ABSTRACT
Introduction
In the past decades, the opioid crisis has heavily impacted parts of the US society and has been followed by an increase in the use of opioids worldwide. It is of paramount importance that we explore the origins of the US opioid epidemic to develop best practices to tackle the rising tide of opioid overdoses.
Areas covered
In this expert review, we discuss opioid (over)prescription, change in perception of pain, and false advertisement of opioid safety as the leading causes of the US opioid epidemic. Then, we review the evidence about opioid dependence and addiction potential and provide current knowledge about predictors of aberrant opioid-related behavior. Lastly, we discuss different approaches that were considered or undertaken to combat the rising tide of opioid-related deaths by regulatory bodies, pharmaceutical companies, and health-care professionals. For this expert review, we considered published articles relevant to the topic under investigation that we retrieved from Medline or Google scholar electronic database.
Expert opinion
The opioid epidemic is a dynamic process with many underlying mechanisms. Therefore, no single approach may be best suited to combat it. In our opinion, the best way forward is to employ multiple strategies to tackle different underlying mechanisms.
1. Background on the opioid crisis
In the United States (US) and other countries worldwide, the use of opioids has risen substantially in the past couple of decades [Citation1]. According to a survey conducted between 1998 and 2016 in Boston, Massachusetts, US, approximately 5% of their inhabitants, representative of the US general population, used an opioid in the 7 days preceding the interview [Citation2]. In addition, opioids were prescribed to about 6% of the Dutch population at some time during a one-year period (2017) [Citation3], to about 8% of the population in any Scandinavian country per year (period from 2006 to 2017) [Citation4], and to about 9% of the general population (of age between 16 and 59 years) in a year’s time in England and Wales (between 2006 and 2019) [Citation5]. Although the use of (prescribed and illicit) opioids in Europe (in absolute numbers) is not as widespread as in the US yet [Citation6], it affects more people each year. According to the European Pain Federation (EFIC), there is no evidence of an opioid crisis across countries in Europe at the present time [Citation6]. However, a clear association between the use of opioids and opioid-involved overdose deaths has been established [Citation7], so the upward trend in prescribing rates warrants prudent opioid prescribing and close monitoring of opioid overdose deaths in Europe and elsewhere. Here, health-care professionals play a key role as they alone can guarantee appropriate, safe opioid therapy when necessary, educate patients about harms, and prevent opioid use when the risks outweigh the benefits and there is no clear indication for prescribing opioids.
In this expert review, we will first discuss the historic events leading to the opioid crisis in the US and its changing characteristics since 1999. The intention here is to understand and reflect upon the events that jointly brought about the health-care crisis in the US (as a case study). We will also discuss addictive properties of opioid medications and factors that are associated with opioid use disorder, although the evidence is not always unambiguous. Lastly, we will discuss the measures that were undertaken to combat the rising tide of opioid overdose deaths in the US, from which we can learn to best prevent the next health-care crisis elsewhere.
1.1. The (three) waves of the US opioid epidemic
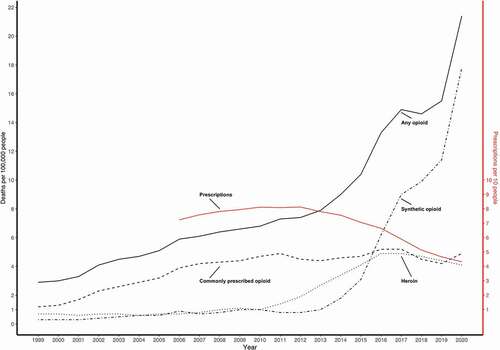
The opioid crisis in the US has been closely monitored since 1999. It is generally accepted that it consists of three distinct waves: a first wave since 1999, a second wave since 2010, and a third wave from 2013 onwards (the three waves are depicted in ) [Citation8–15]. The first wave of the crisis was characterized by an increase in death rates by commonly prescribed opioids (prescription opioid line in ) [Citation8,Citation13]. The next wave of the crisis was triggered by an increase in heroin use [Citation9], and the last wave was initiated by an increase in the use of synthetic opioids (fentanyl and congeners), obtained either by prescription or illicitly [Citation10–12].
In more recent years, the US opioid epidemic seems to have transformed once again. In 2018, a brief decrease in overdose deaths was followed by an increase () that persisted until and including 2020 (the last reported rate of opioid overdose deaths) when over 20 deaths per 100,000 individuals were reported [Citation13,Citation14]. Based on this finding, it has been proposed that another wave, the fourth wave, has commenced in the US [Citation15]. The recent rise in overdose deaths has been characterized by the use of stimulants, methamphetamine and cocaine, and by concomitant use of stimulants and opioids, still mostly synthetic (e.g. benzo dope, a combination of fentanyl and etizolam; tranq dope, a combination of fentanyl and xylazine) [Citation16].
1.2. The US opioid crisis – the perfect storm
Available evidence suggests that the US opioid epidemic was initiated by (over)prescribing of opioids in the 1990s and 2000s [Citation8,Citation17]. Any increase in use of a substance is either stimulated by an increase in demand, e.g. people are in more pain and therefore require more analgesics, or supply has suddenly increased. In the first wave of the US opioid crisis, both demand and supply were altered in a way that has resulted in widespread opioid use.
1.2.1. Changed perception of pain and false reassurance of opioid effectiveness and safety
Since the 1960s, many efforts have been made to prioritize pain management in patient care [Citation18]. The World Health Organization (WHO) added opioids to the Model list of essential medicines in 1977, which further cemented the unique position opioids hold in modern medicine [Citation19]. Later, in 1986, the Expert Committee on Cancer Pain Relief and Active Supportive Care introduced the WHO ‘pain ladder’ for the treatment of malignant pain [Citation20]. The novelty of the WHO Pain ladder was in the stepwise approach to pain management – starting with a non-opioid analgesic, continuing with weak opioids for mild-to-moderate pain, and as a last resort, strong opioids for moderate-to-severe pain. The end goal of the proposed approach was a pain-free patient [Citation20]. Unfortunately, being completely pain-free is unattainable for many chronic pain-inducing conditions. Here, reduction of pain and thus quality of life improvement may be of greater importance to the patients [Citation21–24].
A discussion about the efficacy and particularly the safety of opioids in the treatment of chronic non-malignant pain started with a rather short letter published in the New England Journal of Medicine in 1980, reporting that just 4 out of about 12,000 hospitalized patients (less than 0.1%), who received at least one opioid during their hospitalization and had no prior history of addiction, developed addiction [Citation25]. Unfortunately, the message of this letter was misinterpreted by many, including pharmaceutical companies, and it was falsely assumed that addiction is rare in patients receiving opioids in all settings [Citation26]. Thereafter, at a meeting of the American Pain Society (APS) in 1995, James Campbell gave a talk about the benefits and safety of opioid analgesics in the treatment of chronic non-malignant pain [Citation27]. Later that year, the APS published the ‘Quality improvement guidelines for the treatment of acute and cancer pain,’ further cementing the ‘safe and effective’ policy of opioids in the treatment of chronic non-malignant pain [Citation28]. Furthermore, the APS proclaimed pain as a ‘fifth vital sign’ in 1996, joining body temperature, pulse rate, respiration rate, and blood pressure in the assessment of one’s wellbeing, while other countries followed suit [Citation29]. In 2001, the Joint Commission for Accreditation of Healthcare Organizations (JCAHO; from now on mentioned as the Joint Commission) published a new pain management standard that changed the standard of care by making adequate pain relief a patient’s right, by improving education and training of health-care professionals about pain relief, and by emphasizing the importance of qualitative pain assessment and safe pain management [Citation30]. Although the intention of the JCAHO standard was not to overtreat pain, it did probably have such an effect [Citation31]. A close reader may have noticed that the strategy to combat pain (including educational material) proposed by the WHO never concerned non-malignant pain, but it is still widely used as the goal for the treatment of any type of pain (including non-malignant pain) in medical schools worldwide. Only recently, new guidelines concerning just chronic non-malignant pain are being developed [Citation32,Citation33].
The APS had a key role in the US opioid epidemic – by advising ‘safe and effective’ opioid pain treatment they drove sales of opioid analgesics, manufactured by different pharmaceutical companies, including Purdue Pharma [Citation34,Citation35]. The APS was dissolved in 2019 after facing several lawsuits due to their financial ties with the pharmaceutical industry [Citation36]. Furthermore, the pharmaceutical industry, particularly Purdue Pharma, employed an aggressive marketing strategy to promote oxycodone (OxyContin®) prescription for the treatment of chronic non-malignant pain, while the addictive properties of the medication were downplayed. Addiction to OxyContin® was considered highly unlikely, a claim that was mostly based on the letter by Porter and Jick [Citation25], as well as assumed because of the controlled-release formulation of OxyContin® [Citation37]. However, it has been shown that the controlled-release formulations do not have favorable safety profiles over other formulations [Citation38]. When controlled-release oxycodone was introduced in clinical practice in Ontario, Canada, the associated overdose mortality increased about five-fold between 2000 and 2003 [Citation39]. Physicians were led to believe (by the pharmaceutical industry and the medical and scientific community) that opioids have low addictive potential that provided false reassurance of opioid safety profile in the treatment of chronic non-malignant pain. This is considered to be one of the reasons behind the US opioid epidemic [Citation26].
