk group, where the diagnosis of pneumothorax on CXR or TUS is unclear, confirmation of pneumothorax with CT would be recommended prior to intervention. Furthermore, TUS is a good rule in or rule out test in many cases of pneumothorax, but cannot define the size of pneumothorax which is better assessed using CXR or CT.
2.3. Ultrasound
The use of thoracic ultrasound in diagnostics of pleural disease has transformed the field over the past 3–4 decades. TUS has now become a reliable, portable, and relatively cheap imaging tool to diagnose and assess the breathless patient. TUS can be used to identify chest wall, pleural, and subpleural disease including consolidation, interstitial edema, or thickening (increased B lines), fluid and pleural thickening. Its use is also recommended in international guidelines to reduce the risk of procedural complications[Citation12].
TUS can detect as little as 5 mL of pleural fluid and furthermore can be used to describe pleural fluid in terms of echogenicity and complexity. The echogenicity and complexity of pleural fluid were traditionally thought to represent whether fluid was exudate or transudate. There is disagreement between multiple studies, however. Asciak et al contradict the previously widely held belief that highly echogenic effusions must always be exudates: they found a specificity of only 57.1% in a prospective study of 140 patients[Citation13]. Similarly, Chen and colleagues found that sonographic appearances in biochemically transudative effusions are not always anechoic and simple: 55% of patients had a complex non-septated pattern on TUS [Citation14]. Echogenicity of fluid on TUS should therefore not be used in isolation to make diagnostic conclusions without considering the full clinical picture.
Pleural septations () may occur when there is infected or malignant pleural fluid, or in the presence of a foreign body such as indwelling pleural catheter or previous pleural intervention. Septations are fibrin strands which can form in exudative effusions and are seen on TUS as thin hyperechoic strands which tend to move within the fluid and can progress over time. It is important to recognize that each locule or pocket between septations in an infected pleural space may have different biochemical characteristics and therefore should not be relied upon to decide whether the effusion needs drainage[Citation15].
Figure 1. Chest radiograph (CXR), ultrasound and CT scan from a patietn with empyema, ullustrating the difference in information gleaned from each. A) CXR showing unilateral pleural effusion. B) Ultrasound demonstrating septated, organised pleural space and C) CT showing loculations and visceral pleural enhancement.
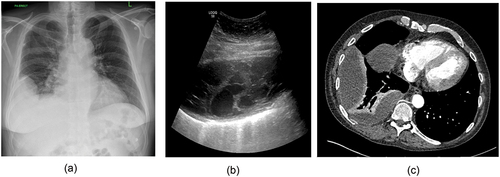
Septated fluid can be seen in inflammatory, infective, or malignant effusions and in some disease states correlates with worse clinical outcomes[Citation16]. Septations may help to predict which patients with infected pleural spaces may need more complex interventions for pleural infection such as intrapleural enzyme treatment (IET) or surgical management [Citation17].
For a diagnosis of malignant pleural effusions, thoracic ultrasound is useful as a rule-in but not rule-out investigation. Qureshi et al found a sensitivity comparable with contrast-enhanced computed tomography (CT), when combining the presence of pleural thickening >1 cm, pleural nodularity, and the presence of hepatic metastases[Citation18]. A 2020 meta-analysis found that the presence of pleural nodularity alone was a pooled sensitivity of 96.9%; however, other features such as pleural thickening were less sensitive[Citation19]. TUS may therefore be used to guide further invasive investigations in patients with suspected malignant effusions and furthermore the best biopsy method may be determined using TUS. It is now well documented that TUS can be used to diagnose septations and predict ‘difficult to access’ pleural spaces prior to attempting local anesthetic single port (medical) thoracoscopy(MT)[Citation20–22]. Use of TUS prior to procedures may reduce unnecessary attempts at MT and guide the clinician to opt for image-guided biopsy instead. TUS may also be used to screen for intercostal vessels prior to attempting pleural interventions, which is thought to reduce bleeding rates, although it is not yet known if this translates to a clinically significant reduction in complications (and it is unlikely that such a study will be conducted, as significant pleural hemorrhage is a rare complication)[Citation23].
TUS can therefore assist in diagnosis of malignant effusion, guide highest yield invasive investigations and reduce complications. More advanced TUS may be used to predict non-expansile lung (NEL) prior to therapeutic thoracentesis. Salamonsen et al used motion (M) mode of ultrasound to determine the transmission of cardiac impulse through the atelectatic lung and found a specificity oF 85% in predicting NEL[Citation24]. TUS may also be used to predict pleurodesis success. The SIMPLE trial showed absence of lung sliding sonographically, rather than conventional pleural fluid output, to guide timing of chest tube removal following talc slurry can reduce length of stay without reducing efficacy of pleurodesis at 3 months[Citation25]. It is in this area, as a combined diagnostic and therapeutic tool, that represents perhaps the most exciting direction for TUS in the management of pleural disease.
2.4. Computed tomography (CT)
Despite its many benefits, TUS cannot completely replace cross-sectional imaging in the diagnosis of pleural disease as CT can provide a three-dimensional representation of the thoracic and pleural anatomy including central structures. As previously noted, in cases of difficulties interpreting CXR or TUS, CT is the gold standard investigation to diagnose pneumothorax as well as defining bronchopleural fistulae, tethered pneumothorax, and esophageal leak. For workup of other pleural pathology, a ‘pleural phase’ venous contrast phase CT is recommended. This optimizes imaging of the pleura with administration of iodinated intravenous contrast 60–90s prior to CT[Citation26]. In cases of esophageal pathology additional oral contrast can identify site of perforation and degree of pleural and mediastinal contamination[Citation27]. It is important to note that for diagnostic purposes, CT does not need to be performed following therapeutic thoracentesis: a retrospective study at a large tertiary hospital showed no significant additional diagnostic information when a CT was performed before and after pleural drainage[Citation28].
Whilst CT may not be recommended as a routine part of evaluation of all patients with pleural effusion, it is crucial in the evaluation of malignant pleural effusion and pleural infection. CT can be used to distinguish simple versus complicated parapneumonic effusions[Citation29]. The ‘split pleura’ sign – visceral and parietal pleural thickening with separation – is traditionally seen as diagnostic of empyema but may only be seen in 68% of patients[Citation30]. In a retrospective study of patients in the ‘MIST’ trial, parietal pleural thickening and enhancement were noted in 98.7% of patients with pleural infection[Citation31]. Complicated effusions may appear as lentiform homogenous fluid, rather than obeying gravity if they are free flowing[Citation32]. CT may also be used to determine drain placement and plan for future surgery in the case of later stage empyema or complicated parapneumonic effusion.
