Abstract
Purpose
Neuromuscular disorders are characterised by muscle weakness that limits upper extremity mobility, but can be alleviated with dynamic arm support devices. Current research highlights the importance and difficulties of evidence-based recommendations for device development. We aim to provide research recommendations primarily concerning upper extremity body functions, and secondarily activity and participation, environmental and personal factors.
Methods
Evidence was synthesised from literature, ongoing studies, and expert opinions and tabulated within a framework based on a combination of the International Classification of Functioning, Disability and Health (ICF) model and contextual constructs.
Results
Current literature mostly investigated the motor capacity of muscle function, joint mobility, and upper body functionality, and a few studies also addressed the impact on activity and participation. In addition, experts considered knowledge on device utilisation in the daily environment and characterising the beneficiaries better as important. Knowledge gaps showed that ICF model components and contextual constructs should be better integrated and more actively included in future research.
Conclusions
It is recommended to, first, integrate multiple ICF model components and contextual constructs within one study design. Second, include the influence of environmental and personal factors when developing and deploying a device. Third, include short-term and long-term measurements to monitor adaptations over time. Finally, include user satisfaction as guidance to evaluate the device effectiveness.
Synthesized evidence will support future research and development of dynamic arm supports.
Tabulated evidence stresses the importance of integrating ICF model components and contextual constructs to fill the knowledge gaps.
Presented knowledge gaps and proposed steps guide the set up of future studies on dynamic arm supports
IMPLICATIONS ON REHABILITATION
Introduction
Neuromuscular disorders (NMD) are characterised by muscle weakness that limits upper extremity mobility and can affect people of all ages [Citation1]. The worldwide prevalence of NMD is 160 per 100,000, which is similar to that of Parkinson’s disease and double that of multiple sclerosis [Citation2]. People with NMD commonly experience difficulties to perform movements against gravity, such as lifting the arms or reaching for an object [Citation3]. These functional limitations translate into problems with activities of daily life (ADL) and participation in society. Various types of dynamic arm support devices (DASs) have been developed that improve engagement in ADL by providing gravity compensation [Citation4,Citation5]. Generally, such devices relieve upper extremity limitations that stem from muscular weakness [Citation6–8]. However, the impact might differ between devices intended for rehabilitation and research and wearable devices intended for daily life. As approximately 7–24% of the Dutch population with NMD, and 8.5% of people with Duchenne Muscular Dystrophy (DMD) worldwide [Citation9], use a DAS in daily life [Citation10], it is relevant to understand how and for what purposes the DAS are used on a daily basis.
The perceived benefits of a DAS vary from complete satisfaction to no perceived added value, where daily life usage has been reported to be discontinued over time [Citation11–13]. Current research highlights the importance of evidence-based recommendations for DAS development and prescription. However, determining recommendations has proven to be difficult [Citation4], due to the diversity in NMD, DAS, and study designs. This is further complicated by the lack of standardised and validated evaluation tools in current research [Citation4,Citation13,Citation14]. To optimise DAS development and its use in daily life, it is important to investigate the users’ characteristics, DAS function, and resulting user-device interactions in the short and long term. According to the Consortium for Assistive Technologies Outcome Research framework, used by Heide et al. the WHO’s International Classification of Functioning, Disability and Health (ICF) model could act as the primary guidelines [Citation4,Citation15,Citation16].
Current research indicates that DAS impact the users abilities across all ICF components [Citation4]. However, it is commonly assumed that a DAS primarily affects body functions through gravity compensation, which shapes the effects on activity and participation. Furthermore, Heide et al., indicated that the ability of a DAS to support ADLs does not guarantee higher performance or even utilisation in a home environment [Citation4]. Therefore, it is important to account for the contextual differences in which the activities are performed. For example, there is a difference in what people are able to do in a standardised environment by following instructions of an examiner compared to what they actually do in their daily life. Holsbeeke et al. described three constructs, or concepts, for these contextual differences: motor capacity (can do in a standardised environment), motor capability (can do in a daily environment), and motor performance (actually do in a daily environment) [Citation17]. We propose that a combination of the ICF model as primary guidelines and the three contextual constructs as secondary guidelines would provide a suitable framework to structure the evidence of DAS evaluation and recommendations for future development.