1.2.2. Further deterioration of opioid use
Given the above, it is evident that the stage was set for the supply of opioids to follow the increasing demand, creating the perfect storm. In addition to over-prescription of opioids, drug diversion, i.e. use for other purposes than intended by the prescribing physician, contributed to uncontrolled opioid use in the US [Citation40,Citation41]. Diversion happened both at the level of a patient and of a prescriber. First, the patients were able to acquire a prescription from a second physician when the initial opioid treatment was stopped by their personal physician (‘doctor shopping’) [Citation42], and second, some medical professionals (physicians and pharmacists) identified the increased demand for opioids as an ideal business opportunity. They began selling opioid prescriptions and opioids themselves (‘pill mills’) [Citation43]. However, the transition toward problematic opioid use did not stop there; patients to whom opioids were prescribed began distributing their analgesic medication to family and friends with an intention to help them ease their pain or for financial gains. Kennedy-Hendricks et al. [Citation44] reported that about 20% of all participants in their study shared their prescribed opioids with others, mostly with the intention to help alleviate their pain. Abusers of prescription opioids considered their behavior to be safer compared with the use of illicit opioids, e.g. heroin, because their opioids were licensed by the medication authority and are therefore ‘legal.’ Furthermore, they contained predictable doses (unlike illicit drugs) so their overdose potential was considered lower [Citation45].
Subsequently, probably due to prescription monitoring programs and efforts to close ‘pill mills’ [Citation45], the use of heroin and synthetic opioids increased, and the number of opioid overdose deaths associated with them quadrupled () [Citation13]. Initially, heroin and illicit synthetic opioids were used by those initially misusing prescription opioids [Citation46]. However, the increase in demand did not go unnoticed by manufacturers of illicit drugs, and they increased the supply of illicit opioids. In 2015, first-time opioid users were 4-times as likely to initiate opioid use with heroin than there were in 2005 [Citation47]. Since the first wave of the opioid epidemic in 1999, it has been estimated that collectively more than 800,000 people died from a drug overdose in the US [Citation48]. Currently, opioids are the main cause of drug overdose deaths, with opioid overdoses accounting for about 70% of all drug overdose deaths in 2019 [Citation49]. In 2016 alone, more than 60,000 lives were lost due to an opioid overdose, after which the US opioid epidemic was declared a national emergency by President Donald Trump [Citation50,Citation51].
However, opioid use is associated not only with fatal opioid overdoses but also with non-fatal opioid overdose [Citation52], increased risk of motor vehicle accidents [Citation53], falling from standing height [Citation54], addiction [Citation55], tolerance [Citation56], and many more. Besides that, opioid use disorder impairs the physical and mental components of the quality of life [Citation57] and causes members of the active population to miss on average 29 workdays per year (work absenteeism) compared with those without an opioid use disorder [Citation58]. Finally, the cost of opioid epidemic in the US was estimated to be about one trillion US dollars in 2017 alone [Citation59].
Although the decision to include opioids within the armamentarium of pain management for chronic non-malignant pain was not based on sound scientific evidence [Citation32], opioids are often prescribed to treat pain not related to cancer for longer periods of time despite the clear and well-known association between prolonged opioid use (more than 3 months) and opioid dependence and abuse [Citation60].
2. What makes opioids prone to abuse?
Modern medicine relies heavily on opioids; without opioids, anesthesia and management of postoperative pain would be more difficult and perhaps even impossible. The chemical structure of opioids shares many similarities with endogenous opioid receptor ligands. These ligands specifically bind to opioid receptors that are ubiquitously present throughout the central nervous system [Citation61–63]. The biological effects of opioids are considerable, and the individual biologic response to them varies considerably [Citation64–66]. The complexity and the role of the pharmacokinetics and pharmacodynamics of opioids in the development of analgesic and adverse effects have been given much attention in the literature and is discussed in detail elsewhere [Citation67–70]. Here, we provide an overview of the mechanisms that are involved in short- and long-term adaptations to repeated activation of opioid receptors and other targets, to guide the discussion about the potential of opioids to produce tolerance and addiction.
2.1. Short- and long-term adaptations to opioid use
2.1.1. Tolerance
Cellular changes in response to opioid use begin immediately after the initial exposure. Opioids bind to opioid receptors, which are G-protein-coupled receptors, that upon activation regulate many downstream biochemical pathways [Citation71]. Both cytoplasmic G-protein subunits of the receptor interact with several cellular-effector mechanisms, inhibiting adenylyl cyclase and voltage-gated calcium channels, and stimulating inwardly rectifying potassium channels (GIRKs) and phospholipase C beta (PLCB) [Citation72,Citation73]. Ultimately, these biochemical changes are inhibitory on a cellular level, but can produce diverse effects based on receptor location (i.e. at pre- or post-synaptic neurons) [Citation72,Citation74]. Although four different opioid receptor subtypes have been identified, the analgesic and adverse actions of morphine (and morphine-like agonists) require predominantly activation of the mu-opioid receptor (MOR) subtype, as demonstrated in knockout mice models [Citation75].
Various receptor and cellular, short- and long-term, adaptations during (repeat) opioid exposure are associated with the development of tolerance. One such adaptation is receptor desensitization that can occur within seconds to minutes after the initial opioid exposure. This particular mechanism includes the cytoplasmic decoupling of the effector (G-protein) from the opioid receptor by phosphorylation (by different kinases) and recruitment of beta-arrestin (and other proteins) and is followed either by receptor endocytosis, degradation, or recovery [Citation73,Citation74,Citation76]. Initially, the receptors are able to quickly recover from acute desensitization, but upon repeat activation (by prolonged opioid use), the recovery potential is attenuated, and desensitization is accelerated, probably by up-regulation of intracellular kinases and beta-arrestin [Citation76]. This ultimately shifts the equilibrium between active and desensitized MORs and eventually leads to acute and long-term tolerance [Citation74,Citation77]. Other mechanisms involved in the development of opioid tolerance are increased adenylate cyclase activity, activation of N-methyl-D-aspartate (NMDA) receptors, and glia cell activation, which all strive to restore the signaling process despite continued opioid exposure [Citation78,Citation79].
2.1.2. Reward
The addictive potential of opioids most probably originates from long-term adaptations in neuronal circuits that receive input from dopaminergic midbrain neurons [Citation80,Citation81]. Natural rewards and addictive substances (including opioids) are able to influence behavior by increasing extracellular dopamine levels within the mesocorticolimbic system [Citation72,Citation82,Citation83] that is involved in reward and establishment of behavioral changes necessary to experience reward [Citation81]. After an initial surge in dopamine levels, the concentration of dopamine returns to baseline levels. However, it has been proposed that chronic exposure to addictive substances changes the homeostatic dopamine set point outside of its normal range [Citation84,Citation85]. This hypothesis has been further supported by results from imaging studies [Citation85,Citation86]. In a positron emission tomography (PET) imaging study by Volkow et al. [Citation86], it was observed that 2 weeks after discontinuation of substance use, dopamine levels in the basal ganglia were depleted in individuals with an opioid (heroin) use disorder.
2.2. Opioid use disorder: clinical considerations
Tolerance, defined as the need to increase drug dose over time to produce the same biological effect, and physical dependence can develop within days of opioid treatment (short-term effects) [Citation87]. Dependence is characterized by withdrawal symptoms that can present as irritability, dysphoria, insomnia, diarrhea, runny nose, shivering, loss of weight, tremor, writhing, agitation, and aggression [Citation88,Citation89] and may last for several days, even weeks [Citation90]. Although the withdrawal symptoms upon discontinuation of opioids may be perceived as severe, they are not life-threatening and can be reduced by opioid tapering [Citation32]. Furthermore, tolerance can not only affect opioid analgesia but can also influence the adverse effect potential [Citation91].
According to the most recent edition of the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association [Citation92], the clinical manifestation of opioid tolerance, dependence, and addiction is summarized in ‘opioid use disorder’ and defined as a disorder that ‘includes signs and symptoms that reflect compulsive, prolonged self-administration of opioid substances that are used for no legitimate medical purpose or, if another medical condition is present that requires opioid treatment, that are used in doses greatly in excess of the amount needed for that medical condition.’ [Citation92]. The clinical picture will differ between patients depending on personal characteristics and the duration of opioid treatment, which is reflected in a wide range of symptoms included in the diagnostic criteria. Furthermore, when opioids are used under appropriate medical supervision, symptoms of tolerance and withdrawal (dependence) are not considered in the evaluation of the disorder [Citation92].