CT evidence of pleural thickening >10 mm, pleural nodularity, mediastinal thickening, or circumferential thickening are all suggestive of malignant disease. These characteristics have a high sensitivity but low specificity, and in the presence of pleural fluid, mediastinal, or circumferential involvement is less sensitive[Citation33,Citation34]. One retrospective study showed the positive predictive value of a malignant CT report was 80%, but the negative predictive value only 65%[Citation35]. This demonstrates the need to pursue a tissue diagnosis in high-risk patients and to consider radiological and clinical follow up in those with CT-labeled benign pleural disease. CT imaging of the abdomen and pelvis should also be considered: a recent prospective study found clinically significant subdiaphragmatic findings in nearly a quarter of patients who were investigated for unilateral pleural effusion[Citation36].
2.5. Positron emitted tomography (PET-CT)
PET-CT remains controversial as an investigation for malignant pleural disease. False positives are known to occur in pleural infection and previous talc pleurodesis whilst low-grade cancers including epithelioid mesothelioma may not be detected on PET-CT. There is a lack of evidence for PET-CT in addition to CT and pleural fluid cytology for diagnosis or prognostication of MPE[Citation37]. Meta-analyses have shown a reasonable sensitivity (81–95%) but low specificity (63–82%) for detection of malignancy[Citation38–40]. Whilst there is not enough evidence for routine use of PET-CT in workup of pleural effusion, it may have a clearer role when used in combination with other imaging modalities to highlighting metabolically active targets for biopsy in patients with mixed mesothelioma and benign pleural thickening.
2.6. Magnetic resonance imaging (MRI)
MRI is often less accessible and more expensive than CT and is limited due to interference from movement. It has a role in identification, diagnosis, and follow up of soft tissue tumors such as solitary pleural fibromas and lipomas. Furthermore, it may help to diagnosis of malignant disease particularly in those patients with contrast allergy, although CT remains the first-line imaging of choice[Citation41].
2.7. Use of radiology in the high-risk patient
High-risk pneumothorax cases, such as bilateral pneumothoraces or large pneumothorax with radiological evidence of tension, can be identified quickly with CXR. In the era of rapid trauma-series CTs, it is now not uncommon to have CT imaging suggestive of ‘occult’ tension pneumothorax, although the authors acknowledge this to be somewhat of an oxymoron [Citation42]. Portable TUS can similarly quickly identify high-risk septated pleural effusion in pleural infection, and therefore initiate further diagnostics and chest tube drainage in high-risk empyema patients. Pleural phase CT can be used to identify patients with a high chance of malignancy, such as with presence of pleural nodularity and circumferential pleural thickening.
3. Pleural aspiration (Thoracocentesis) and pleural fluid analysis
Pleural aspiration involves the removal of up to 1.5 L of pleural fluid from the pleural space for both diagnostics and temporary therapeutic benefit in the case of symptomatic effusions. Pleural aspiration has, for decades, formed the cornerstone of pleural diagnostics, with pleural fluid providing the opportunity for biochemical, microbiological, and cytological laboratory analysis. It is typically advocated in the majority of cases of new unilateral pleural effusion. There are occasions however in which pleural aspiration may not be required. Typically this would occur in the case of bilateral pleural effusions with a known, non-malignant cause that would explain the presentation, such as cardiac failure[Citation43]. In cases such as these, management of the underlying cause (such as diuresis) is a reasonable first step and advocated by international guidelines[Citation43]. It is important to note however that cardiac failure, while extremely common (annual incidence in the USA oF 500 000) only presents with bilateral effusions in 60% of cases in the largest datasets available, and in the case of asymmetrical effusions non-responsive to medical therapy, pleural aspiration, and biopsy should be re-considered[Citation44,Citation45]. This is particularly relevant as pleural effusions resulting from a combination of malignant and non-malignant causes are increasingly recognized[Citation46].
Pleural fluid obtained should be sent for, as a minimum, protein, lactate dehydrogenase (LDH), microbiology, culture and sensitivity (MCS), and cytology.
3.1. Pleural fluid biochemistry
Pleural fluid biochemistry is a key test in the pleural diagnostic pathway, with the key advantages of being a rapid turnaround test with typical patterns in specific disease states.
Despite being devised half a century prior, light’s criteria for differentiating exudates from transudates is the first step in interpreting pleural fluid biochemistry. Light’s criteria defines exudates as pleural effusions with any of: pleural fluid (PF) protein to serum ratio as >0.5, PF LDH to serum ratio >0.6 or PF LDH as two-thirds the upper limit of the reference range for normal serum LDH[Citation47–49]. The identification of exudates are clinically important as the typical exudative conditions (listed in ) include malignancy and infection, with distinct treatment paradigms, in contrast to transudates, which broadly reflect systemic fluid overloaded states.
Table 1. Causes of pleural effusions[Citation47–49].
Light’s criteria, although widely used in pleural diagnostics, should not be regarded as infallible, with up to 10% of transudates by light’s resulting from malignancy in retrospective studies[Citation50]. Additionally, the issue of discordant pleural effusions (with a low LDH and high protein or vice versa) have been assessed in a large retrospective study (n = 992), with a suggestion that patients with discordant biochemistry may represent a distinct population, with a higher incidence of fluid overloaded states (11% in discordant group vs 2% in concordant group) and increasing discordance with older age[Citation51].
Pleural fluid biochemistry often forms a key element in the diagnosis of pleural infection. While positive microbiology is the gold standard in diagnosing pleural infection, blood, and fluid cultures often require days to establish and are negative in over one-third of patients with pleural infection[Citation52]. The presence of frank pus is diagnostic for empyema, however in the absence of this, rapid biochemical parameters of pH<7.2, glucose<2.2 mmol/L, and LDH>1000IU/L to determine inflammatory pleural effusions can direct treatment for pleural infection in the appropriate clinical circumstances. Pleural fluid pH has proven to be the most specific, despite being first described by Light et al in 1973[Citation53]. Data from our own institution have shown that a significant proportion of patients with glucose<2.2 and LDH>1000 do so secondary to non-infective diagnoses, the most common being malignancy and connective tissue disease[Citation54].