Technological advances in DAS, such as wearable robotics, are developing rapidly and it is expected that they will become more pervasive in daily life support systems [Citation9,Citation10]. Yet the research on DAS evaluation in patients is relatively new and under development [Citation4,Citation14]. Perspectives on the state of the art from third parties who are either engaged in development and prescription of these devices, or are end users, could provide important insights which are often lacking in the literature. The current study aims to synthesise the literature with expert opinions in order to provide an overview of current evidence and identify knowledge gaps that may limit the development of DAS. A secondary aim is to provide research recommendations to establish a standardised and validated approach for DAS evaluation in people with NMD.
Methods
Literature search
Inclusion criteria for the literature review focussed on studies that evaluated a DAS intended for daily life situations that supported the lower arm through gravity compensation. Studies with healthy participants only were included if the DAS was a finalised prototype designed for daily use by people with NMD. Furthermore, studies needed to report measures involving body functions described as neuromusculoskeletal and movement-related functions [Citation15]. Other inclusion and exclusion criteria can be found in the Appendix Table 4. Both scientific and non-peer reviewed literature were searched.
Table 4. Literature search terms, criteria, and strings.
Scientific literature was searched in PubMed and Web of Science in August 2018 and updated in July 2020. The search strategy for each database can be found in the Appendix Table 4. Titles and abstracts were independently screened by two researchers (JE, AP) where remaining articles were compared and finally in-/excluded. Included articles’ authors and reference lists were then searched for additional articles.
Non-peer reviewed literature published in the past 5 years (2015–2020) was searched from a goverenment clinical trial database (clinicaltrials.gov), DAS suppliers’ websites identified from previously included articles, and research mentioned by the experts. The search strategy for the goverenment clinical trial source consisted of the combination of “Neuromuscular Disorders” or “Neuromuscular Diseases” with either “Robot,” “Exoskeleton”, or “Arm support.” One researcher (JE) gathered the information and another researcher (AP) checked for consistency with the in-/exclusion criteria. Any inconsistency was discussed until agreement was reached.
Focus groups
Five focus groups were formed from a patient community, DAS developer, clinical, rehabilitational, and research setting. The groups consisted out of fifteen experts in total: two members of a patient community with a neuromuscular disorder, five DAS developers/suppliers, one physician, five therapists, and two researchers. At that time, one member with a NMD used a DAS in daily life and the other was orientating themself. The experts were most experienced with muscular weakness from atrophy/dystrophy or lesions to the central nervous system. Two developers/suppliers also had direct contact with some clients. Eight people were involved in research: two full-time researchers, one member with a NMD evaluated research proposals in a funding comity, and two developers, two therapists, and one physician were partially involved in projects involving DAS development, training, and improvement of DAS selection procedure.
The groups were interviewed by three researchers (JE, EP, ACW) in person on separate occasions. The interviews were in Dutch, semi-structured, and guided by the (originally Dutch) questions from the Appendix. The questions were formed based on preliminary review of the literature and discussion between the research team. Information was distilled of the focus groups’ views on (1) current impairments/limitations of people with NMD, (2) effectiveness and requirements of a DAS linked to the previous, (3) previous research projects and remaining questions, and (4) research priorities. The interviews were audiotaped in support of the keywords which were noted during the interview by at least two researchers and evaluated afterwards. Keywords were formulated based on the terminology used in previous literature and descriptions were added for clarification purposes.
Evidence synthesis
Fundamental topics from literature and interviews were tabulated and summarised to synthesise current evidence with expert opinions. The tabulation framework was formed with the ICF model components (body functions, activity and participation, and environmental and personal factors) as rows and contextual constructs (motor capacity, – capability, and – performance) as columns. Furthermore, ICF model components were further divided as categories and sub-categories to cover the different movement and impairment aspects. Each table cell represents a unique combination of one sub-category and one contextual construct. Current evidence matching expert opinions were indicated in each table cell by the reference number. Cells where current evidence was lacking, despite experts’ interests, were considered knowledge gaps and indicated by a dash symbol. We formulated our synthesised current evidence, knowledge gaps, and research recommendations according to the terminology used for conceptual modelling of assistive technology device outcomes [Citation16].