Until recently, it seemed improbable that an opioid use disorder (formerly named ‘addiction’) could be present in a clinical setting because the compulsive need for opioids, with disregard of any negative consequences, was rarely observed in patients [Citation93]. However, dependence and complementary withdrawal symptoms are neither necessary nor sufficient for the manifestation of opioid use disorder in a clinical setting [Citation94,Citation95]. For example, it is common for dependence to occur without a concomitant opioid use disorder, in the treatment of malignant pain [Citation96]. Still, recent evidence suggests that opioid use disorder may be common among cancer survivors and patients in remission [Citation97,Citation98]. The presence of substance use disorder in any clinical setting is not improbable and may very well be more prevalent than originally considered. In a 2015 review study [Citation55], 38 different studies on opioid misuse and ‘addiction’ from diverse clinical settings were included. The authors concluded that the rates of ‘addiction’ varied between 8% and 12% and appeared to be highest in pain clinics.
The probability of substance use disorder increases with the increase in their availability [Citation99]. Above, we described that the availability and ease with which the substance can be procured, especially opioids, has increased considerably in the US since the 1990s [Citation100]. Exposure is, in itself, the single most important risk factor for any substance use disorder, including obviously opioid use disorder. For example, it has been demonstrated by a large US population-based study that the respondents of the survey (n = 9,279) who use prescribed opioids had an increased risk (odds ratio of 3.1 after correcting for confounding variables) of any opioid misuse compared with nonusers [Citation101]. Moreover, the daily dose of prescribed opioids, the number of filled opioid prescriptions, and prolonged opioid use are all positively associated with the risk of opioid misuse [Citation102–104], although the benefit of prolonged exposure to opioids for the treatment of chronic non-malignant pain (in comparison to nonsteroidal anti-inflammatory agents, NSAIDs) has not been supported in a well-conducted randomized clinical trial [Citation105].
For advances in safe opioid treatment, it is of paramount importance to assess the individual patient’s predisposition for opioid use disorder before an opioid is prescribed [Citation106]. Furthermore, when prolonging opioid treatment is deemed necessary, the risk of aberrant opioid-related behavior needs to be continuously evaluated, and the opioid treatment properly tailored to ensure safe and effective treatment [Citation107].
3. Who is at risk for opioid misuse, abuse, and addiction?
Although not to prescribe opioids may protect from an opioid use disorder, in many clinical scenarios this option is simply not feasible and the uncontrolled pain itself may further exacerbate the potential for aberrant opioid-related behavior [Citation108]. We must therefore prescribe opioids with careful consideration of the individual patient’s characteristics [Citation107,Citation109].
3.1. Predictors of opioid use disorder
Several risk factors of aberrant opioid-related behavior have been identified. They may be grouped by demographic differences, psychiatric comorbidities (presence versus absence), substance misuse factors, and other factors [Citation107,Citation108].
Evidence on demographic factors of aberrant opioid-related behavior is particularly highly heterogeneous, and population, setting, and outcome definition dependent [Citation110]. Although more women are being prescribed opioids than men [Citation111], it appears that illicit opioid misuse is more prevalent in the younger age groups and is associated with male sex [Citation112,Citation113]. For example, when population-based data on opioid-related hospital admissions and deaths in the Netherlands were examined, it was found that patients with opioid prescriptions were, on average, ten-year older and more often women (54.4%) than in those without an opioid prescription (male sex in 66.3% of cases) [Citation116]. Women are also more likely to report substance use and abuse than men but that does not necessarily translate into prevalence of misuse [Citation115]. In addition to age and sex, other variables, for example, gender identity, ethnicity, marital, and socio-economic status, may be important, but the evidence is sparse, and many population groups were not included in studies [Citation110].
An association between chronic pain, concurrent psychiatric comorbidities, and opioid misuse has been identified [Citation109]. A small double-blind, placebo-controlled randomized trial (n = 81 with a 25% drop out rate) on negative affect, a constellation of anxiety, depression, and a catastrophizing cognitive style, found that patients with chronic low back pain with high negative affect during 6 months of follow-up were likely to be prescribed higher doses of opioids, had lesser improvement in pain, and greater rate of opioid misuse than those with low negative affect [Citation116]. Depression, in particular, increases the risk of abuse of prescription opioids [Citation117], but a similar increase in risk of prescription opioid abuse was also identified in patients with an anxiety disorder [Citation118], panic attacks, post-traumatic stress disorder, and personality disorders [Citation119]. However, a well-treated psychiatric disorder is considered a protective factor for opioid misuse in adolescents [Citation17].
Above all other risk factors, a personal history of substance misuse and abuse preceding a long-term opioid treatment is a strong predictor of aberrant opioid-related behavior [Citation120]. A study in which electronic health records were investigated for signs of opioid dependence in patients with chronic non-malignant pain predicted an increased risk of current dependence, particularly in patients with a history of severe dependence and prescription opioid abuse (odds ratio 56) [Citation121]. Personal history of any substance (alcohol, tobacco, or marijuana) abuse is associated with an aberrant opioid-related behavior [Citation122]. The non-opioid abusive substances serve as introductory drugs to prescription opioids. In a study in adolescent cannabis and tobacco users (age 14 years), a positive association with opioid use at age 19 years was identified [Citation123]. Furthermore, it is now widely accepted that prescription opioids serve as a gateway drug toward the abuse of heroin and other illicit opioids [Citation46]. In the US, the majority of heroin users report having started their addiction trajectory with prescription opioids [Citation46,Citation124]. Besides the history of personal substance abuse, familial substance abuse is also an important risk factor [Citation125]. In families where one of the parents was a current marijuana user, the offspring had a higher risk of binge alcohol, tobacco, and marijuana consumption [Citation126].
Other risk factors of substance abuse include sexual abuse, particularly in the preadolescent period, legal problems and being a victim of an injury, and genetic factors (although genetic screening is currently not implemented in routine clinical practice) [Citation120,Citation127–129]. In a recent study, 55 pregnant women who were opioid users were interviewed about childhood trauma and abuse. When childhood sexual abuse was reported, the risk of current opioid misuse in pregnancy was increased (odds ratio 3.5) [Citation130]. Similar findings were observed for any type of childhood abuse, including physical and emotional abuse [Citation131]. As we already established that non-opioid abusive substances are often introductory drugs to prescription opioid misuse, it may be worthwhile to enforce efforts of drug awareness and prevention programs in children of all ages.
3.2. Why are research findings on predictors of opioid misuse diverse?
There is much attention given to research on the safety of opioid use. The breadth of provided evidence can be appreciated by a quick Medline search; an algorithm consisting of keywords ‘risk factors,’ ‘opioid,’ ‘misuse,’ ‘addiction,’ and ‘abuse’ yields nearly 200,000 hits with exponential growth in number since the 1990s. However, the general lack of high-quality evidence and highly heterogeneous findings have been recognized by many authors [Citation110,Citation132]. Findings depend not only on the internal validity of the study (considering confounding, information, and selection bias) but also on the population under observation (children, adolescents, adults, and elderly), country of origin (with differences in health-care systems), year of research, setting (surgery, intensive care unit, pain clinic, street), and others, thus limiting generalizability of the study findings. Furthermore, conditional on the type of opioid misused (prescription or illicit drugs), the operational definition of the outcome under observation, and on the type of pain studied (malignant versus non-malignant pain versus no pain), predictors and other outcomes found to be associated with aberrant opioid-related behavior may differ substantially [Citation110,Citation133].
To improve our understanding of mechanisms behind opioid misuse, abuse, and addiction and to develop valid, useful clinical tools to aid in recognizing high-risk patients in practice, we need to especially improve internal validity of opioid safety research, which is particularly challenging in observational studies, since clinical trials are mostly insufficiently powered to detect safety signals [Citation134]. Based on our experience in conducting large-scale observational research on opioid safety, we recognized the presence of confounding by indication to be challenging to control for in this research field. Furthermore, the information on opioid use, outcomes, and other variables in registry-based studies is imperfect, which could have a profound impact on detecting safety signals [Citation135].
The majority of opioid safety studies utilize an inactive comparator (no use) to study the safety profile of opioids. The ‘no use’ is there as an observational equivalent of a placebo control in a randomized clinical trial; however, in that setting the randomization ensures that if the two arms differ, it is only by chance. This does not hold for observation comparisons: patients to whom opioids were prescribed must be different from those not requiring such medication; opioid users typically have an indication for opioid use. We can correct for these differences by controlling for them with various proposed techniques, e.g. multivariable regression models, propensity score adjustments, and matching, but these may be insufficient at completely removing group differences in prognostic factors. Even though advanced methods have been proposed, e.g. high dimension propensity score, self-controlled series, and external confounding adjustment [Citation136–138], which are promising a high degree of control over measured and unmeasured (by proxy) confounding variables, they are seldom utilized [Citation132]. Another approach would be to make use of an active comparator design [Citation139]. However, it remains unclear what would the optimal comparator be in the research on opioid safety. A choice of an active comparator in opioid research very much depends on the research question, and even then it may well be that one specific opioid (or another analgesic) is preferentially prescribed to more vulnerable patients that also have a poorer outcome prognosis.