Other biochemical patterns that are typically useful in diagnosing the cause of pleural effusions are listed in , with biochemistry often being a reliable diagnostic test to define chylothorax, pseudochylothorax, and effusions related to pancreatitis amongst others.
3.2. Pleural fluid microbiology
Microbiological culture is an essential diagnostic component to determine causative organisms in pleural infection and guide antibiotic treatment choice. As noted above, a systematic review in this area found the PF culture positivity rate to be only 56%[Citation55]. This can be increased by 20% by inoculating pleural fluid in blood culture bottles compared to standard methods[Citation56]. Pleural biopsy can also improve yield, and this is discussed in the ‘pleural biopsy’ section. As such, when diagnosing pleural infection, clinicians must use clinical, and radiological features in combination with pleural sampling. It is also necessary to be aware of the common causative organisms, as treatment is often empirical. illustrates the most common organisms identified according to healthcare or community acquisition.
Table 2. Frequency of bacterial organisms isolated on routine pleural fluid culture[Citation55].
It is notable that the lung parenchyma and the pleural space differ in host environment, leading to a different microbiological profile between pneumonia and pleural infection[Citation57]. When considering empirical antibiotic choice, it is important to tailor these to local prevalence, and indeed causative organisms vary according to country, age, and community or hospital setting[Citation58]. The largest dataset in this regard is a recent systematic review of over 10,000 patients with positive diagnostic microbiology. Overall, the most common organism isolated was Staphylococcus Aureus, followed by Viridans Streptoccocus group, with Klebsiella and Pseudomonas also commonly isolated. Within S.Aureus isolates, only 67% of community acquired and 42% of hospital acquired infections were methicillin sensitive, a cause for concern amongst treating physicians. From the community acquired pleural infection group, pneumococci, and viridans were common isolates, suggesting a link to oral flora, although the mechanisms by which these gain access to the pleura are not entirely clear. Possible mechanisms include hematogenous spread in the context of poor dental hygiene and recurrent aspiration[Citation55].
An evolving area to improve diagnostic microbiological yield in pleural infection lies in metagenomic studies using bacterial DNA sequencing, which is more sensitive than standard culture[Citation59]. Studies to date have suggested that significant cases of pleural infection are polymicrobial, and while ascertaining such information at the point of care would be clinically useful, high costs, and availability remain a barrier to adoption in daily practice[Citation60].
3.3. Pleural fluid cytology – still the first choice?
The most basic information gleaned from pleural fluid cytology is that of cell count differentials. Although rarely the sole parameter to achieve diagnosis, these can be of utility when specific patterns are identified. Neutrophil dominant effusions typically reflect acute pathology, such as pulmonary embolus, acute infection, or parapneumonic effusion. Lymphocytic effusions (with >50% lymphocytes) can be seen in tuberculosis (often very high lymphocyte count), congestive cardiac failure or hematological malignancy[Citation61]. Finally eosinophilic effusions are a comparatively less common presentation, but result from a broad range of differentials, including malignancy, inflammatory, and drug induced[Citation62]. Idiopathic eosinophilic pleuritis is also a well-documented phenomenon although remains a diagnosis of exclusion[Citation63].
The use of PF cytology has long been the initial step in diagnosing malignant pleural effusion (MPE), although many recent studies have brought into question the diagnostic utility of PF cytology alone. The diagnostic sensitivity of PF cytology is relatively low at 37–47% in patients with proven MPE[Citation64,Citation65]. Additionally, there is huge variation by cancer type, with diagnostic rates in malignant pleural mesothelioma (MPM) only 6%, as many MPMs do not release tumor cells into adjacent pleural fluid. lists the diagnostic sensitivity of pleural fluid according to malignancy type. Another key factor when appraising the utility of cytology is the frequency with which PF cytology can guide specific, personalized oncological treatment. This is particularly relevant in the current era of targeted immuno-modulating treatment and the requirement for molecular marker identification on tumor cells to guide this. This is exemplified in lung adenocarcinoma, wherein current international guidelines recommend the testing for multiple gene mutations such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) in advance of initiating systemic therapy[Citation66]. A recent retrospective dataset has found the molecular marker yield of PF cytology to be only 20%, and as such direct to biopsy strategies have been advocated, particularly in cases when pleural fluid yield is likely to be poor (such as in patients with history and imaging suggestive of mesothelioma)[Citation67–69].
Table 3. Pleural fluid cytological sensitivity by cancer type [Citation64].
4. Pleural biopsies
With the aforementioned limitations o pleural fluid alone, there has been great interest in defining optimal methods to obtain pleural biopsy as a definitive diagnostic strategy. Pleural biopsies typically form the gold standard in the diagnosis of malignant pleural effusions and can increase the microbiological yield in both pleural infection and TB. Diagnoses of exclusion such as benign asbestos-related pleural effusion (BAPE) and eosinophilic pleuritis also require pleural biopsy before they are finalized (alongside compatible imaging and clinical features). The optimal technique for obtaining pleural biopsies is the subject of numerous studies and these are discussed here.
4.1. Blind and image-guided pleural biopsy techniques
Closed pleural biopsies can be obtained using reversed-bevel closed needles, (Abrams and Cope) named after their respective inventors. The diagnostic sensitivities for malignancy (in the absence of image guidance) are relatively low for these techniques, with randomized trial data suggesting this to be under 50%, corroborated by a large retrospective study[Citation70,Citation71]. Performance does appear to improve in the diagnosis of pleural tuberculosis to 69–90%[Citation72]. There has been suggestion in limited case series that the addition of CT or ultrasound guidance can improve diagnostic yield; however, the complication rate associated with closed pleural biopsy is relatively high (15% pneumothorax), meaning that these techniques have been largely superseded by image guided cutting needle biopsy or medical thoracoscopy as resources allow[Citation48,Citation73].
Image-guided biopsy using a cutting needle () has gained increasing adoption in pleural centres as an additional technique to achieve pleural biopsy. This typically involves the use of an 18 gauge needle with a 2 cm serrated cutting area, which can target areas of pleural abnormality visualized either by ultrasound or CT imaging (typically parietal pleural nodularity or thickening). The logistical appeal of cutting needle biopsy lies in the minimally invasive nature of the technique, and the ability to deploy directly alongside an ultrasound-guided pleural aspiration with minimal added time or equipment (in contrast to medical thoracoscopy). Diagnostic sensitivity is high when used with ultrasound guidance at over 90% for malignancy in a small retrospective cohort, and 84.6% in a recent systematic review encompassing 30 studies[Citation74,Citation75]. Prospective studies are lacking in this area however, and when successful molecular marker analysis is considered, the diagnostic sensitivity drops below 80%[Citation67]. This element is of great importance to consider; ultrasound-guided cutting needle biopsy should not be used to replace medical thoracoscopy, but rather used as an adjunctive tool to be deployed in patients who may not be eligible for thoracoscopy due to poor performance status or lack of accessibility to the pleural space.
