Abstract
In this paper, we study a two-species competitive system where both the species produce toxin against each other at some cost to their growth rates. A much wider set of outcomes is possible for our system. These outcomes are important contrasts to competitive exclusion or bistable attractors that are often the outcomes for competitive systems. We show that toxin helps to gain an advantage in competition for toxic species whenever the cost of toxin production remains within some moderate value; otherwise it may result in the extinction of the species itself.
1. Introduction
The allelopathic effect on plankton communities has become an important subject from the environmental perspective. DeCandolle Citation8 was probably the first person to suggest the possibility that many plants may excrete something from their roots which is injurious to other plants. The term allelopathy was proposed by Molisch Citation25 and defined by Rice Citation32 as the effect of one plant on the growth of another through the release of chemical compounds into the environment. In 1996, the International Allelopathy Society revised the definition of allelopathy to include any process involving secondary metabolites produced by plants, algae, bacteria and fungi that influence the growth and development of biological and agricultural systems Citation18. This includes both the positive (stimulatory) and negative (inhibitory) effect. Though, some biologists restrict the use of this term to negative effects only Citation17. There are several examples of allelopathic interactions among marine algal species both in vitro and in situ Citation5 Citation10 Citation11 Citation14 Citation23 Citation28 Citation29 Citation33 Citation39 Citation40 Citation42. A review of different aspects of chemical ecology of microalgae, including allelopathy, can be found in Cembella Citation3.
The first mathematical representation of allelopathic interaction was probably proposed by Maynard-Smith Citation24. The author considered a two species Lotka-Volterra competitive system by assuming that each species produces an allelopathic substance at a constant rate to the other, but only when the other is present. Concerning allelopathy among phytoplankton, several models have examined and generalized the model proposed by Maynard-Smith Citation24. An exhaustive analysis of stability of a general two-species competition system including allelopathic interactions was carried out by Chattopadhyay Citation6. Since the production of the allelopathic substance is not instantaneous, but occurs after some discrete time lag required for the maturity of both species, Mukhopadhyay et al. Citation26 modified the system by considering the time delays due to maturities of the species. Further developments of this basic model to include effects of environmental fluctuations together with spatial and temporal heterogeneity in plankton populations also have been studied Citation27 Citation38. Sole et al. Citation36 modified the allelopathic interaction term and proved that the role of allelopathy appears to be important only for high concentrations of the toxic algae. Recently, using simple models, Jonsson et al. Citation19 supported the previous observation and confirmed that allelopathic (hereafter called a toxin) effect does not play any role to the initiation of algal blooms.
Though toxin provides a benefit to the toxic species by suppressing the growth of its competitors, a cost is always associated with the production of toxin. Naturally, the study of benefit and cost of toxin production is in an important issue in research. In a laboratory experiment, Pintar and Starmer Citation30 investigated the effect of cost in producing killer toxin for the yeast species, Pichia kluyveri, which is able to produce and excrete extracellular toxic potents that can kill other specific sensitive yeasts. Wolch-Salamon et al. Citation44 also presented a study of the combined effect of dispersal and nutrient availability on the cost and benefit of toxin production. Hsu and Waltman Citation16 considered mathematical model for two competing populations competing for a single resource where one of the competitors can produce a toxic chemical against its opponent at some cost to its reproductive abilities. Braselton and Waltman Citation2 investigated the role of density-dependent inhibitor production for two competing populations, one of which is toxic and a fraction of its potential growth is devoted to the production of toxin. Frank Citation13 also developed models for the dynamics of competition between producer and susceptible strains. He assumed that producer strains pay a cost for toxin production or plasmid carriage in terms of a relatively lower intrinsic rate of increase, but gain the benefit of killing a fraction of the susceptible competitors at a specified rate. But the interaction between two toxic species, where a cost is associated with the production of toxin, still has not been explored. Initiation of such theoretical study will encourage experimental ecologists to perform some experimental studies in this direction.
In this paper, we have concentrated upon the model considered by Chattopadhyay Citation6, where two toxin-producing phytoplankton species interact with each other. We have assumed that both phytoplankton can produce toxic chemicals against each other at some expense to their own growth. We have found that benefits of toxic killing outweigh their costs only when the cost of toxin production remains within some moderate value. We have also shown that the coexistence and extinction of species are regulated by these cost functions.