Data utilized in opioid safety studies have rarely been collected for the purpose of scientific research. Therefore, we must assume a high variability in the reporting of opioid-related outcomes, opioid use, and other variables within and between medical centers. Although the information about opioid use is most often gained by examination of pharmacy claims that tend to be quite accurate, even the most sophisticated algorithms used to identify the duration of opioid treatment fail to address the issue of compliance with therapy being prescribed. Therefore, we do not know whether a patient actually ingested the medication, whether illicit opioids are used, or whether patients are buying opioids over the counter [Citation132]. Although availability of opioids as an over the counter medicine may vary between countries and the exposure prevalence due to over the counter opioids is assumed to be small compared with prescription opioids and may therefore not have substantial impact on the effect estimates [Citation140], the structure of misclassification (and its association with other errors) introduced by over the counter use may be difficult to anticipate [Citation141]. Similarly, various disease classifications (most often international classification of diseases, ICD) are utilized to identify outcomes and even populations in different settings and countries. For example, the F-series are not used for coding of drug-related deaths in the US, whereas in Europe this is standard practice [Citation7]. When a new study, based on data collected in Europe, is being planned, but the code series from US are utilized, a serious underestimation of outcomes will occur. Furthermore, to identify individual opioid-related outcomes, a set of codes or even individual codes are used, e.g. heroin poisoning. This may lead to serious misclassification since the probability of accurate reporting may be reduced and the identification of individual opioid poisoning by a physician may be challenging (e.g. due to unreliable urine testing) [Citation109,Citation142,Citation143]. Incomplete or missing information on the exposure, outcome, or other variables, and the underlying mechanism that led to the inaccurate information may have various consequences for the investigated outcome of interest that even the most experienced researchers may misjudge [Citation144], and therefore needs to be formally explored [Citation145].
4. What can be done to prevent further escalation or another opioid crisis?
Many interventions have been developed to counter the opioid epidemic, but several of them only targeted misuse of prescription opioids. Therefore, despite the fact that the number of opioid prescriptions has declined for over a decade now, the number of opioid deaths in the US is still rising. This ‘opioid paradox’ [Citation146] shows clearly that the myriad preventive measures that were implemented over that same decade, did not have the desired effect.
4.1. Regulatory solutions
Because the opioid crisis was initially perceived as a public health problem [Citation147], many of the first preventive measures were legislative and regulatory, aimed at decreasing the number of prescriptions and indirectly the number of pills available for misuse. In several health-care settings, prescription drug monitoring programs (PDMPs) were intensified or expanded. These mostly automated systems with usually state-wide coverage enable prescribers to check whether a patient has already received a recent prescription for a certain drug. Use of these PDMPs prior to prescription of a monitored drug is now mandatory in many parts of the US. This has limited the number of drugs prescribed [Citation148,Citation149]. However, PDMPs intentionally targeted the prescription rates of opioids and did not have an influence on non-medical use of opioids and might even unintentionally have increased the use of heroin and other illicit opioids [Citation150].
An important, nationwide step was taken when the Centers for Disease Control (CDC) published their ‘Opioid prescribing guideline’ in 2016 [Citation32], focused on the treatment of chronic non-malignant pain with opioids. This guideline gave a series of recommendations on whether or not to initiate opioid therapy for chronic pain, on which opioids to prescribe (it states a preference for immediate-release opioid formulations as opposed to extended-release formulations), which dose and for how long to prescribe (as low a dose as possible for the shortest period of time), and how to assess the risk of opioid related harm (e.g. not prescribing to patients with a history of substance abuse or concomitant use of benzodiazepines). Similarly, some countries in Europe updated or developed new prescription guidelines, as, for example, the Netherlands [Citation151] and the United Kingdom [Citation33], that either rely more heavily on opioids in the postoperative period (the Netherlands) or were developed specifically for chronic non-malignant pain and therefore support also non-pharmacological interventions.
In the wake of the US guidelines, which were first and foremost intended as a set of clinical recommendations, many US states implemented laws limiting the duration of opioid prescriptions, and in some cases even the dose that could be prescribed [Citation152]. Furthermore, restrictions were placed on ‘doctor shopping’ [Citation153], and high-volume prescribers were sent letters informing them of their unusual prescription behavior [Citation154]. These legal limitations have affected the prescription rates of opioids (the red line in ) and although they might have curbed the increasing rate of opioid overdoses associated with prescribed opioids (), they have done little so far to limit the overall number of overdose deaths (these are now mainly driven by illicit opioids), and the question remains whether they are effective at all [Citation155].
4.2. Pharmacological solutions
Pharmacological solutions to the opioid problem have also been presented over the past two decades. When the first signs of opioid misuse were starting to surface, several new pharmacological opioid formulations, targeted at decreasing abuse potential (so-called abuse deterrent formulations or ADFs), entered the market. Furthermore, novel opioid-receptor agonists and of course new formulations of naloxone became used.
4.2.1. Abuse deterrent formulations
There are several ways in which a drug can be formulated in an abuse deterrent way, as described by the Food and Drugs Administration (FDA) [Citation156]: adding a physical or chemical barrier to the drug in question, combining agonist/antagonist combinations, decreasing a drug’s likability by including aversive substances that deter users from using the drug in large amounts, and novel technologies such as unconventional delivery systems or using prodrugs that can only be activated by ingestion.
The best-known example of the first category, adding a chemical-physical barrier, is a reformulation of OxyContin® (ADF OxyContin®). The drug was marketed with a new shell, which made crushing and extraction of the drug difficult. This decreased the number of opioid overdoses due to oxycodone [Citation157], but only for a short while. A plateau was reached within a few years after reformulation, for which there are several possible explanations. First, it is possible that users used different ways to ingest the drug (orally as opposed to snorting and injecting), which would eventually lead to the same incidence of oxycodone overdoses. It is also possible that users changed their drug preference and simply started to snort and inhale/inject other types of opioids. This would then decrease the number of oxycodone overdoses, but not the number of overall opioid overdoses. It is important to note that the number of heroin overdoses rose between 2010 and 2014 [Citation158]. A study into the abuse of ADF OxyContin® in a large cohort of patients with an opioid use disorder showed that in a subsample only a small percentage of users stopped abusing oxycodone altogether [Citation159]. Some switched to a different drug (heroin) but most did not change their behavior after the reformulation. The evidence for a massive switch to heroin is inconclusive: one study reported that the odds of heroin initiation did not change after the introduction of ADF OxyContin® [Citation160], others have shown no decrease in overall opioid overdose deaths after the introduction of ADF OxyContin® [Citation161,Citation162], consistent with the idea that users simply switched to other opioid drugs. After the introduction of ADF OxyContin® several other abuse deterrent formulations were marketed [Citation163]. We note, however, that not all ADF formulations hold the same physicochemical properties that facilitate or deter alternative routes of administration [Citation163].
Another way of deterring abuse is by combining antagonists with agonists. This has an interesting pharmacological rationale. Naloxone, together with naltrexone, still the most important opioid antagonist, has poor bioavailability when swallowed orally, due to its high first-pass effect. An opioid user swallowing the tablet as intended would not suffer from the effects of the added naloxone, but if one were to snort or inject a crushed tablet, naloxone would work and limit the opioid’ effects or even cause withdrawal symptoms. The use of agonist/antagonist combinations to deter opioid misuses goes back even further than addition of a physicochemical barrier: already in the early 2000s a combination tablet of buprenorphine and naloxone was released [Citation164]. Since then, several other formulations, combining oxycodone or morphine with either naloxone or naltrexone, became available [Citation163].
4.2.2. Opioid alternatives
There are few true alternatives to the use of opioids for moderate-to-severe pain. When non-steroidal anti-inflammatory drugs (NSAIDs) have fallen out of favor because of their undesirable cardiovascular side-effect profile or because of limitation in the health-care budget assigned to this widely used medication group (as, for example, in the Netherlands [Citation114]), there are few opioid alternatives to alleviate both acute and chronic pain. The ultimate goal in opioid research, finding an opioid with all the advantages but none (or fewer) of the disadvantages, has thus far proven elusive. The opioid analgesics currently available all exert their main actions through the mu-opioid receptor (MOR) as opposed to the kappa and delta opioid receptors [Citation75,Citation165]. This receptor activation is responsible for both the desired (analgesic) and unwanted (respiratory depressant) effects of opioids and therefore for the overdose deaths. A new investigative pathway has opened up a possible future pain therapy – biased opioid receptor ligands. After mu opioid receptor (MOR) activation, the analgesic effect is mostly mediated through the activation of the G protein, while it is assumed (but not fully proven) that the majority of side effects, such as respiratory depression, are mediated through the activation of an auxiliary cytoplasmic transduction MOR protein, beta-arrestin [Citation166,Citation167]. Any pharmacological compounds favoring the G protein pathway over the beta-arrestin pathway would theoretically have analgesic properties while lowering the risk of side-effects: the biased ligands. Several candidate molecules have been tested in pre-clinical and clinical trials [Citation168,Citation169], from which oliceridine was the first to receive FDA approval for in-hospital use.