Figure 2. Pleural biopsies using A) cutting needle under ultraound guidance and B) thoracoscopic technique. Arrow indicates position of cutting needle, star indicates pleural effusion.
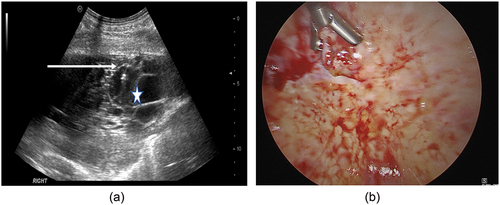
Whether to undertake cutting needle biopsies preferentially under ultrasound or CT guidance is a question not fully answered by the available data on diagnostic yield, and local expertise will likely determine which is more utilized at present. There are however significant advantages with ultrasound regarding waiting times and the patient pathway. The aforementioned systematic review demonstrated the diagnostic yield to be greater in CT-guided cutting needle biopsy at 93%[Citation75], however, a retrospective study of 273 patients found similar (>95%) diagnostic accuracy of both CT and US guidance. This study also highlighted the faster, less expensive nature of an US-guided approach[Citation76]. Clearly, the factors leading to this finding speak to the relative deliverability of each – US-guided procedures can occur alongside therapeutic aspiration, even as part of a ‘First’ pleural procedure, can be delivered by physicians (versus radiologists with CT) and do not require CT scanning time or expose the patient to ionizing radiation.
Pooled data between US- and CT-guided techniques suggest that the complication rate is low, with a 3.6% pneumothorax rate, and 1% empyema rate[Citation77].
While the focus for the above section has been pertaining to malignancy and TB, it is should be noted that the AUDIO study has shown that ultrasound-guided cutting needle biopsy at the time of drainage in pleural infection can increase microbiological yield by 25% compared to pleural fluid analysis only[Citation78]. A future definitive study could determine whether pleural biopsy becomes a new standard of care in pleural infection diagnostics.
4.2. Thoracoscopic pleural biopsy – Medical vs surgical approaches
Pleural biopsies under direct vision using a fibre-optic camera () to assess the macroscopic appearance of the pleura, diaphragm, and lung and guide biopsy targets remains the gold standard investigation for diagnosing unexplained exudative pleural effusions. Medical thoracoscopy (MT or local anesthetic thoracoscopy) is typically undertaken by pleural or respiratory physicians, with conscious sedation in a spontaneously breathing patient and usually occurs as a day case procedure. Surgical thoracoscopy (video-assisted thoracoscopic surgery, VATS) is conducted under general anesthetic and requires single lung ventilation, and thus requires a patient that is fit enough to survive these. Both techniques allow for highly effective diagnostic pleural sampling and inspection of the pleural space, however key differences arise in regard to the technical feasibility between the two, length of stay associated and the extent of therapeutic interventions that can be delivered contemporaneously.
MT requires the insertion of either a single or dual ports for access with an endoscope (with the second port for biopsy forceps where required, so-called two port technique). The diagnostic yield of MT in the literature for unexplained pleural effusion, is between 91 and 95% with the most common diagnoses assessed being MPE and TB [Citation79–81]. Despite this, peri-procedural planning around MT is necessary to ensure access to the pleural space is adequate. In the case of large, free flowing pleural effusions, this is rarely a significant problem, however becomes more relevant in effusions that are heavily septated or organized (wherein the lung may not deflate allowing sufficient space to maneuvre the endoscope, or preclude adequate inspection of the parietal pleura), and in patients who have imaging based pleural abnormality (such as pleural thickening) but no discernible pleural effusion. In the latter scenario, a Boutin needle is required to induce a pneumothorax to allow port and endoscope access. Pneumothorax induction is safe and effective when deployed by trained operators, with one case series reporting no adverse events and a success rate of 87%[Citation82]. Peri-procedural ultrasound assessment can also help predict the likelihood of successful pneumothorax induction, by identifying ‘lung sliding’ or the movement of the parietal pleura over visceral pleural surface with ventilation[Citation83]. The authors would recommend the use of ultrasound to risk stratify patients prior to pneumothorax induction thoracoscopy, and in cases with a high risk of failure (signs of a pleurodesed space with lack of lung sliding), consider referral for VATS wherein increased options to achieve a working space are available (see below). In conjunction with the high diagnostic yield, robust data showing MT to be a safe procedure is available. The British Thoracic Society pleural guideline evaluated over 4500 thoracoscopic procedures and found the major complication rate to be low at 1.8%, with mortality 0.3%[Citation84]. The key major complications operators should be aware of include persistent post-operative air leak, empyema, and procedural tract metastases.
VATS is a surgical procedure, often involving a three port technique and requiring general anesthetic, accompanied by a short post-operative inpatient stay. The significant advantage over MT lies in the ability to deal with an organized, septated pleural space and convert to open thoracotomy where required. The diagnostic sensitivity of VATS is also high, and comparable to MT, at 90–95%. The complication rate is much more challenging to define, given the different patient group undergoing VATS and the more extensive therapeutic interventions delivered (such as decortication, pleurectomy, or abrasion). Overall, despite this the major complication rate is likely to be similar where the nature of intervention and patient population is matched, and the literature reveals rates of between 1.2 and 4% for major complications [Citation80,Citation85].
A nuanced view regarding the optimal approach is required when evaluating how to best utilize the three high yield pleural biopsy techniques of image-guided pleural biopsy, MT and VATS. Clearly local expertise and familiarity will impact heavily. However, in centres with access to all three options, the authors would discourage triaging patients to VATS, MT, and image-guided biopsy (in that order) based on decreasing performance status alone as this does not fully capture the utility of each. We suggest that image-guided biopsy can be utilized as an early biopsy technique in patients with targetable sonographic pleural thickening alongside initial pleural aspiration (with the option to then undertake thoracoscopy thereafter if negative), or in patients who are likely to not have an accessible pleural space by MT either prior to VATS or if not medically suitable for surgery. This is illustrated in .
Figure 3a. Suggested algorithm for the diagnosis of pleural infection.