Results
Synthesised evidence
Current evidence and knowledge gaps were synthesised per category based on literature findings and expert opinions and presented in . There were five categories identified for body functions, three for activity and participation, and one for environmental and personal factors. The user-device interaction was considered handling an object and therefore categorised under activity and participation. Body functions was consequently linked to user-device interaction as a sub-category. Literature covered roughly nine out of 51 cells within the body functions component, which also covered eight out of 19 cells within the activity and participation component, and two out of four cells within the environmental and personal factors.
Table 3. Tabulation and summary of literature (L) and expert views (E) and synthesis of research recommendations.
Literature review
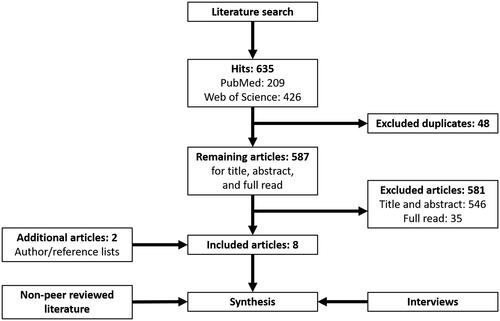
The literature search resulted in 635 hits (PubMed: 209, Web of Science: 426) of which 587 unique articles (). Then, 546 articles were excluded based on title and abstract, and another 35 after a subsequent full read of the article. Two articles were added after reference cross cheques. Finally, eight articles were included for reviewing after the inclusion process [Citation18–25] (). Two studies, ongoing until 31 December 2020 and 2019, respectively, were identified from the goverenment clinical trial source [Citation26,Citation27] ().
Table 1. Characteristics of included studies.
Table 2. Characteristics of ongoing studies.
Dynamic arm support evaluations
The evaluated DAS were a prototype A-gear [Citation19,Citation28], and commercially available devices Armon Ayura [Citation26,Citation27,Citation29], Armon Edero [Citation25,Citation29], Gowing [Citation24,Citation30], JAECO MultiLink Arm with Elevation Assist [Citation25,Citation31], JAECO WREX [Citation21,Citation23,Citation26,Citation27,Citation31], SLING [Citation20,Citation22,Citation30], and Top-Help [Citation22,Citation30], and a JAECO WREX modified with a trunk support prototype [Citation18] (). These DAS provide gravity compensation through adjustable counter-weights (SLING) or springs (A-gear, Armon Edero, JAECO MultiLink Arm with Elevation Assist, JAECO WREX, Top-Help) with an additional actuator that adjusts the springs’ tension (Armon Ayura, Gowing). All studies investigated the body functions, several also investigated activity and/or participation [Citation22,Citation26,Citation27], and one the satisfactory levels of using the DAS [Citation24]. All study designs were set up to cross-sectionally investigate motor capacity during functional and ADL tasks with and without (w/o) a DAS. Effects were mostly quantified for muscle function, joint mobility, and upper body functionality. Main effect identified were: DAS can lower up to 15% activity of the Trapezius muscle in healthy people; DAS can increase arm elevation from 25 up to 100° and elbow flexion from 10 up to 120° in people with NMD; Performance of the Upper Limb (PUL) scores were improved by 20 up to 30% with a device in people with NMD and showed a large effect (>1.15) in the modified Upper Extremity Performance Test for the Elderly (TEMPA); and the ability to perform ADL w/o a support device was participant specific where a device could have a limiting and beneficial effect. The ongoing studies perform longitudinal interventions of 8 and 5 weeks, respectively, to field-test two DAS [Citation26,Citation27] (). We considered these studies to investigate the motor capacity, – capability and – performance regarding upper body functionality and activity/participation levels.