4.2.3. Naloxone for home use
Finally, a different way of preventing the loss of life from opioid overdoses is to treat overdoses promptly. An opioid overdose is easily treated when discovered early. Administration of 0.4 to 4 mg of naloxone (via intravenous, intramuscular, or intranasal routes), depending on the opioid used and dose, can reverse opioid-induced respiratory depression and thus prevent coma, cardiac arrest, and death. The caveat here is that the availability of naloxone – while naloxone is readily available in hospitals and physician practices, it is not available in those places where most overdoses happen. An idea already developed in the early years of this century [Citation170,Citation171], to provide communities with improvised naloxone kits for home use, was more widely introduced in the early 2010s. In 2014, the WHO issued a guideline on community management of opioid overdose, stating ‘Naloxone needs to be available to anyone likely to witness an opioid overdose in the pre-hospital setting’ [Citation172]. To this effect, the so-called ‘take home naloxone’ formulations (THN), such as an auto-injector pen and a nasal spray, were introduced. In their opioid prescription guideline, the CDC [Citation173] and US Surgeon General Public Health Advisory [Citation174] recommend prescribing any form of THN to any patient with a high risk of overdose (i.e. a patient with a history of overdose or opioid use disorder, a patient with a high opioid dose or concurrent benzodiazepine use, or any individual using illegal opioids). MacDonald et al. [Citation175] conducted a systematic review of the observational evidence available for THN schemes. Not only did they show that THN schemes are successful in decreasing opioid overdose deaths, but they also showed that they are cost-effective, have a low risk of adverse events, and are easily implemented over a wide range of social settings. They therefore conclude that THN distribution should be introduced as a standard of care in prevention of opioid overdose deaths [Citation175].
4.3. Patient-centered solutions
Patients’ expectations of both their pain levels and the effect of the analgesic therapy should be carefully managed by the physician. Patient education in pain and pain therapy during a pre-operative visit might be able to help decrease opioid need after the surgery [Citation176,Citation177]. Similarly, someone who receives an opioid prescription for non-surgical pain should be informed of possible side-effects and the potential for misuse by both the prescriber and the pharmacist dispensing the medication [Citation178,Citation179]. Patient awareness of the risks of opioid use might help with decreasing opioid use and consequent misuse.
Tailoring prescriptions, for example post-surgery, to the specific patient will also help in reducing leftover pills [Citation180–182]. Any pills left at home are a risk for non-medical use, be it for self-medication, or diversion to others. Patients are likely to hold on to their leftover pills, for their own or other people’s future use [Citation183]. Furthermore, the return of opioid tablets to the pharmacy (or the hospital) should be as easy as possible and might even need to be financially rewarded [Citation146] also to decrease the number of pills available for misuse.
5. Expert opinion
As we have tried to demonstrate in this review, the opioid crisis is a complex problem, and there does not seem to exist one definite solution. Not only has the general opinion on pain and what amount of pain is bearable changed but also doctors’ attitudes and possibilities, as well as possibilities of health-care facilities. The rise in the number of opioid-related fatalities continues year upon year and shows no sign of slowing. As physicians, we are at least partially responsible for this ‘rising tide of deaths’ [Citation184], and it is therefore also our responsibility to help find a solution for this problem. However, modern medicine without opioids is currently unthinkable. We are limited in therapeutic options when a patient is in serious pain. Anesthesia without opioids is very difficult and possibly unsafe [Citation185]. We need to convince ourselves, but also all of our colleagues, as well as our patients that there is a fine line between responsible opioid use and misuse. In this respect, it is important to note that the need for opioids in pain therapy is subject to a high amount of variability. It is therefore difficult to develop a one-size-fits-all strategy for opioid therapy in both acute and chronic non-malignant pain settings. It is of paramount importance that therapy is individualized, and a good relationship between patient and prescriber is key here. Initiation of opioid therapy warrants close contact between patient and physician to enable monitoring of opioid effect, possible side-effects or signs of misuse. Where possible, prescriptions should be short-termed and refills only possible after close contact with the physician. Ideally, opioids should only be used as a ‘pain circuit breaker’ in non-malignant pain, much like a course of antibiotics. Cancer pain patients should, on the other hand, have access to opioid therapy when required, also on a long-term basis, but again with careful consideration of appropriate opioid therapy and with acknowledging the side effects. Opioid use should not be, however, extended beyond the intended indication (for example, after cancer patients enter remission or are cured) to prevent opioid use disorder in this patient group. Where continuation of analgesic therapy is unavoidable in the treatment of non-malignant pain, possible alternatives for opioid therapy (such as NSAIDs and antidepressants or antiepileptics) should be considered. In this indication, prolonged opioid use should be avoided at all costs, as little scientific evidence has been provided to support continued opioid use in chronic non-malignant pain [Citation105]. Additionally, when appropriate, complementary approaches such as physical therapy, psychological support, and rehabilitation programs should be considered. Not only can these non-pharmacological treatments help in alleviating chronic non-malignant pain but can also aid patients to deal with the pain and accept it. It has been demonstrated that a multidisciplinary approach to pain management is more beneficial for a patient than a conventional one. Patients treated by such a team reported having reduced pain intensity, improved psychological well-being, quality of sleep, and physical functioning [Citation186]. Furthermore, patient empowerment in the treatment of chronic non-malignant pain will provide the necessary information to the patient, so they can make an informed decision about the initiation of the opioid treatment and be alerted for possible side effects [Citation187]. Additionally, it can aid in detecting opioid misuse when an opioid is already prescribed [Citation188]. Unfortunately, these alternative approaches are not always reimbursed by health insurance nor are the lengthy patient consultations that are required[Citation189, Citation190].
As we have shown, due to the complexity of the opioid crisis, there is not one universal cure. A combination of measures, aimed at different underlying mechanisms behind the opioid crisis, and always in concordance with all parties involved, is the best way forward.
Article highlights
Overprescribing of opioids was the initial cause of the US opioid epidemic
Prescription rate of opioids is increasing in many countries worldwide
There are many risk factors associated with opioid use and opioid use disorder that may depend on the opioid type (prescribed vs illicit) and clinical setting
Many approaches, each targeting a certain aspect of the opioid epidemic, have been considered
There is no universal solution for the rising tide of opioid overdoses
Opioids should be prescribed for the shortest duration of time with the lowest but still effective dose (similarly to a course of antibiotics)
Declaration of interest
A Dahan received/receives funding from AMO Pharma Ltd., Bedrocan BV, Grünenthal GmbH, Medasense Biometrics Ltd., Medtronic, MSD Nederland BV and Trevena Inc; received consultancy and/or speaker fees from Enalare Therapeutics Inc., Grünenthal BV, Medasense Biometrics Ltd., Trevena Inc., MSD Nederland BV; received grants/awards from ZonMW (The Hague, The Netherlands) and US Food and Drug Administration (Washington DC, USA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
A Bedene, FR Rosendaal, A Dahan, and ELA van Dorp provided substantial contribution to conception and designed of the study. A Bedene and ELA van Dorp drafted the manuscript and all authors read, provided critical revisions, approved the final submitted version.
Acknowledgments
Tackling and Preventing the Opioid Epidemic (TAPTOE) is a collaborative project between Utrecht University (NL), SIR Institute for Pharmacy Practice and Policy (NL), Leiden University Medical Center (NL), and Radboud University Medical Center (NL). The TAPTOE consortium has also received grants from the Canisius-Wilhelmina Hospital, Sint-Maartenskliniek, National Healthcare Institute (ZIN), Trimbos Institute, the Royal Dutch Pharmacists’ Association (KNMP) and the Dutch Medicines Evaluation Board (CBG-MEB).
Additional information
Funding
References
- United Nations Office on Drugs and Crime (UNODC). Understanding the global opioid crisis. Global SMART Update 2019.
- Kelly JP, Cook SF, Kaufman DW, et al. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008 Sep 15;138(3):507–513.
- Bedene A, Lijfering WM, Niesters M, et al. Opioid prescription patterns and risk factors associated with opioid use in the Netherlands. JAMA Network Open. 2019 Aug 2;2(8):e1910223.
- Muller AE, Clausen T, Sjogren P, et al. Prescribed opioid analgesic use developments in three Nordic countries, 2006-2017. Scand J Pain. 2019 Apr 24;19(2):345–353.