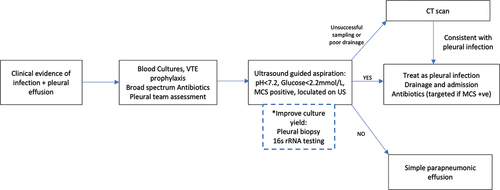
Figure 3b. Authors suggested pathway for the investigation and management of suspected malignant pleural effusion.
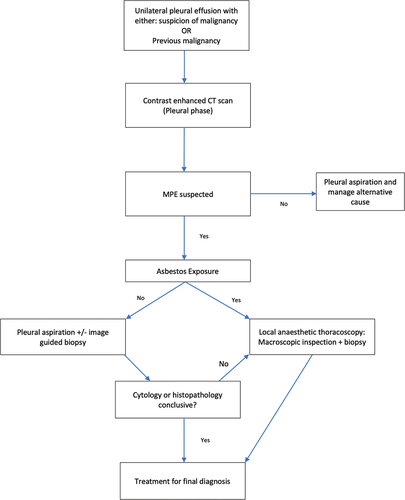
Thoracoscopic approaches remain the diagnostic standard, and even more so in suspected malignant pleural mesothelioma, wherein diagnostic confidence is generally proportional to biopsy size, and thoracoscopy allows for the acquisition of deeper and larger biopsies including fat and/or muscle to assess tumor invasion[Citation86,Citation87]. MT should be used as the definitive diagnostic test for malignancy or rule out causes of pleural pathology when biopsy is required and this is feasible, requiring fewer resources and reduced patient hospital stay. The therapeutic value of MT however is limited to fluid drainage and talc pleurodesis, with much more uncertain utility in pleural infection[Citation88,Citation89]. The use of VATS may be guided by the complexity of the pleural space and the likely requirement for more extensive therapeutic intervention such as washout or decortication. Of course, patient choice must also be considered, and a subsect may make a choice based on either a preference for or against general anesthesia.
5. Novel diagnostic directions and other pleural biomarkers
The above review addresses the current evidence base in best-practice diagnostics for pleural disease, necessarily focusing on the areas with the highest impact data. There are a number of promising early-phase diagnostic tests and other biomarkers that may currently or in the near future be carried out alongside the above well-established paradigms.
Within pleural infection, there has been work to optimize the specificity of diagnosis in patients with equivocal imaging and symptoms (for example with an increasingly septated pleural space and high inflammatory markers), and procalcitonin has been postulated to be a more specific marker of infection in this cohort[Citation90]. Another area with interest is to combine diagnostics with the ability to guide treatment, and in this regard pleural fluid soluble urokinase plasminogen activator (suPAR) has shown promising results in predicting requirement for surgery[Citation91]. More recently pleural fluid Plasminogen-Activator Inhibitor-1 (PAI-1) has been shown to correlate with septation formation and even independently predict length of hospital stay and 12-month mortality[Citation92].
Pleural fluid adenosine deaminase (ADA) is a biomarker that is used widely in the diagnosis of pleural TB. Cautions should be applied, given a high false positivity in rheumatoid disease and MPE, however ADA (typically a value of <40IU/L) retains a strong negative predictive value in excluding pleural TB in low incidence regions[Citation48,Citation93].
In the field of MPE, one area for potential to improve the diagnostic yield of ultrasound-guided pleural biopsies is the use of intravenous contrast. Sonographic pleural thickening may be a theoretical correlate of tumor presence; however, this is not always the case as fibrotic/inflammatory tissue may appear thickened on US[Citation74]. The administration of intravenous contrast has shown promising results in parenchymal lung lesion biopsies[Citation94]. Contrast-enhanced ultrasound has potential to increase diagnostic yield in pleural biopsy, as it provides real-time information on microvascular perfusion, highlighting areas of high metabolic tumor activity which are most likely to provide a molecular diagnosis[Citation95].
There has been growing interest in pleural manometry (the change in intrapleural pressure during drainage) in recent years, in particular to diagnose the presence of non-expansile lung (NEL). NEL occurs when an excessive negative pleural pressure is generated during pleural aspiration, often causing pain during the procedure. In MPE, this occurs due to either obstructive endobronchial lesions or visceral pleural thickening, preventing lung re-expansion. Pleural manometry has shown promising results in predicting NEL, although does not appear to reduce procedural pain[Citation96,Citation97]. Early identification of NEL has the potential to guide definitive interventions earlier, as those patients with NEL and thus lack of pleural apposition require indwelling pleural catheter insertion and attempts at chemical pleurodesis are likely to be unsuccessful.
6. Expert opinion
The area of pleural diagnostics encompasses a broad range of techniques in order to achieve a diagnosis in a highly heterogenous disease spectrum. Over the past decade, an ever-increasing number of studies has helped hone and refine the field with high-quality evidence, and pleural physicians now have an extensive dataset to guide best practice. These are incorporated into the authors’ suggested diagnostic algorithms for two of the most common pleural conditions – suspected pleural infection and suspected malignant pleural effusion, illustrated in , respectively.
The future of the field will lie beyond assessment of diagnostic sensitivities on a per-condition basis, moving toward personalizing the diagnostic strategy to the individual patient, reducing total number of procedures by combining definitive diagnostic and therapeutic interventions and finally, the use of novel diagnostic biomarkers to improve precision in diagnosis and predict outcome.
Practically, there are steps toward all of the above, with data in the literature to support a direct to MT approach for patients with suspected mesothelioma (triaged based on asbestos exposure and compatible CT imaging)[Citation69]. This can be further extrapolated to a targeted approach for other causes of MPE, whereby a patient with, for example, high suspicion of ovarian cancer (or a historic diagnosis of gynaecological malignancy), in which cytological yield would be >90%, it would be reasonable to proceed with a lower risk, less invasive pleural aspiration. In the truly undiagnosed effusion with little imaging or patient features to guide, adopting a combination ‘First procedure’ with ultrasound-guided biopsy and therapeutic aspiration may allow for a middle ground between minimal service impact and increasing the chances of successful diagnosis. However, robust data are needed to demonstrate not only a higher diagnostic yield and shorter diagnostic pathway, but also patient centred benefit, and such studies are planned. Real-world impacts of such a pathway must be considered, and these include the potential for a reduction in patient waiting time for diagnosis and by extension, treatment. Delivering more complex pleural procedures earlier does, however, rely on earlier input from pleural specialists, in turn necessitating the expansion of pleural services in many hospitals.