Expert opinions
Experts expressed their opinions mainly in identifying DAS effects on muscle function, joint mobility, and upper body functionality ( and Appendix Table 5). They viewed people with NMD as impaired in these aspects of body functions and believed a DAS could compensate specific impairments. For example, a DAS improves joint mobility and lowers muscle efforts. This combination allows a user to perform multiple repetitions in a greater workspace needed in self-care activities such as eating and drinking. However, a DAS often does not fully support the complete hand to mouth motion and is then considered limited in their effectiveness. Therefore, experts believe further research is necessary to support DAS development not only on body functions but on all ICF components and contextual constructs. Furthermore, experts advocated to expand and improve the descriptions of the (active) population and disease progression. Specifically, device utilisation in the daily environment was of interest to bridge the knowledge between motor capacity, motor capability, and motor performance.
Table 5. Expert views as tabulated keywords from the collective focus group interviews. DAS: Dynamic Arm Support, w/o: with and without.
Discussion
Current evidence and knowledge gaps
The aim of this scoping review was to provide research recommendations for DAS evaluation based on a synthesis of literature and expert opinions. We primarily focussed on body functions and secondarily on other ICF model components: activity and participation, and environmental and personal factors. To structure the evidence and identify gaps we used a framework that combined the ICF model components with contextual constructs: motor capacity, capability, and performance. Most included studies focussed on the user-device interaction within the framework cells of body functions and motor capacity. Typically, they studied the introductory phase of using a DAS, with just a few studies addressing the long-term adaptations. The lack of standardised evaluation tools posed difficulties in creating comparable evidence [Citation24] and the synthesis of current evidence [Citation4]. The following knowledge gaps were identified: first, we poorly understand the adaptations that may ensue following skill acquisition, fatigue, or disease, which alter the support requirements over time. Second, it is yet unclear how abilities across ICF components and contextual constructs are related. For instance, it is unclear how changes in body functions influence the activity and participation and how these changes are, in turn, the result of environmental setting and task requirements. Finally, various aspects, such as comfort levels, were considered important by the experts that have not yet gained sufficient attention in the scientific literature.
Adaptations over time
From this review it is clear that the ability to adapt following skill acquisition, fatigue, or disease’s progression, have not yet been properly investigated over time. In order to benefit from a DAS, it is crucial that the user acquires the skills to operate the device and retains them over time. Previous studies have shown that training with a DAS is feasible in people with NMD [Citation32,Citation33]. However, it is currently unclear which skills are needed and which ones need to be learned to increase a device’s benefits. Moreover, due to the significant loss in upper extremity functionality and increasing fatigue due to the progressive nature of some NMDs, handling the device can become increasingly difficult over time [Citation11–13,Citation25]. The perceived benefits, which vary between users, can even decrease so much over time that the user decides to stop using the device completely. Similarly, a recent systematic review on the short-term benefits of wearable devices found that as the disability level changes the device benefits change as well [Citation34]. To prevent discontinuation, researchers and developers are promoting intuitive and adaptable DAS that counteract pathological changes due to disease progression [Citation5,Citation19]. However, such developments require extensive insights into a user’s ability to adapt motor capacity, capability, and performance over time. Activity monitoring through wearable sensors might provide a solution to acquire evidence over such long periods and some recent advances have been made in this field [Citation26,Citation27,Citation35].
Relations across framework cells
Evidence from experts suggests that the ICF model and contextual constructs are considered during the design process and the formulation of device requirements, however, this is not reflected in the literature findings. It is commonly assumed that a DAS primarily affects body functions, which influences performance in the activity and participation. However, most literature focussed mainly on the technical and design requirements necessary to overcome body function impairments, mostly neglecting the relationship between motor ability and ADL performance. Only one study directly investigated the relation between joint mobility and the ability to perform common ADLs, concluding that improvements in joint mobility alone does not directly translate to changes in ADL performance in a home environment [Citation22]. For instance, increased arm elevation with a device from 26.4° to 67.1° did not result in the ability to comb one’s hair, while peers could execute the same task at an elevation angle of 44.6° with and without a device. Furthermore, Heide et al. also indicated that environmental and personal factors, such as adjustments in the home setting and compensatory movements, have an important influence on ADL performance in multiple studies [Citation4,Citation11,Citation22]. These factors could affect the relationship between changes in joint mobility and the ability to complete tasks. In addition, Cruz et al. 2020, stated that lack of or delay in funding, lack of support from experts, and lack of proper device integration with the wheelchair resulted in discontinuation of the DAS. The authors therefore recommended to include multiple factors when evaluating the effectiveness of a DAS, especially in a home environment. As a result, we propose that future research considers the device requirements of multiple ICF model components and contextual constructs, i.e., motor ability, capability and performance, within the same study design.