- Government of the United Kingdom. United Kingdom drug situation 2019: focal point annual report. 2021.
- Hauser W, Buchser E, Finn DP, et al. Is Europe also facing an opioid crisis? A survey of European Pain Federation chapters. Eur J Pain. 2021 Sep;25(8):1760–1769.
- Chen T-C, Knaggs RD, Chen L-C. Association between opioid-related deaths and prescribed opioid dose and psychotropic medicines in England: a case-crossover study. Br J Anaesth. 2021 Nov;127(5):789–797.
- Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers-United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011 Nov 4;60(43):1487–1492.
- Rudd RA, Paulozzi LJ, Bauer MJ, et al. Increases in heroin overdose deaths – 28 States, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014 Oct 3;63(39):849–854.
- Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths – 27 states, 2013–2014. MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65(33):837–843.
- O’Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region – United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017 Sep 1;66(34):897–903.
- O’Donnell JK, Halpin J, Mattson CL, et al. Deaths involving fentanyl, fentanyl analogs, and U-47700-10 states, July–December 2016. MMWR Morb Mortal Wkly Rep. 2017 Nov 3;66(43):1197–1202.
- Centers for Disease Control and Prevention (CDC). Data from: overdose death rates involving opioids, by type, United States, 1999-2020 (Deaths per 100,000 people) [dataset]. 2022 [updated 2022 May 22; [cited 2022 July 18]. Available from: https://www.cdc.gov/drugoverdose/data/OD-death-data.html
- O’Donnell J, Gladden RM, Mattson CL, et al. Vital signs: characteristics of drug overdose deaths involving opioids and stimulants – 24 states and the district of Columbia, January–June 2019. MMWR Morb Mortal Wkly Rep. 2020 Sep 4;69(35):1189–1197.
- Jenkins RA. The fourth wave of the US opioid epidemic and its implications for the rural US: a federal perspective. Prev Med. 2021;152:106541.
- Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry. 2021 Jul 1;34(4):344–350.
- Volkow ND, Jones EB, Einstein EB, et al. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76(2):208–216.
- Jones MR, Viswanath O, Peck J, et al. A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther. 2018 Jun;7(1):13–21.
- World Health Organization (WHO). The selection of essential drugs: a report of a WHO Expert Committee. World Health Organization Technical Report Series 1977. p. 1–36.
- World Health Organization (WHO). Cancer pain relief and palliative care: report of a WHO expert committee. In: Expert committee on cancer pain relief and active supportive care, editor. Geneva: World Health Organization Technical Report Series; 1990. p. 1–76.
- Andrew R, Derry S, Taylor RS, et al. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Pract. 2014 Jan;14(1):79–94.
- Lamé IE, Peters ML, Vlaeyen JWS, et al. Quality of life in chronic pain is more associated with beliefs about pain, than with pain intensity. Eur J Pain. 2005;9(1):15–23.
- Ang J-Y, Leong EL, Chan H-K, et al. Health-related quality of life of Malaysian patients with chronic non-malignant pain and its associated factors: a cross-sectional study. BMC Musculoskelet Disord. 2022;23(1):400.
- Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1):1–13.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980 Jan 10;302(2):123.
- Leung PTM, Macdonald EM, Stanbrook MB, et al. A 1980 letter on the risk of opioid addiction. N Engl J Med. 2017 Jun 1;376(22):2194–2195.
- Campbell JN. APS 1995 Presidential address. Pain Forum. 1996;5(1):85–88.
- Max MB, Donovan M, Miaskowski CA, et al. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874–1880.
- Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain. 2016 Jan;157(1):65–69.
- Phillips DM, Phillips DM. JCAHO Pain Management. Standards are unveiled. JAMA. 2000;284(4):428–429.
- Rathmell JP, Wu CL, Sinatra RS, et al. Acute post-surgical pain management: a critical appraisal of current practice, December 2–4, 2005. Reg Anesth Pain Med. 2006 Jul-Aug 31;31(4 Suppl 1):1–42.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA. 2016 Apr 19;315(15):1624–1645.
- National Institute for Health and Care Excellence (NICE). Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain, NICE guideline [NG193]. 2021.
- Gourd E. American Pain Society forced to close due to opioid scandal. Lancet Oncol. 2019 Jul;20(7):e350.
- Ornstein C, Weber T. American Pain Foundation shuts down as senators launch investigation of prescription narcotics 2012 [cited 2022 Mar 10]. Available from: https://www.propublica.org/article/senate-panel-investigates-drug-company-ties-to-pain-groups
- Clark C. American Pain Society seeks ok to call it quits. MedPage Today. 2019 [cited 2022 March 10]. Available from: https://www.medpagetoday.com/painmanagement/painmanagement/80054
- Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009 Feb;99(2):221–227.
- Rischitelli DG, Karbowicz SH. Safety and efficacy of controlled-release oxycodone: a systematic literature review. Pharmacotherapy. 2002 Jul;22(7):898–904.
- Dhalla IA, Mamdani MM, Sivilotti MLA, et al. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. Can Med Assoc J. 2009;181(12):891–896.
- Berge KH, Dillon KR, Sikkink KM, et al. Diversion of drugs within health care facilities, a multiple-victim crime: patterns of diversion, scope, consequences, detection, and prevention. Mayo Clin Proc. 2012;87(7):674–682.
- National Academies of Sciences Engineering Medicine Health; Medicine Division; Board on Health Sciences; Policy Committee on Pain Management; Regulatory Strategies to Address Prescription Opioid Abuse. Evidence on strategies for addressing the opioid epidemic: regulating/restricting conditions of lawful access to approved drugs. In: Phillips JK, Ford MA, Bonnie RJ, editors. Pain management and the opioid epidemic: balancing societal and individual benefits and risks of prescription opioid use. Washington (DC): National Academies Press (US); 2017.
- Sansone RA, Sansone LA. Doctor shopping: a phenomenon of many themes. Innov Clin Neurosci. 2012 Nov;9(11–12):42–46.
- Kennedy-Hendricks A, Richey M, McGinty EE, et al. Opioid overdose deaths and Florida’s crackdown on pill mills. Am J Public Health. 2016 Feb;106(2):291–297.
- Kennedy-Hendricks A, Gielen A, McDonald E, et al. Medication sharing, storage, and disposal practices for opioid medications among US adults. JAMA Intern Med. 2016;176(7):1027–1029.
- Cicero TJ, Ellis MS. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues Clin Neurosci. 2017 Sep;19(3):259–269.
- Cicero TJ, Ellis MS, Surratt HL, et al., The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821–826.
- Cicero TJ, Ellis MS, Kasper ZA. Increased use of heroin as an initiating opioid of abuse. Addict Behav. 2017 Nov;74:63–66.
- Wide-ranging ONline Data for Epidemiologic Research (WONDER). [Internet]. Atlanta (GA): CDC, National Center for Health Statistics. 2020 [cited 2022 Mar 10]. Available from: http://wonder.cdc.gov.
- Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021 Feb 12;70(6):202–207.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants – United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018 Mar 30;67(12):349–358.
- McCarthy M. US declares opioid epidemic a “national emergency.” BMJ. 2017 Aug;14(358):j3881.
- Kalkman GA, Kramers C, van Dongen RT, et al. Trends in use and misuse of opioids in the Netherlands: a retrospective, multi-source database study. Lancet Public Health. 2019 Oct;4(10):e498–e505.
- Li G, Chihuri S. Prescription opioids, alcohol and fatal motor vehicle crashes: a population-based case-control study. Inj Epidemiol. 2019;6(1):11.
- Soderberg KC, Laflamme L, Moller J. Newly initiated opioid treatment and the risk of fall-related injuries. A nationwide, register-based, case-crossover study in Sweden. CNS Drugs. 2013Feb;27(2):155–161.
- Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015 Apr;156(4):569–576.
- Mercadante S, Arcuri E, Santoni A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs. 2019 Oct;33(10):943–955.
- Rhee TG, Rosenheck RA. Association of current and past opioid use disorders with health-related quality of life and employment among US adults. Drug Alcohol Depend. 2019 Jun;1(199):122–128.
- Goplerud E, Hodge S, Benham T . A substance use Cost calculator for US employers with an emphasis on prescription pain medication misuse. J Occup Environ Med. 2017 Nov;59(11):1063–1071.
- Florence C, Luo F, Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021 Jan 1;218:108350.
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014 Jul;30(7):557–564.
- Fenalti G, Zatsepin NA, Betti C, et al. Structural basis for bifunctional peptide recognition at human δ-opioid receptor. Nat Struct Mol Biol. 2015;22(3):265–268.
- Koehl A, Hu H, Maeda S, et al. Structure of the µ-opioid receptor-G(i) protein complex. Nature. 2018 Jun;558(7711):547–552.
- Stein C. Opioid receptors. Annu Rev Med. 2016;67(1):433–451.