2. Model construction
The model, studied and analysed by Chattopadhyay Citation6 is the following
It is reasonable to consider the toxic concentration as a separate entity in the model formulation. We would like to mention that the inclusion of this mechanism has already been applied in chemostat models Citation2 Citation16. In 2008, Chakraborty et al. Citation4 also investigated the effect of nutrient limitation on the toxin production by considering the toxin as a different variable. It is to be noted here that photoxidation can cause rapid degradation of toxic compounds Citation10 Citation22 and the toxins are quickly inactivated by visible light in the 400–510 mm range and by ultraviolet light in the 225 mm range Citation7 Citation35.
The above observations encouraged us to modify system Equation(1). In the present study, we have considered a two species competitive system where each species, N
1 and N
2, produces a substance toxic to the other. We have represented the toxins produced by both the species, by separate variables, P
1 and P
2, respectively. d
1 and d
2 are the degradation of toxic substances produced by the first and second species, respectively. It is known that, for algal populations, there is a trade-off between growth rate and the metabolic costs associated with the changes produced for their own protection Citation41. In our model, we have made the assumption that a portion of the growth rates,
and
, respectively, of both the species is devoted to producing toxic chemicals.
Recent studies have revealed that allelopathic interactions among plankton become important only at the high cell concentrations typical for well-developed plankton blooms Citation12
Citation19
Citation33
Citation36. In 2005, Sole et al., modelled allelopathic effects among marine microalgae and quantified the importance of this interaction using experimental data. They suggested that the allelopathic effect should be a non-linear function of the amount of toxin present in the medium or, in other words, of the amount of toxic cells. They modified the mathematical form of the allelopathic interaction term proposed by Chattopadhyay Citation6
to
for non-toxic alga N
1 and toxic alga N
2, which suggests that the non-toxic alga is significantly affected by the allelopathy term only after the toxic alga has reached relatively high concentrations. In our study, we also consider a similar type of allelopathic interaction terms as
and
, which reveals that allelopathic interactions become important only when the amount of toxin produced by some species reaches high levels.
With these assumptions, system Equation(1) reduces to
Since all the parameters of system Equation(2) are non-negative and the right-hand side of system Equation(2)
is continuous variables (N
1, N
2, P
1, P
2) with the positive vector
, it can be easily shown that they are local Lipschitzian as well as positive invariance in R
4
+. Hence the solutions of EquationEquation (2)
with non-negative initial conditions exist for all future time and are unique. It can be easily shown that system Equation(2)
is bounded also.
The system Equation(2) possesses the following equilibria: E
0=(0, 0, 0, 0),
,
and the interior equilibrium point
. The equilibrium points E
1 and E
2 always exist for the assumptions k
1<1 and k
2<1. Ecologically, this is an appropriate assumption in the sense that plankton species do not devote all efforts to produce toxin only. We now seek the region of parameter space for which the model Equationequation (2)
admits feasible interior equilibria. Any feasible equilibria must correspond to a positive root N
1* of the equation f(N
1)=0, where
EquationEquation (3) has atleast one positive real root if A
5<0 which is satisfied when
. Therefore the sufficient condition for the existence of interior equilibrium point is as follows:
3. Local stability analysis
Now we observe the stability nature of the system Equation(2) around different biologically feasible equilibria. In the text, we state only the results and all proofs are appended in the appendix.
Theorem 1
The system
Equation(2)
is unstable around E
0
for all parametric values.
Proof
The proof is obvious and hence omitted. ▪
Theorem 2
The system
Equation(2)
is locally asymptotically stable (LAS) around E
1
if
where
and LAS around E
2
if
where
.
Proof
The proofs are given in the appendix. ▪
Theorem 3
The sufficient conditions for the LAS of the system
Equation(2)
around unique E* are as follows:
Proof
The proof is given in the appendix. ▪
Now, we are in a position to present a comparative observations between systems Equation(1) and Equation(2)
. To visualize the comparison, at a glance, we present a table (). We observe the following key points:
Table 1. Comparison of the analytical results between the systems Equation(1) and Equation(2)
and Equation(2) .
.