Unaddressed framework cells
Our mixed-method approach revealed that literature focuses on selected framework cells which are often considered separately, thus limiting the evidence on the interaction between cells. For instance, current evidence shows that muscle activity and joint mobility are both influenced by load reduction [Citation18–23]. However, as also pointed out by experts, the interaction between (1) muscle activity and joint mobility and (2) how this is affected by disease and (3) how this could be restored by the device should be investigated. Stabilising and facilitating the shoulder girdle requires relatively complex muscle coordination, which is affected in people with NMD [Citation36–38]. Experts believe that insights into how muscle coordination is affected would benefit the development of a suitable DAS and to optimise a device to fit the individual requirements [Citation5,Citation39]. Other symptoms, such as stiffness, pain, and early fatigue, were also regarded as important factors by experts and were present in the literature findings, however these topics were not clearly addressed in the study designs. Three studies investigated comfort levels with limited evidence on stiffness, pain, or fatigue [Citation19,Citation24,Citation25] and were therefore not represented in the respective cells. In contrast, while pain and stiffness are highly prevalent and should be reduced to comfortable levels, they might not be the limiting factors for ADL performance in people with NMD [Citation10]. Bergsma et al. 2017, found that participants who had high pain and stiffness levels also reported relatively few activity limitations, which indicates an overuse in their body functions. However, it is unclear whether a DAS positively relieves these symptoms and how effects differ across motor capacity, capability, and performance. Therefore, future research should consider the importance of these unaddressed cells for device development as possible influencers or as main device requirements.
Research recommendations
From our analysis it is clear that integration and inclusion of ICF components and contextual constructs are needed to bridge the knowledge gaps in the development and evaluation of DAS. To realise these two tasks, we propose four steps with each a focus point, examples from our analysis, and suggestions for the design of future studies.
First, we propose that future research incorporates multiple ICF components and contextual constructs within one study design. It is commonly assumed that body functions are primarily affected by a DAS, which shapes the effects on activity and participation. Therefore, we suggest to focus on the relation between these two components before proceeding to examine the effect of environmental and personal factors. Our analysis shows that muscle activity and joint mobility are affected by load reduction, but their relation has not been investigated nor linked to ADL performance. Gandolla et al., deducted similar conclusions from their focus on activity and participation [Citation34]. Therefore, we recommend to investigate the relation between the two ICF components in a biomechanical framework under various levels of load reduction during functional tasks to optimise effort reduction and mobility improvements during common ADLs.
Secondly, the influence of environmental and personal factors should be investigated when deploying a device. Barriers within these factors have been ascribed to personal preferences, such as performing ADLs without support or conserving energy altogether, and home setting, such as limited space or a fixed location of the device, but also to a lack of funding or support from experts [Citation11,Citation25].
Thirdly, research should include short term, such as within- and between-day repeatability, and longitudinal measurements, such as yearly follow ups, to monitor adaptations over time. For example, limited evidence indicated that a DAS delays fatigue onset and reduces fatigue, but it is unclear if and how this affects ADL performance throughout the day. From two ongoing studies and previous literature we consider activity monitoring a method to quantify device utilisation and a proxy for motor performance [Citation26,Citation27,Citation35,Citation40]. In addition, characteristics of ADLs, such as a diminished frequency and variety, can be used to monitor adaptations in activity and participation [Citation35]. Furthermore, we propose to include muscle strength and joint mobility measures to monitor disease progression [Citation41,Citation42]. A longitudinal cohort study should investigate the relationship between disease progression and adaptations in daily activity of people with NMD over the course of a year. Disease progression and daily activity could be sampled every few months. Daily activity should then be averaged over the timespan of several days to include a range of ADL. The relationship could be expressed as a correlation between disease progression factors, muscle strength and joint mobility, and device utilisation, and motor performance.