The future of diagnostic biomarkers may also hold great value in the risk stratification of patients and the aim in this regard will be to not only achieve diagnosis but predict early the eventual treatment modalities required. Examples in pleural infection include biomarkers to predict likelihood of drainage and surgery, and in malignancy diagnostic biomarkers to triage patients according to the chance of pleurodesis success, thus allowing earlier triage to chemical pleurodesis or IPC. The author’s opinion is that In MPE, the eventual direction of research would be to ascertain a diagnostic biomarker of pleural fluid output volume, which could in turn be targeted pharmacologically to reduce pleural fluid production and thus reduce the number of pleural procedures required for both diagnosis and treatment.
Finally, more radical shifts in Pleural medicine may centre around delivering (in MPE) definitive therapeutic interventions at the same time as definitive diagnostic testing at the first meeting with a Pleural physician. As an example, the patient with imaging and background history suggesting of mesothelioma may progress directly to a thoracoscopy+ talc or IPC (or all three) and trials are underway to evaluate components of such a strategy (TACTIC clinical trial, ISRCTN11058680).
While the last decade has seen great progress, the coming years should aim to bring pleural medicine in line with other respiratory subspecialties such as asthma in personalized, tailored care to the individual rather than generic diagnostic strategies. The authors would suggest that this would revolve around earlier pleural biopsy and sophisticated biomarker identification that would provide patients with prognostic information, and in the fullness of time could be targeted pharmacologically.
Article highlights
Pleural diseases represent a diagnostic challenge, with heterogenous clinical presentation and over 50 diseases know to affect the pleura.
Imaging is often necessary in the diagnosis of pleural pathology, and, over the past decade, ultrasound has transformed the safety, speed and accuracy of diagnostics in pleural diseases.
Despite the advances in imaging, often pleural fluid sampling is required to ascertain a diagnosis. Clinicians should send for analysis, as a minimum, the pleural fluid protein, lactate dehydrogenase, glucose, cytology, and microbiology.
Pleural biopsy is an increasingly required test, in particular for suspected malignant pleural effusion, wherein negative pleural fluid cytology alone is insufficient to rule out malignant pleural disease. Pleural biopsy can also improve the microbiological yield in pleural infection.
The future of pleural diagnostics lies in reducing the number of pleural procedures patients require by deploying higher yield tests (such as pleural biopsy) earlier in the pathway. In addition, the discovery and utilization of biomarkers to guide management is likely to be a key area of future research in this area.
Declaration of interest
Dinesh Addala is funded by the UK National Institute for Health and Care Research (NIHR) as a NIHR Doctoral Research Fellow and Najib Rahman receives funding from the NIHR Biomedical Research Council. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Sahn SA. Pleural Fluid analysis. In: Light RW, Lee YCG, editors. Textbook of pleural diseases. 2nd ed. London: Arnold Press; 2008. p. 209–226.
- Mummadi SR, Stoller JK, Lopez R, et al. Epidemiology of adult pleural disease in the United States. Chest. 2021;160(4):1534–1551.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. clinical Infectious Diseases. 2007;45(11):1480–1486.
- Hooper CE, Lee YCG, Maskell NA. Setting up a specialist pleural disease service. Respirology. 2010;15:1028–1036.
- Bhatnagar R, Maskell N. Developing a ‘pleural team’ to run a reactive pleural service. Clin Med (Lond). 2013;13:452–456.
- Blackmore CC, Black WC, Dallas RV, et al. Pleural Fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103–109.
- Chiles C, Ravin CE. Radiographic recognition of pneumothorax in the intensive care unit. Crit Care Med. 1986;14:677–680.
- Ball CG, Kirkpatrick AW, Fox DL, et al. Are occult pneumothoraces truly occult or simply missed? J Trauma. 2006;60:294–298. discussion 298-299.
- Ding W, Shen Y, Yang J, et al. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140:859–866.
- Lichtenstein D, Mezière G, Biderman P, et al. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26:1434–1440.
- Slater A, Goodwin M, Anderson KE, et al. COPD can mimic the appearance of pneumothorax on thoracic ultrasound. Chest. 2006;129:545–550.
- Reddy CB, DeCamp MM, Diekemper RL, et al. Summary for clinicians: clinical practice guideline for management of malignant pleural effusions. Ann ATS. 2019;16:17–21.
- Asciak R, Hassan M, Mercer RM, et al. Prospective Analysis of the Predictive Value of Sonographic Pleural Fluid Echogenicity for the Diagnosis of Exudative Effusion. Respiration. 2019;97:451–456.
- Chen H-J, Tu C-Y, Ling S-J, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34:362–369.
- Maskell NA, Gleeson FV, Darby M, et al. Diagnostically significant variations in pleural fluid ph in loculated parapneumonic effusions. Chest. 2004;126:2022–2024.
- Banka R, Terrington D, Mishra EK. Management of septated malignant pleural effusions. Curr Pulmonol Rep. 2018;7:1–5.
- Chen KY, Liaw YS, Wang HC, et al. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19:837–843.
- Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64:139–143.
- Shiroshita A, Nozaki S, Tanaka Y, et al. Thoracic ultrasound for malignant pleural effusion: a systematic review and meta-analysis. ERJ Open Res. 2020;6:00464–02020.
- Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. CHEST. 2015;147:1008–1012.
- Cassanelli N, Caroli G, Dolci G, et al. Accuracy of transthoracic ultrasound for the detection of pleural adhesions†. Eur J Cardiothorac Surg. 2012;42:813–818.
- Wei B, Wang T, Jiang F, et al. Use of Transthoracic Ultrasound to Predict Pleural Adhesions: a Prospective Blinded Study. Thorac Cardiovasc Surg. 2012;60:101–104.
- Bedawi EO, Talwar A, Hassan M, et al. Intercostal vessel screening prior to pleural interventions by the respiratory physician: a prospective study of real world practice. Eur Respir J. 2020;55:1902245.
- Salamonsen MR, Lo AKC, Ng ACT, et al. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146:1286–1293.
- Psallidas I, Hassan M, Yousuf A, et al. Role of thoracic ultrasonography in pleurodesis pathways for malignant pleural effusions (SIMPLE): an open-label, randomised controlled trial. Lancet Respir Med. 2022;10:139–148.