The following analytical results are immediate from .
| • | Both | ||||
| • |
| ||||
| • |
| ||||
| • | The necessary and sufficient condition for LAS of the interior equilibrium point,E*(0), of the system Equation(1) | ||||
4. Numerical experiment
For our numerical simulation, most of the fixed parameter values are taken from various literature sources and are given in . First we would show that the selected set of parameter values is numerically compatible with the experimental observations performed by Schmidt and Hansen Citation33. Schmidt and Hansen Citation33 investigated the importance of cell concentration, growth phase and pH to the harmful effects of Chrysochromulina polylepis on the dinoflagellate Heterocapsa triquetra. They also studied the effect of the initial concentration of C. polylepis on the growth response of H. triquetra in mixed cultures. They observed a decrease in the H. triquetra population on Day 4 in the culture with the highest initial C. polylepis concentration (); but, with the other two cultures which were initiated at lower C. polylepis concentrations, the decrease in H. triquetra numbers appeared some days later (Days 6 and 7). They also found that irrespective of the initial concentration of C. polylepis, the decline in H. triquetra numbers always began when the C. polylepis concentration reached ca 3×104 cells ml−1. In our system also (system Equation(2)), we have observed similar kind of observations for the set of parameter values taken from (). We have considered three different initial concentrations of N
1 (3, 5, and 7 cells ml−1) keeping fixed the initial concentrations of the other variables
. We observe that, for higher initial concentrations of N
1 (7 cells ml−1), N
2 starts to decrease on Day 3, but for the cases in which N
1 starts with lower initial concentrations (3 and 5 cells ml−1), decrease of N
2 starts a few days later (Days 4 and 5, respectively). We also observe that the concentration of N
2 starts to decrease only when the concentration of N
1 reaches 12 cells ml−1 irrespective of the initial concentrations of N
1. Therefore, the output of the numerical simulation of our model qualitatively resembles the experimental observations of Schmidt and Hansen Citation33.
Figure 1. Figure taken from Schmidt and Hansen Citation33.
Figure 2. Comparison of the outcome of system Equation(2) for different initial concentrations of N
1. The dotted curve is due to the initial condition (7, 0.1, 0.1, 0.1), thin line is due to (5, 0.1, 0.1, 0.1) and the bold line is for (3, 0.1, 0.1, 0.1). Parameter values used: k
1=0.4; k
2=0.2; d
1=0.3; d
2=0.1 and the other parameter values are taken from .
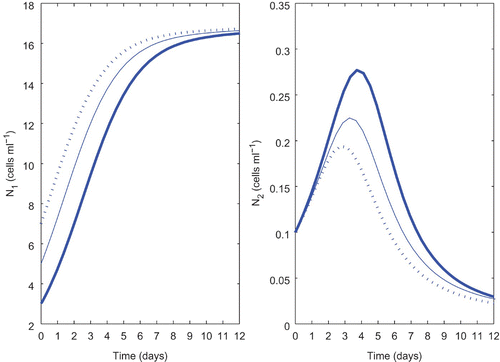
Table 2. The set of fixed parameter values taken from various literature sources.
Since it was quite unclear from the analytical results, we use numerical method to get a clear view regarding the coexistence and extinction of species for different k
1 and k
2. Here, mainly we investigate the effect of k
1 and k
2 upon the stability of system Equation(2). Specifically, the stability of the system is calculated numerically by testing the Routh–Hurwitz criteria. For this, we plot the stability of different equilibrium points in (k
1, k
2) space, where the the other parameter values are fixed as in (). Then for different parameter set-up, we locate the rest points, determine the eigenvalues of the Jacobian evaluated at the rest points, and solve the ordinary differential equation for different initial conditions.
Figure 3. Domains of stability and instability of system (2). Parameter values are taken from . The horizontal axis represents the fraction of potential growth devoted to production of toxin for N 1 (i.e. k 1). The vertical axis represents the fraction of potential growth devoted to production of toxin for N 2 (i.e. k 2).