Lastly, experts and literature agree that user satisfaction, such as perceived benefits and comfort, should be taken as guidance to evaluate the device effectiveness. Cruz et al., and Gandolla et al., also recently promoted the use of objective and subjective measures as both measures provide equally important evidence of the functional status of the user [Citation25,Citation34]. Therefore, device requirements should align with the needs and goals of the user and additionally aim to relieve symptoms of pain, stiffness, and fatigue. Furthermore, future research should validate and incorporate subjective measures related to the respective ICF model components and contextual constructs. A possible longitudinal study could include a questionnaire that links symptoms of pain, stiffness, and fatigue in people with NMD to the motor capability and capacity to perform ADL w/o a DAS and perceived benefits over the course of a year.
Recommendations for developing evaluation tools
When the above evidence is combined, it will provide the basis for understanding how standardised device benefits result in daily device utilisation accounting for changes in disease progression and users’ needs and goals over time. Evaluation tools developed along these insights should be standardised and validated with focus on international consensus, as indicated by recent research [Citation14]. We suggest several minimal requirements for the development of such tools. First, the tools require an integration of translatable cells that cover at least two of the contextual constructs. Second, the tools should be applicable alongside the development and after deployment of DAS. Third, subjective measures, such as perceived benefits and comfort, should be included in a device’s evaluation of effectiveness near the product final development stages.
Conclusion
Three knowledge gaps were identified and given synthesised research recommendations based on the integration and inclusion of ICF model components and contextual constructs. First, adaptations due to altered support requirements over time are poorly understood. Second, relations between ICF model components and contextual differences are limited. Finally, several framework cells, such as comfort levels, were brought to our attention by experts that were not covered sufficiently in scientific literature. We promote the use of multiple ICF model components and contextual constructs within research to benefit the development of DAS. Research should quantify device benefits and daily device utilisation with respect to disease progression and users’ needs and goals over time. Furthermore, we suggest several minimal requirements for the development of evaluation tools of DAS. The tools are required to cover multiple framework cells and to be applicable in various environments, for various users, and on multiple time points. Moreover, the tools should integrate objective and subjective measures to evaluate device effectiveness.
Acknowledgements
The authors would like to thank the focus groups for their contributed expertise, and Evy Paulussen and Anna-Carolin Wijnands for their contributions in data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Deenen JC, van Doorn PA, Faber CG, et al. The epidemiology of neuromuscular disorders: age at onset and gender in the Netherlands. Neuromuscul Disord. 2016;26(7):447–452.
- Deenen JC, Horlings CG, Verschuuren JJ, et al. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis. 2015;2(1):73–85.
- Bergsma A, Cup EH, Janssen MMHP, et al. Upper limb function and activity in people with facioscapulohumeral muscular dystrophy: a web-based survey. Disabil Rehabil. 2017;39(3):236–243.
- van der Heide L, Gelderblom G, de Witte L. Effects and effectiveness of dynamic arm supports. Am J Phys Med Rehabil. 2015;3:256.
- van der Heide L, van Ninhuijs B, Bergsma A, et al. An overview and categorization of dynamic arm supports for people with decreased arm function. Prosthet Orthot Int. 2014;38(4):287–302.
- Coscia M, Cheung VCK, Tropea P, et al. The effect of arm weight support on upper limb muscle synergies during reaching movements. J NeuroEngineering Rehabil. 2014;11(1):22.
- Ellis MD, Sukal T, DeMott T, et al. Augmenting clinical evaluation of hemiparetic arm movement with a laboratory-based quantitative measurement of kinematics as a function of limb loading. Neurorehabil Neural Repair. 2008;22(4):321–329.
- Prange GB, Jannink MJA, Stienen AHA, et al. Influence of gravity compensation on muscle activation patterns during different temporal phases of arm movements of stroke patients. Neurorehabil Neural Repair. 2009;23(5):478–485.
- Janssen MMHP, Bergsma A, Geurts ACH, et al. Patterns of decline in upper limb function of boys and men with DMD: an international survey. J Neurol. 2014;261(7):1289–1290.