- Eddy NB, Halbach H, Braenden OJ. Synthetic substances with morphine-like effect: relationship between analgesic action and addiction liability with a discussion of the chemical structure of addiction-producing substances. Bull World Health Organ. 1956;14(3):353–402.
- Lötsch J. Opioid metabolites. J Pain Symptom Manage. 2005;29(5,Supplement):10–24.
- Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012 Feb;6(1):11–16.
- Flood P, Rathmell JP, Schafer S, et al. Stoelting’s pharmacology and physiology in anesthetic practice. 5th. Philadelphia: Wolters Kluwer Health; 2015.
- Drewes AM, Jensen RD, Nielsen LM, et al. Differences between opioids: pharmacological, experimental, clinical and economical perspectives. Br J Clin Pharmacol. 2013 Jan;75(1):60–78.
- Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613–624.
- Rang HP, Dale MM, Ritter J, et al. Rang and Dale’s pharmacology. 7th ed. Edinburgh [etc.]: Elsevier/Churchill Livingston; 2012.
- Harrison C, Smart D, Lambert DG. Stimulatory effects of opioids. Br J Anaesth. 1998 Jul;81(1):20–28.
- Williams JT, Christie MJ, Cellular MO. Synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299–343.
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73(1):953–990.
- Williams JT, Ingram SL, Henderson G, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013 Jan;65(1):223–254.
- Matthes HW, Maldonado R, Simonin F, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996 Oct 31;383(6603):819–823.
- Allouche S, Noble F, Marie N. Opioid receptor desensitization: mechanisms and its link to tolerance. Front Pharmacol. 2014;5:280.
- Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008 May;154(2):384–396.
- Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594.
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997 Oct 3;278(5335):58–63.
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005 Nov;8(11):1481–1489.
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005 Aug;162(8):1414–1422.
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–5278.
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12(1):54–67.
- Koob GF, Le Moal M, Addiction D. Dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129.
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652.
- Volkow ND, Fowler JS, Wang GJ, et al., Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6): 557–569.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003 Nov 13;349(20):1943–1953.
- Cushman P, Dole VP. Detoxification of rehabilitated methadone-maintained patients. JAMA. 1973 Nov 12;226(7):747–752.
- Pergolizzi JV, Raffa RB, Rosenblatt MH. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: current understanding and approaches to management. J Clin Pharm Ther. 2020;45(5):892–903.
- World Health Organization (WHO). Clinical guidelines for withdrawal management and treatment of drug dependence in closed settings. Vol. 4. Geneva: Wihtdrawal Management; 2009.
- Algera MH, Olofsen E, Moss L, et al. Tolerance to opioid-induced respiratory depression in chronic high-dose opioid users: a model-based comparison with opioid-naïve individuals. Clin Pharmacol Ther. 2021 Mar;109(3):637–645.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed.; 2013. d o i:https://doi.org/10.1176/appi.books.9780890425596.
- Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342–1343.
- O’Brien CP, Childress AR, Ehrman R, et al. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22.
- Jage J. Opioid tolerance and dependence – do they matter? Eur J Pain. 2005 Apr;9(2):157–162.
- McQuay H. Opioids in pain management. Lancet. 1999 Jun 26;353(9171):2229–2232.
- Loren AW. Harder to treat than leukemia – opioid use disorder in survivors of cancer. N Engl J Med. 2018 Dec 27;379(26):2485–2487.
- Preux C, Bertin M, Tarot A, et al. Prevalence of opioid use disorder among patients with cancer-related pain: a systematic review. J Clin Med. 2022 Mar 14;11(6):6.
- Barrett ME, Joe GW, Simpson DD. Availability of drugs and psychological proneness in opioid addiction. Int J Addict. 1990;25(10):1211–1226.
- National Institute on Drug Abuse. Increased drug availability is associated with increased use and overdose. National Institute on Drug Abuse website. 2022 [cited 2022 Feb 23]. Available from: https://nida.nih.gov/publications/research-reports/prescription-opioids-heroin/increased-drug-availability-associated-increased-use-overdose
- Edlund MJ, Sullivan M, Steffick D, et al. Do users of regularly prescribed opioids have higher rates of substance use problems than nonusers? Pain Med. 2007;8(8):647–656.
- Groenewald CB, Rabbitts JA, Gebert JT, et al. Trends in opioid prescriptions among children and adolescents in the United States: a nationally representative study from 1996 to 2012. Pain. 2016 May;157(5):1021–1027.
- Klimas J, Gorfinkel L, Fairbairn N, et al. Strategies to identify patient risks of prescription opioid addiction when initiating opioids for pain: a systematic review. JAMA Network Open. 2019;2(5):e193365–e193365.
- Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691.
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the space randomized clinical trial. JAMA. 2018 Mar 6;319(9):872–882.
- Stanos S. Evolution of opioid risk management and review of the classwide REMS for extended-release/long-acting opioids. Phys Sportsmed. 2012 Nov;40(4):12–20.
- Pergolizzi JVsJr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443–451.
- Stumbo SP, Yarborough BJH, McCarty D, et al. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat. 2017;73:47–54.
- Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. 2017 Nov;125(5):1741–1748.
- Voon P, Karamouzian M, Kerr T. Chronic pain and opioid misuse: a review of reviews. Subst Abuse Treat Prev Policy. 2017 Aug 15;12(1):36.
- Campbell CI, Weisner C, LeResche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547.
- Bagley SM, Gai MJ, Earlywine JJ, et al. Incidence and characteristics of nonfatal opioid overdose among youths aged 11 to 24 years by sex. JAMA Network Open. 2020;3(12):e2030201–e2030201.
- Sullivan MD, Edlund MJ, Fan M-Y, et al. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and Medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332–339.
- Bedene A, Van Dorp ELA, Faquih T, et al. Causes and consequences of the opioid epidemic in the Netherlands: a population-based cohort study. Sci Rep. 2020 Sep 17;10(1):15309.
- Green TC, Grimes Serrano JM, Licari A, et al. Women who abuse prescription opioids: findings from the addiction severity index-multimedia version connect prescription opioid database. Drug Alcohol Depend. 2009 Jul 1;103(1–2):65–73.
- Wasan AD, Michna E, Edwards RR, et al. Psychiatric comorbidity is associated prospectively with diminished opioid analgesia and increased opioid misuse in patients with chronic low back pain. Anesthesiology. 2015;123(4):861–872.
- Sullivan MD. Depression effects on long-term prescription opioid use, abuse, and addiction. Clin J Pain. 2018;34(9):878–884.
- Rogers AH, Zvolensky MJ, Ditre JW, et al. Association of opioid misuse with anxiety and depression: a systematic review of the literature. Clin Psychol Rev. 2021;84:101978.
- Wilsey BL, Fishman SM, Tsodikov A, et al. Psychological comorbidities predicting prescription opioid abuse among patients in chronic pain presenting to the emergency department. Pain Med. 2008;9(8):1107–1117.
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008 Jul-Aug;24(6):497–508.
- Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776–1782.
- Jones CM, Clayton HB, Deputy NP, et al. Prescription opioid misuse and use of alcohol and other substances among high school students – youth risk behavior survey, United States, 2019. MMWR Suppl. 2020 Aug 21;69(1):38–46.
- Thrul J, Rabinowitz JA, Reboussin BA, et al. Adolescent cannabis and tobacco use are associated with opioid use in young adulthood—12-year longitudinal study in an urban cohort. Addiction. 2021;116(3):643–650.
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013 Sep 1;132(1–2):95–100.
- Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55(11):973–979.
- Madras BK, Han B, Compton WM, et al. Associations of parental marijuana use with offspring marijuana, tobacco, and alcohol use and opioid misuse. JAMA Network Open. 2019;2(11):e1916015–e1916015.
- Cheatle MD, Compton PA, Dhingra L, et al. Development of the revised opioid risk tool to predict opioid use disorder in patients with chronic nonmalignant pain. J Pain. 2019;20(7):842–851.
- Brown RT, Deyo B, Nicholas C, et al. Screening in Trauma for Opioid Misuse Prevention (STOMP): results from a prospective cohort of victims of traumatic injury. Drug Alcohol Depend. 2022;232:109286.
- Berrettini W. A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues Clin Neurosci. 2017;19(3):229–236.
- Kors S, Kurdziel-Adams G, Towers C, et al. Sexual abuse as a risk factor for opioid misuse in pregnancy. J Child Sex Abuse. 2022;31(5):538–549.
- Austin AE, Shanahan ME, Zvara BJ. Association of childhood abuse and prescription opioid use in early adulthood. Addict Behav. 2018;76:265–269.
- Ranapurwala SI, Naumann RB, Austin AE, et al. Methodologic limitations of prescription opioid safety research and recommendations for improving the evidence base. Pharmacoepidemiol Drug Saf. 2019 Jan;28(1):4–12.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015 Feb 17;162(4):276–286.