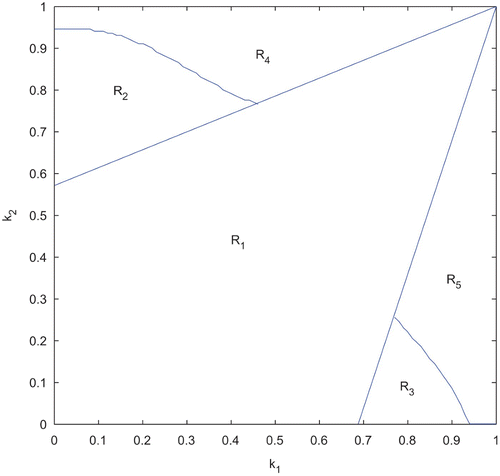
shows that, the whole parameter space is divided into five non-overlapping regions: R 1, R 2, R 3, R 4 and R 5. Note that, each of the regions bears some distinct unique characteristics regarding the stability of the equilibrium points and are listed below.
-
(1) R 1: E* is LAS.
-
(2) R 2: E* and E 1 are both LAS.
-
(3) R 3: E* and E 2 are both LAS.
-
(4) R 4: E 1 is LAS.
-
(5) R 5: E 2 is LAS.
It is clear from that, whenever k 1 and k 2 lie within some moderate values, coexistence occurs. But when the value of k 1 or k 2 becomes sufficiently high, it results the extinction of the first or second species, respectively. These findings are in accordance with our analytical observations as well.
Now, we take points from each of the three regions, R 1, R 2, and R 3 for analysing the existence, stability, and instability behaviour of the equilibrium points. First, we choose k 1=0.4 and k 2=0.4 from the region R 1 and the other parameter values are kept same as in . For such values, we find three interior equilibrium points among which two are stable and two boundary equilibrium points which are unstable in nature. The eigenvalues of the corresponding equilibrium points are indicated in . A plot of the trajectories is shown in . Since two interior equilibria are very close to two boundary equilibria, the enlarged version of these two portions are given in and . In this case, all the trajectories converge to either of the stable equilibrium points depending on the initial conditions.
Figure 4. Trajectories of the system (2) for k 1=0.4 and k 2=0.4 taken from the region R 1 of and the other parameter values are taken from . We observe the existence of three interior and two boundary equilibrium points among which two interior equilibrium points are LAS. (b) and (c) Two enlarged portions of (a).
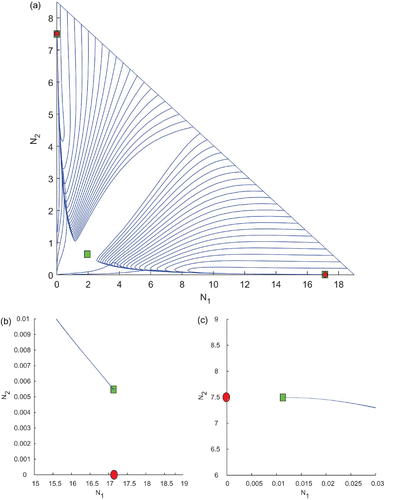
Table 3. Equilibrium points and their stability at k 1=0.4 and k 2=0.4.
Now, we move to region R 2 for the same purpose. We choose k 1=0.2 and k 2=0.8 from the region R 2, and keep the other parameter values same as in . This gives two biologically feasible interior equilibrium points and two boundary equilibrium points. Interestingly, we observe one positive equilibrium point and one boundary equilibrium point E 1 are stable. Whereas, the other interior equilibrium point and the boundary equilibrium point E 2 are unstable saddles. The basic computations are given in and a plot of the trajectories is shown in . Some trajectories converge to the interior equilibrium point, some of them converge to E 1 as shown in . An enlarge version of the portion where one interior and one boundary equilibrium point are close is given in . It is worthwhile to mention here that the trajectories of attractors are determined by the initial conditions.
Figure 5. Trajectories of the system Equation(2) for k
1=0.2 and k
2=0.8 taken from the region R
2 of and the other parameter values are taken from . We observe the existence of two interior and two boundary equilibrium points among which one interior and one boundary equilibrium points are LAS. (b) An enlarged portion of (a).