- Bergsma A, Janssen MMHP, Geurts ACH, et al. Different profiles of upper limb function in four types of neuromuscular disorders. Neuromuscul Disord. 2017;27(12):1115–1122.
- van der Heide L, de Witte L. The perceived functional benefit of dynamic arm supports in daily life. J Rehabil Res Dev. 2016;53(6):1139–1150.
- Rahman T, Sample W, Seliktar R, et al. Design and testing of a functional arm orthosis in patients with neuromuscular diseases. IEEE Trans Neural Syst Rehabil Eng. 2007;15:244–251.
- Shank TM, Wee J, Ty J, et al. Quantitative measures with WREX usage. IEEE Int Conf Rehabil Robot. 2017;2017:1375–1380.
- Janssen MMHP, Lobo-Prat J, Bergsma A, et al. 2nd Workshop on upper-extremity assistive technology for people with duchenne: effectiveness and usability of arm supports. Neuromuscular Disorders: NMD. 2019;29(8):651–656.
- World Health Organization. 2001. International classification of functioning, disability and health: ICF. Geneva: World Health Organization. Available from: https://apps.who.int/iris/handle/10665/42407
- Jutai JW, Fuhrer MJ, Demers L, et al. Toward a taxonomy of assistive technology device outcomes. Am J Phys Med Rehabil. 2005;84:294–302.
- Holsbeeke L, Ketelaar M, Schoemaker MM, et al. Capacity, capability, and performance: different constructs or three of a kind? Arch Phys Med Rehabil. 2009;90(5):849–855.
- Dunning AG, Janssen MMHP, Kooren PN, et al. Evaluation of an arm support with trunk motion capability. J Med Device. 2016;10:1–4.
- Kooren PN, Dunning AG, Janssen MMHP, et al. Design and pilot validation of A-gear: a novel wearable dynamic arm support. J NeuroEngineering Rehabil. 2015;12(1).DOI:https://doi.org/10.1186/s12984-015-0072-y
- Essers J, Meijer K, Murgia A, et al. An inverse dynamic analysis on the influence of upper limb gravity compensation during reaching. Int Conf Rehabil Robot. 2013;2013:6650368.
- Estilow T, Glanzman AM, Powers K, et al. Use of the Wilmington robotic exoskeleton to improve upper extremity function in patients with Duchenne muscular dystrophy. Am J Occup Ther. 2018;72(2):7202345010p1.
- van der Heide L, Ramakers I, Essers JMN, et al. Is it possible to assess the effects of dynamic arm supports on upper extremity range of motion during activities of daily living in the domestic setting using a portable motion capturing device? – A pilot study. TAD. 2017;29(1–2):91–99.
- Haumont T, Rahman T, Sample W, et al. Wilmington robotic exoskeleton: a novel device to maintain arm improvement in muscular disease. J Pediatr Orthop. 2011;31:1–5.
- Lebrasseur A, Lettre J, Routhier F, et al. Evaluation of the usability of an actively actuated arm support. Assist Technol. 2019;1–7.DOI:https://doi.org/10.1080/10400435.2019.1629124
- Cruz A, Callaway L, Randall M, et al. Mobile arm supports in Duchenne muscular dystrophy: a pilot study of user experience and outcomes. Disabil Rehabil-Assist Technol. 2020.DOI:https://doi.org/10.1080/17483107.2020.1749892
- Bendixen RM. Use of dynamic arm support devices for upper limb function in non-ambulatory men with Duchenne Muscular Dystrophy (DMD) ClinicalTrials.gov2019. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03531788
- Pedrocchi A. USEFUL: User-centred assistive SystEm for arm Functions in neUromuscuLar Subjects ClinicalTrials.gov2019. Available from: https://clinicaltrials.gov/ct2/show/NCT03127241.
- Flextension [July 2020]. Available from: https://flextension.nl.
- Armon Products [July 2020]. Available from: https://armonproducts.com.
- Focal Meditech B.V. [July 2020]. Available from: https://focalmeditech.nl.
- JAECO Orthopedic [July 2020]. Available from: https://jaecoorthopedic.com.