- Duerden L, Benamore R, Edey A. Radiology: what is the role oF chest radiographs, CT and PET in modern management?In Maskell NA, Laursen CB, Lee YCG, et al., editors Pleural Disease (ERS Monograph) [Internet]. Sheffield: European Respiratory Society; 2020: 48–72. doi: 10.1183/2312508X.10023819
- Søreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the First 24 hours. Scand J Trauma Resusc Emerg Med. 2011;19:66.
- Corcoran JP, Acton L, Ahmed A, et al. Diagnostic value oF radiological imaging pre- and post-drainage of pleural eFFusions. Respirology. 2016;21:392–395.
- Porcel JM, Pardina M, Alemán C, et al. Computed tomography scoring system For discriminating between parapneumonic eFFusions eventually drained and those cured only with antibiotics: CT For parapneumonic effusions. Respirology. 2017;22:1199–1204.
- Waite RJ, Carbonneau RJ, Balikian JP, et al. Parietal pleural changes in empyema: appearances at CT. Radiology. 1990;175:145–150.
- Franklin J, Talwar A, Addala D, et al. CT appearances of pleural inFection: analysis of the Second Multi-centre Intra-pleural Sepsis Trial (MIST 2) cohort. Clin Radiol. 2021;76:436–442.
- Henschke CI, Davis SD, Romano PM, et al. Pleural eFFusions: pathogenesis, radiologic evaluation, and therapy. J Thorac Imaging. 1989;4:49–60.
- Traill ZC, Davies RJ, Gleeson FV. Thoracic computed tomography in patients with suspected malignant pleural eFFusions. Clin Radiol. 2001;56:193–196.
- HalliFax RJ, Talwar A, Wrightson JM, et al. State-of-the-art: radiological investigation of pleural disease. Respir Med. 2017;124:88–99.
- HalliFax RJ, Haris M, Corcoran JP, et al. Role oF CT in assessing pleural malignancy prior to thoracoscopy. Thorax. 2015;70:192–193.
- Syer T, Arnold DT, Patole S, et al. Investigation oF a unilateral pleural effusion: what CT scan coverage is optimal? Thorax. 2020;75:503–505.
- Brun C, Gay P, Cottier M, et al. Comparison of cytology, chest computed and positron emission tomography Findings in malignant pleural effusion From lung cancer. J Thorac Dis. 2018;10:6903–6911.
- de Fonseka D, Underwood W, Stadon L, et al. Randomised controlled trial to compare the diagnostic yield of positron emission tomography CT (PET-CT) TARGETed pleural biopsy versus CT-guided pleural biopsy in suspected pleural malignancy (TARGET trial). BMJ Open Respir Res. 2018;5:e000270.
- Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic accuracy of 18F-FDG-PET and PET/CT in the differential diagnosis between malignant and benign pleural lesions: a systematic review and meta-analysis. Acad Radiol. 2014;21:11–20.
- Porcel JM, Hernández P, Martínez-Alonso M, et al. Accuracy oF Fluorodeoxyglucose-PET Imaging For Differentiating Benign From Malignant Pleural Effusions: a Meta-analysis. CHEST. 2015;147:502–512.
- Hierholzer J, Luo L, Bittner RC, et al. MRI and CT in the differential diagnosis of pleural disease. Chest. 2000;118:604–609.
- Naumann DN, Sellon E, Mitchinson S, et al. Occult tension pneumothorax discovered Following imaging For adult trauma patients in the modern major trauma system: a multicentre observational study. BMJ Mil Health. 2022;e002126. DOI:10.1136/bmjmilitary-2022-002126.
- Maskell N. British thoracic society pleural disease guidelines - 2010 update. Thorax. 2010;65:667–669.
- Light RW. Pleural eFFusions. Med Clin North Am. 2011;95:1055–1070.
- Morales-Rull JL, Bielsa S, Conde-Martel A, et al. Pleural effusions in acute decompensated heart Failure: prevalence and prognostic implications. Eur J Intern Med. 2018;52:49–53.
- BintcliFFe OJ, Hooper CE, Rider IJ, et al. Unilateral pleural effusions with more than one apparent etiology. A prospective observational study. Ann ATS. 2016;13:1050–1056.
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513.
- Hooper C, Lee GYC, Maskell NA. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii4–ii17.
- Janda S, Swiston J. Diagnostic accuracy of pleural Fluid NT-pro-BNP For pleural effusions of cardiac origin: a systematic review and meta-analysis. BMC Pulm Med. 2010;10:58.
- Porcel JM, Alvarez M, Salud A, et al. Should a cytologic study be ordered in transudative pleural effusions? Chest. 1999;(116):1836–1837.
- Addala D, Mercer RM, Lu Q, et al. P102 Discordant exudative pleural eFFusions: demographics and aetiology. Thorax. 2019;74:A146–A146.
- Maskell NA, Batt S, Hedley EL, et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med. 2006;174:817–823.
- Light RW, MacGregor MI, Ball WC, et al. Diagnostic SigniFicance of Pleural Fluid pH and PCO2. CHEST. 1973;64:591–596.
- Addala D, Mercer RM, Lu Q, et al. P101 InFlammatory pleural eFFusions: diFFerentiating the diagnosis. Thorax. 2019;74:A145–A146.
- Hassan M, Cargill T, Harriss E, et al. The microbiology of pleural infection in adults: a systematic review. Eur Respir J. 2019;54:1900542.
- Menzies SM, Rahman NM, Wrightson JM, et al. Blood culture bottle culture of pleural Fluid in pleural infection. Thorax. 2011;66:658–662.
- Wrightson JM, Wray JA, Street TL, et al. Absence of atypical pathogens in pleural infection. Chest. 2015;148:e102–e103.
- Lin Y-C, Chen H-J, Liu Y-H, et al. A 30-month experience oF thoracic empyema in a tertiary hospital: emphasis on differing bacteriology and outcome between the medical intensive care unit (MICU) and medical ward. South Med J. 2008;101:484–489.
- Dyrhovden R, Nygaard RM, Patel R, et al. The bacterial aetiology of pleural empyema. A descriptive and comparative metagenomic study. Clin Microbiol Infect. 2019;25:981–986.
- Kanellakis NI, Wrightson JM, Gerry S, et al. The bacteriology of pleural infection (TORPIDS): an exploratory metagenomics analysis through next generation sequencing. Lancet Microbe. 2022;3:e294–e302.
- Mercer RM, Corcoran JP, Porcel JM, et al. Interpreting pleural Fluid results. Clin Med. 2019;19:213–217.