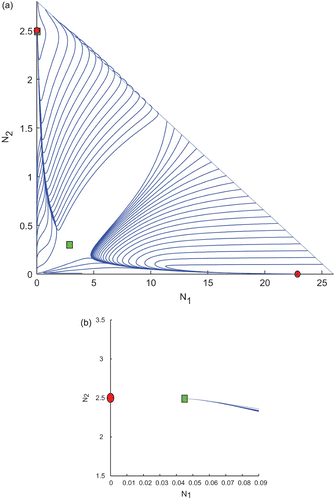
Table 4. Equilibrium points and their stability at k 1=0.2 and k 2=0.8.
Now, we take k 1=0.8 and k 2=0.1, from the region R 3. Here, the basic computations and the plot of the trajectories are given in and , respectively. Here, we also find the existence of two interior equilibrium points and two boundary equilibrium points among which one of the interior equilibrium points and the boundary equilibrium point E 2 are stable and the others are unstable saddles. In this case also, we would like to mention that the trajectories of attractors are determined by the initial conditions.
Figure 6. Trajectories of the system Equation(2) for k
1=0.8 and k
2=0.1 taken from the region R
2 of and the other parameter values are taken from . We observe the existence of two interior and two boundary equilibrium points among which one interior and one boundary equilibrium points are LAS. (b) An enlarged portion of (a).
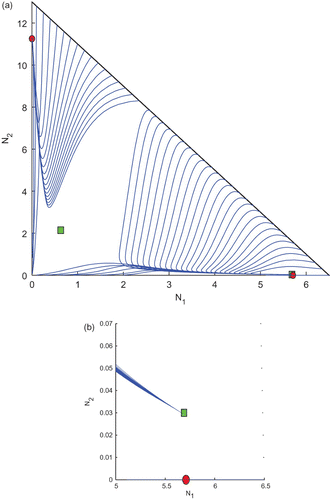
Table 5. Equilibrium points and their stability at k 1=0.8 and k 2=0.1.
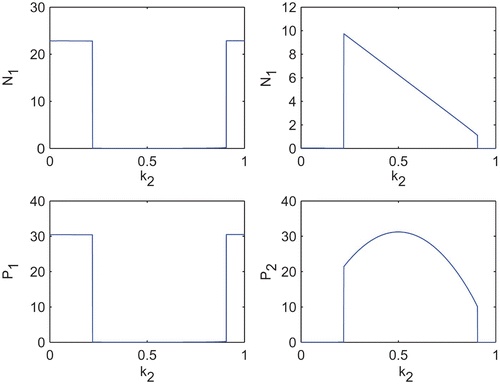
Next, we shall observe the effect of k 1 and k 2 upon the equilibrium abundances of both the species. For this, first we fix k 2=0.2 and vary k 1 (). We observe that, when the toxin production rate (in other words, cost of toxin production) remains very low, abundance of N 1 as well as its toxin production remains low. Whenever the toxin production rate increases and remains within some moderate values, N 1 abundance together with its amount of toxin production becomes significantly high and due to such high toxicity, N 2 abundance remains low. Now, again when the toxin production rate becomes very high, the metabolic cost due to toxin production also becomes very high and affects its growth; competitive advantage due to toxin production cannot compensate for the loss in growth and we observe a drastic fall in N 1 abundance. Though the toxin production rate remains high, but due to low abundance of N 1, the toxic effect on N 2 becomes low which helps to increase N 2. We also observe the similar behaviour of N 1 and N 2 but in an inverse way, when we vary k 2 and fix k 1=0.2 ().
Figure 7. Equilibrium abundances of the variables of the system Equation(2) for different k
1. Parameter values are taken from and k
2=0.2.
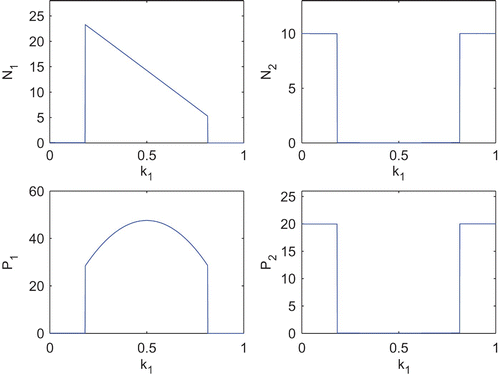
Figure 8. Equilibrium abundances of the variables of the system Equation(2) for different k
2. Parameter values are taken from and k
1=0.2.