- Heutinck L, Jansen M, van den Elzen Y, et al. Virtual reality computer gaming with dynamic arm support in boys with Duchenne muscular dystrophy. J Neuromuscul Dis. 2018;5(3):359–372.
- Jansen M, Burgers J, Jannink M, et al. Upper limb training with dynamic arm support in boys with Duchenne muscular dystrophy: a feasibility study. Int J Phys Med Rehabil. 2015;3:256–264.
- Gandolla M, Antonietti A, Longatelli V, et al. The effectiveness of wearable upper limb assistive devices in degenerative neuromuscular diseases: a systematic review and meta-analysis. Front Bioeng Biotechnol. 2020;7:1–16.DOI:https://doi.org/10.3389/fbioe.2019.00450
- van der Geest A, Essers JMN, Bergsma A, et al. Monitoring daily physical activity of upper extremity in young and adolescent boys with Duchenne muscular dystrophy: a pilot study. Muscle and Nerve. 2019;61(3):293–300.
- Essers JMN, Peters AA, Meijer K, et al. Superficial shoulder muscle synergy analysis in facioscapulohumeral Dystrophy during humeral elevation tasks. IEEE Trans Neural Syst Rehabil Eng. 2019;27:1556–1565.
- Bergsma A, Murgia A, Cup EH, et al. Upper extremity kinematics and muscle activation patterns in subjects with facioscapulohumeral dystrophy. Arch Phys Med Rehabil. 2014;95(9):1731–1741.
- de Baets L, Jaspers E, Janssens L, et al. Characteristics of neuromuscular control of the scapula after stroke a first exploration. Front Hum Neurosci. 2014;8:933.
- Trigili E, Grazi L, Crea S, et al. Detection of movement onset using EMG signals for upper-limb exoskeletons in reaching tasks. J NeuroEngineering Rehabil. 2019;16(1):1–16.DOI:https://doi.org/10.1186/s12984-019-0512-1
- Koene S, Dirks I, van Mierlo E, et al. Domains of daily physical activity in children with mitochondrial disease: a 3D accelerometry approach. JIMD Rep. 2016;6:7–17.
- Janssen MMHP, Harlaar J, Koopman B, et al. Unraveling upper extremity performance in Duchenne muscular dystrophy: a biophysical model. Neuromuscul Disord. 2019;29(5):368–375.
- Han JJ, De Bie E, Nicorici A, et al. Reachable workspace reflects dynamometer-measured upper extremity strength in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2015;52(6):948–955.
Appendix 1
Appendix
Literature search terms, criteria, and strings
Interview questions
Describe your relation to the population?
What are the most common problems within the population?
How are these problems solved by the currently available dynamic arm supports?
Which problems are not solved by the currently available dynamic arm supports?
Describe your relation to the respective research field?
Give a short description of the previous/current research projects?
What are the current unanswered research questions that are not being investigated?
What are your interests regarding using dynamic arm supports?
How would you regard (in)effective device usage? Please describe a respective measurement unit?
What would a user’s interests of use be? Considered over which time period: hour, (partial) day, week, month, year?
What are the minimum requirements of dynamic arm supports? What are currently available dynamic arm supports capable of and what are opportunities for improvement?
What are the current gaps in knowledge for further dynamic arm support development? How would you approach these gaps to generate knowledge? Combine this question with #13.
How familiar are you with the following examples of evaluation techniques?
Questionnaires and scoring tests:
MAS: Modified Ashworth scale
FMA: Fugl-Meyer Assessment
ARAT: Action Research Arm Test
Jebsen test of hand function
WMFT: Wolf Motor Function Test
MAL: Motor Activity Log
SIS: Stroke Impact Scale
ABILHAND
ULFI: Upper-Limb Functional Index
PUL: Performance of the upper limb scale
RMA: Rivermead arm score
Barthel index
Biomechanical tests:
Muscle activation (electromyography); amplitude, timing, synergy, fatigue.
Kinematics; workspace, Range of Motion, tracking error, movement accuracy, joint angles.
Kinetics; torque, force-interaction.
Activity monitoring:
Activity sensors; compliance, (in)active, area under curve, peak, bouts.
Diaries; usage duration, type of activities.
Other remarks?
Expert views