- Oba Y, Abu-Salah T. The prevalence and diagnostic signiFicance of eosinophilic pleural effusions: a meta-analysis and systematic review. Respiration. 2012;83:198–208.
- Shrestha TM, Nepal G, Shing YK, et al. Idiopathic eosinophilic pleural effusion treated successfully with corticosteroid therapy: a clinical case report. Cureus. 2019;11:e3975.
- Arnold DT, De Fonseka D, Perry S, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J. 2018;52(5):1801254.
- Mercer R, Varatharajah R, Shepherd G, et al. Critical analysis of the utility of initial pleural aspiration in the diagnosis and management of suspected malignant pleural effusion. BMJ Open Respir Res. 2020;7:e000701.
- Ahmadzada T, Kao S, Reid G, et al. An update on predictive biomarkers for treatment selection in non-small cell lung cancer. J Clin Med. 2018;7:153.
- Sundaralingam A, Aujayeb A, Akca B, et al. Achieving molecular profiling in pleural biopsies: a multicenter, retrospective cohort study. Chest. 2022;S0012-3692(22):04169–1. DOI:10.1016/j.chest.2022.11.019.
- Tsim S, Stobo DB, Alexander L, et al. The diagnostic performance of routinely acquired and reported computed tomography imaging in patients presenting with suspected pleural malignancy. Lung Cancer. 2017;103:38–43.
- Tsim S, Paterson S, Cartwright D, et al. Baseline predictors of negative and incomplete pleural cytology in patients with suspected pleural malignancy – data supporting ‘Direct to LAT’ in selected groups. Lung Cancer. 2019;133:123–129.
- Maskell NA, Gleeson FV, Davies RJO. Standard pleural biopsy versus CT-guided cutting-needle biopsy For diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361:1326–1330.
- Zhang T, Wan B, Wang L, et al. The diagnostic yield of closed needle pleural biopsy in exudative pleural effusion: a retrospective 10-year study. Ann Transl Med. 2020;8:491.
- Koegelenberg CFN, Irusen EM, von Groote-Bidlingmaier F, et al. The utility oF ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax. 2015;70:995–997.
- Botana-Rial M, Leiro-Fernández V, Represas-Represas C, et al. Thoracic ultrasound-assisted selection For pleural biopsy with Abrams needle. Respir Care. 2013;58:1949–1954.
- HalliFax RJ, Corcoran JP, Ahmed A, et al. Physician-based ultrasound-guided biopsy For diagnosing pleural disease. Chest. 2014;146:1001–1006.
- Mei F, BoniFazi M, Rota M, et al. Diagnostic yield and safety of image-guided pleural biopsy: a systematic review and meta-analysis. Respiration. 2021;100:77–87.
- SconFienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology. 2013;266:930–935.
- Lin Z, Wu D, Wang J, et al. Diagnostic value of ultrasound-guided needle biopsy in undiagnosed pleural effusions: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e21076.
- Psallidas I, Kanellakis NI, Bhatnagar R, et al. A pilot feasibility study in establishing the role of ultrasound-guided pleural biopsies in pleural infection (The AUDIO Study). Chest. 2018;154:766–772.
- Grosu HB, Kern R, Maldonado F, et al. Predicting malignant pleural effusion during diagnostic pleuroscopy with biopsy: a prospective multicentre study. Respirology. 2022;27:350–356.
- Harris RJ, Kavuru MS, Mehta AC, et al. The impact of thoracoscopy on the management of pleural disease. Chest. 1995;107:845–852.
- Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy For diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest. 2010;137:1362–1368.
- Corcoran JP, Psallidas I, HalliFax RJ, et al. Ultrasound-guided pneumothorax induction prior to local anaesthetic thoracoscopy. Thorax. 2015;70:906–908.
- ARL M, Agrawal S, Bennett JA, et al. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts Fibrous septation. Respirology. 2010;15:804–808.
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: british thoracic society pleural disease guideline 2010. Thorax. 2010;65:ii54–ii60.
- ARL M, Awan YM, Marchbank A, et al. Diagnostic and therapeutic performance of video-assisted thoracoscopic surgery (VATS) in investigation and management of pleural exudates. Ann R Coll Surg Engl. 2008;90:597–600.
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52:1800349.
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v31–39.
- Bhatnagar R, Laskawiec-Szkonter M, Piotrowska HEG, et al. Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry (TAPPS trial): protocol of an open-label randomised controlled trial. BMJ Open. 2014;4:e007045.
- Kheir F, Thakore S, Mehta H, et al. Intrapleural fibrinolytic therapy versus early medical thoracoscopy for treatment of pleural infection. Randomized controlled clinical trial. Ann Am Thorac Soc. 2020;17:958–964.
- Dixon G, Lama-Lopez A, BintcliFFe OJ, et al. The role of serum procalcitonin in establishing the diagnosis and prognosis of pleural infection. Respir Res. 2017;18:30.
- Arnold DT, Hamilton FW, Elvers KT, et al. Pleural fluid supar levels predict the need for invasive management in parapneumonic effusions. Am J Respir Crit Care Med. 2020;201:1545–1553.
- Bedawi EO, Kanellakis NI, Corcoran JP, et al. The biological role of pleural fluid PAI-1 and sonographic septations in pleural infection: analysis of a prospectively collected clinical outcome study. Am J Respir Crit Care Med 2022. DOI:10.1164/rccm.202206-1084OC. https://www.atsjournals.org/doi/10.1164/rccm.202206-1084OC
- Sivakumar P, Marples L, Breen R, et al. The diagnostic utility of pleural Fluid adenosine deaminase For tuberculosis in a low prevalence area. Int J Tuberc Lung Dis. 2017;21:697–701.
- Sun W, Zhou Y, Yang C, et al. Contrast-enhanced ultrasound guided pleural biopsy improves diagnostic confidence For pleural based lesions: a 3-year prospective study. BMC Pulm Med. 2021;21:224.
- Dudau C, Hameed S, Gibson D, et al. Can contrast-enhanced ultrasound distinguish malignant from reactive lymph nodes in patients with head and neck cancers? Ultrasound Med Biol. 2014;40:747–754.
- Martin GA, Tsim S, Kidd AC, et al. Pre-EDIT: a randomized feasibility trial of elastance-directed intrapleural catheter or talc pleurodesis in malignant pleural effusion. Chest. 2019;156:1204–1213.
- Lentz RJ, Lerner AD, Pannu JK, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7:447–455.