5. Discussion
Phytoplankton species compete with each other for limiting resources, and consequently, they have evolved different strategies to better exploit a resource. Phytoplankton may compete by directly interfering with each other through the release of allelopathic compounds. Allelopathic compounds are secreted into the environment and conferring an advantage to the toxin producing species by killing or inhibiting the growth of other species. One of the main reasons for interest in allelopathy is that it could be one of the main factors that promotes the dominance of marine and freshwater harmful algal bloom (HAB)-forming species over other phytoplankton species Citation22. Though, the toxin provides a benefit to the toxin-producing species by suppressing the growth of its competitors, a metabolic cost is always associated with the production of toxin. These costs are energetically based. The energy and cellular machinery used to produce toxin cannot be used for reproduction and results in the reduction of population growth rate Citation30. In order for producers to invade a population of other species, these costs must therefore be compensated by benefits resulting from killing. On the other hand, the costs and benefits of toxin production depend on some environmental conditions Citation9 Citation13 Citation30 Citation44. Thus, the costs and benefits of toxin production may have some influence on the HAB dynamics. But, it is still not too clear under what conditions do the benefits of toxic killing outweigh the metabolic costs involved. In this study, emphasis has been placed on determining to what extent in toxin production can the toxic species inhibit its competitors.
To demonstrate this, we have considered a two species competitive system considering that each species produces a toxic chemical which is harmful to the other. We have allocated a cost for producing toxic chemicals and toxin concentrations by separate equations. We have assumed that allelopathic effects become significantly important when the amount of toxin present in the system becomes very high (it has been reported by Sole et al. Citation36; Jonsson Citation19). For numerical simulations, parameter values are taken from various literature sources. We have investigated the importance of initial cell concentrations on the abundance of other species. It is observed that, when a species starts with higher initial concentrations, it receives an advantage due to toxicity in the early days of the experiment. But, if the initial concentration of the species is low, its toxic effect starts to affect the other a few days later. We have also observed that the concentration of one species starts to decrease only when the concentration of another one exceeds some critical level. These findings are in accordance with the laboratory experiment of Schmidt and Hansen Citation33.
There are studies where the effect of costs and benefits of toxin production has been discussed. Both the costs and benefits of toxin production are dependent on environmental conditions Citation9 Citation13 Citation30 Citation44. In nature, plants are constrained to having less than maximum levels of toxin release Citation1 Citation15 Citation20 Citation31 Citation37 Citation43. In this study, we have also found such types of critical levels of toxin production. We observe that each toxic species gain an advantage in competition over the other by producing toxic chemicals until the metabolic costs associated with toxin production remains within some moderate values. Interestingly, within that range, the competitive advantage due to toxin production may to some extent compensate the loss due to the production of toxic chemicals. But, whenever it crosses the critical value, the metabolic cost due to toxin production also becomes very high and affects its growth. In this case, the metabolic cost outweighs the benefit of toxic killing; as a result abundance of that species decreases and further loss can results species extinction. Therefore, the output of our mathematical model supports the natural evidences. On the other hand, whenever the toxin production rate remains below some critical level, the benefit of toxic killing becomes very low, so that the abundance of the species remains very low in spite of its high growth rate. Thus, finally, we can conclude that, by releasing toxic chemicals, toxin-producing species gain an advantage in competition; but the release of toxic chemicals should remain within some moderate values, otherwise it can result the extinction of species itself. It is observed that cost of toxin production first affects the growth of the population, and then it goes to toxin production. Also, the abundance of the toxic cells has a great impact on allelopathic effect. In this respect, our result resembles the experimental outcomes of Schmidt and Hansen Citation33.
Acknowledgements
The authors are grateful to the referees for their extremely valuable comments and suggestion on the previous version of the manuscript.
References
- Baldwin , I. T. and Callahan , P. 1993 . Autotoxicity and chemical defense: Nicotine accumulation and carbon gain in solanaceous plants . Oecologia , 94 : 534 – 541 .
- Braselton , J. and Waltman , P. 2001 . A competition model with dynamically allocated inhibitor production . Math. Biosci. , 173 : 55 – 84 .