- Singh S, Loke YK. Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials. 2012;13(1):138.
- Van Smeden M, Lash TL, Groenwold RHH. Reflection on modern methods: five myths about measurement error in epidemiological research. Int J Epidemiol. 2019;49(1):338–347.
- Schneeweiss S, Rassen JA, Glynn RJ, et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009 Jul;20(4):512–522.
- Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515.
- Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303.
- Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228.
- Schmidt M, Hallas J, Friis S. Potential of prescription registries to capture individual-level use of aspirin and other nonsteroidal anti-inflammatory drugs in Denmark: trends in utilization 1999-2012. Clin Epidemiol. 2014;6:155–168.
- Rothman K, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
- Bertholf RL, Sharma R, Reisfield GM. Predictive value of positive drug screening results in an urban outpatient population. J Anal Toxicol. 2016 Nov;40(9):726–731.
- Green CA, Perrin NA, Janoff SL, et al. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 2017;26(5):509–517.
- Lash TL. Heuristic thinking and inference from observational epidemiology. Epidemiology. 2007 Jan;18(1):67–72.
- Lash TL, Fox MP, MacLehose RF, et al. Good practices for quantitative bias analysis. Int J Epidemiol. 2014;43(6):1969–1985.
- Kharasch ED, Clark JD, Adams JM. Opioids and public health: the prescription opioid ecosystem and need for improved management. Anesthesiology. 2022Jan1;136(1):10–30.
- Pergolizzi JVsJr, Raffa RB, Taylor RsJr, et al. Abuse-deterrent opioids: an update on current approaches and considerations. Curr Med Res Opin. 2018;34(4):711–723.
- Strickler GK, Zhang K, Halpin JF, et al. Effects of mandatory prescription drug monitoring program (PDMP) use laws on prescriber registration and use and on risky prescribing. Drug Alcohol Depend. 2019;1(199):1–9.
- Manders L, Abd-Elsayed A. Mandatory review of prescription drug monitoring program before issuance of a controlled substance results in overall reduction of prescriptions including opioids and benzodiazepines. Pain Physician. 2020 Jun;23(3):299–304.
- Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017 Jun;69:65–77.
- Houweling PL, Molag ML, Van Boekel RLM, et al. Revision of postoperative pain guideline. Ned Tijdschr Geneeskd. 2013;157(49):A7005.
- National Conference of State Legislatures. Prescribing policies: states confront opioid overdose epidemic 2019 [cited 2022 Feb 23]. Available from: https://www.ncsl.org/research/health/prescribing-policies-states-confront-opioid-overdose-epidemic.aspx
- Meara E, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016 Jul 7;375(1):44–53.
- Sacarny A, Yokum D, Finkelstein A, et al. Medicare letters to curb overprescribing of controlled substances had no detectable effect on providers. Health Aff (Millwood). 2016 Mar;35(3):471–479.
- Barnett ML, Gray J, Zink A, et al. Coupling policymaking with evaluation– the case of the opioid crisis. N Engl J Med. 2017 Dec 14;377(24):2306–2309.
- U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Abuse-deterrent opioids – evaluation and labeling, guidance for industry. In: U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), editor. U.S. department of health and human services food and drug administration, Office of Communications Division of Drug Information; 2015 April. Available from: https://www.fda.gov/files/drugs/published/Abuse-Deterrent-Opioids-Evaluation-and-Labeling.pdf
- Sessler NE, Downing JM, Kale H, et al. Reductions in reported deaths following the introduction of extended-release oxycodone (OxyContin) with an abuse-deterrent formulation. Pharmacoepidemiol Drug Saf. 2014 Dec;23(12):1238–1246.
- Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015 Jan 15;372(3):241–248.
- Cicero TJ, Ellis MS. Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States: lessons learned from oxycontin. JAMA Psychiatry. 2015 May;72(5):424–430.
- Wolff C, Dowd WN, Ali MM, et al. The impact of the abuse-deterrent reformulation of extended-release OxyContin on prescription pain reliever misuse and heroin initiation. Addict Behav. 2020;105:106268.
- Alpert A, Powell D, Pacula RL. Supply-side drug policy in the presence of substitutes: evidence from the introduction of abuse-deterrent opioids. Pain Am Econ J Econ Policy. 2018;10(4):1–35.
- Larochelle MR, Zhang F, Ross-Degnan D, et al. Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene. JAMA Intern Med. 2015 Jun;175(6):978–987.
- Litman RS, Pagán OH, Cicero TJ. Abuse-deterrent opioid formulations. Anesthesiology. 2018 May;128(5):1015–1026.
- Strain EC, Stoller K, Walsh SL, et al. Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology (Berl). 2000 Mar;148(4):374–383.
- Dahan A, Sarton E, Teppema L, et al. Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology. 2001 May;94(5):824–832.
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999 Dec 24;286(5449):2495–2498.
- Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005 Sep;314(3):1195–1201.
- Manglik A, Lin H, Aryal DK, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537(7619):185–190.
- Tan HS, Habib AS. Oliceridine: a novel drug for the management of moderate to severe acute pain – a review of current evidence. J Pain Res. 2021;14:969–979.
- Baca CT, Grant KJ. Take-home naloxone to reduce heroin death. Addiction. 2005 Dec;100(12):1823–1831.
- Dettmer K, Saunders B, Strang J. Take home naloxone and the prevention of deaths from opiate overdose: two pilot schemes. BMJ. 2001 Apr 14;322(7291):895–896.
- World Health Organization (WHO). Community management of opioid overdose. Geneva; 2014.
- Centers for Disease Control and Prevention (CDC). Lifesaving naloxone. 2022 [cited 2022 July 20]. Available from: https://www.cdc.gov/stopoverdose/naloxone/
- U.S. Department of Health and Human Services: Oddice of the Surgeon General. U.S. surgeon general’s advisory on naloxone and opioid overdose. 2022. Available from: https://www.hhs.gov/surgeongeneral/reports-and-publications/addiction-and-substance-misuse/advisory-on-naloxone/index.html
- McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction. 2016;111(7):1177–1187.
- Sugai DY, Deptula PL, Parsa AA, et al. The importance of communication in the management of postoperative pain. Hawaii J Med Public Health. 2013 Jun;72(6):180–184.
- Holman JE, Stoddard GJ, Horwitz DS, et al. The effect of preoperative counseling on duration of postoperative opiate use in orthopaedic trauma surgery: a surgeon-based comparative cohort study. J Orthop Trauma. 2014 Sep;28(9):502–506.
- Thakur T, Chewning B. Using role theory to explore pharmacist role conflict in opioid risks communication. Res Social Adm Pharm. 2020;16(8):1121–1126.
- Esposito DB, Desai VCA, Stephenson JJ, et al. Patient knowledge of safe use of ER/LA opioid analgesics following implementation of the class-wide REMS: a Survey Study. Patient Prefer Adherence. 2021;15:431–442.
- Chen EY, Marcantonio A, Tornetta PsIII. Correlation between 24-hour predischarge opioid use and amount of opioids prescribed at hospital discharge. JAMA Surg. 2018;153(2):e174859–e174859.
- Bleicher J, Stokes SM, Brooke BS, et al. Patient-centered opioid prescribing: breaking away from one-size-fits-all prescribing guidelines. J Surg Res. 2021 Aug;264:1–7.
- Schirle L, Stone AL, Morris MC, et al. Leftover opioids following adult surgical procedures: a systematic review and meta-analysis. Syst Rev. 2020;9(1):139.
- Wu PE, Juurlink DN. Unused prescription drugs should not be treated like leftovers. CMAJ. 2014 Aug 5;186(11):815–816.
- Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010 Nov 18;363(21):1981–1985.
- Lavand’homme P, Estebe JP. Opioid-free anesthesia: a different regard to anesthesia practice. Curr Opin Anaesthesiol. 2018 Oct;31(5):556–561.
- Becker N, Sjøgren P, Bech P, et al. Treatment outcome of chronic non-malignant pain patients managed in a Danish multidisciplinary pain centre compared to general practice: a randomised controlled trial. Pain. 2000 Feb;84(2–3):203–211.
- Nichols VP, Toye F, Eldabe S, et al. Experiences of people taking opioid medication for chronic non-malignant pain: a qualitative evidence synthesis using meta-ethnography. BMJ Open. 2020;10(2):e032988.
- Wilson M, Roll JM, Corbett C, et al. Empowering patients with persistent pain using an internet-based self-management program. Pain Manag Nurs. 2015;16(4):503–514.
- Centers for Disease Control and Prevention (CDC). Data from: U.S. opioid dispensing rate maps 2022 [cited 2022 July 19]. Available from: https://www.cdc.gov/drugoverdose/rxrate-maps/index.html
- Centers for Disease Control and Prevention (CDC). Opioids: data analysis & resources 2022 [cited 2022 July 18]. Available from: https://www.cdc.gov/opioids/data/analysis-resources.html