- Cembella , A. 2003 . Chemical ecology of eukaryotic microalgae in marine ecosystems . Phycologia , 42 : 420 – 447 .
- Chakraborty , S. , Roy , S. and Chattopadhyay , J. 2008 . Nutrient-limited toxin production and the dynamics of two phytoplankton in culture media: A mathematical model . Ecol. Model. , 213 : 191 – 201 .
- Chan , A. , Andersen , R. , Blanc , M. L. and Harrison , P. 1980 . Algal planting as a tool for investigating allelopathy among marine microalgae . Mar. Biol. , 84 : 287 – 291 .
- Chattopadhyay , J. 1996 . Effects of toxic substances on a two-species competitive system . Ecol. Model. , 84 : 287 – 289 .
- Colins , M. 1978 . Algal toxins . Microbiol Rev. , 42 : 725 – 746 .
- M.A.P. DeCandolle, Physiologic Vegetable, Vol. 111, Bechet Jeune Library Faculty Medicine, 1832
- Dykes , G. A. and Hastings , J. W. 1997 . Selection and fitness in bacteriocin-producing bacteria . Proc. R. Soc. B , 264 : 683 – 687 .
- Fistarol , G. O. , Legrand , C. and Graneli , E. 2003 . Allelopathic effect of Primnesium pervum on a natural plankton community . Mar. Ecol. Prog. Ser. , 255 : 115 – 125 .
- Fistarol , G. , Legrand , C. , Selander , E. , Hummert , C. , Stolte , W. and Graneli , E. 2004 . Allelopathy in alexandrium spp.: Effect on a natural plankton community and on algal monocultures . Aquat. Microb. Ecol. , 35 : 45 – 56 .
- Flynn , K. J. 2008 . Attack is not the best form of defence: Lessons from harmful algal bloom dynamics . Harmful Algae , 8 : 129 – 139 .
- Frank , S. A. 1994 . Spatial polymorphism of bacteriocins and other allelopathic traits . Evol. Ecol. , 8 : 369 – 386 .
- Gentien , P. and Arzul , G. 1990 . Exotoxin production by gyrodinium cf. aureolum (dinophyceae) . J. Mar. Biol. Assoc. , 70 : 571 – 581 .
- Gershenzon , J. and Croteau , R. 1991 . “ Terpenoids ” . In Herbivores: Their Interactions with Secondary Plant Metabolites , 2 , Edited by: Rosenthal , G. A. and Berenbaum , M. R. 165 – 219 . San Diego, CA : Academic Press .
- Hsu , S. B. and Waltman , P. 1998 . Competition in the chemostat when one competitor produces toxin . Japan J. Indust. Appl. Math. , 15 : 471 – 490 .
- Inderjit , D. and Foy , C. 1999 . Principles and Practices in Plant Ecology: Allelochemical Interactions , New York : CRC Press .
- International Allelopathy Society . 1996 . Constitution. Drawn up during first world congress on allelopathy: A science for the future Cadiz, , Spain Available at http://www-ias.uca.es/bylaws.htm # CONSTI
- Jonsson , R. , Pavia , H. and Toth , G. 2009 . Formation of harmful algal blooms cannot be explained by allelopathic interactions . Proc. Natl. Acad. Sci. USA , 106 ( 27 ) : 11177 – 11182 .
- Kuc , J. 1987 . “ Plant immunization and its applicability for disease control ” . In Innovative Approaches to Plant Disease Control , Edited by: Chat , I. 225 – 274 . New York, NY : John Wiley & Sons .
- Lancaster , P. and Tismenetsky , M. 1985 . The Theory of Matrices , 2 , Orlando, FL : Academic Press .
- Legrand , C. , Rengefors , K. , Fistarol , G. O. and Graneli , E. 2003 . Allelopathy in plankton – biochemical, ecological and evolutionary aspects . Phycologia , 42 ( 4 ) : 406 – 419 .
- Maestrini , S. and Granéli , E. 1991 . Environmental conditions and ecophysiological mechanisms which led to the 1988 Chrysocromulina polylepis bloom: An hypothesis . Oecologia Aquat. , 14 : 397 – 413 .
- Maynard-Smith , J. 1974 . Models in Ecology , Cambridge : Cambridge University Press .
- Molisch , H. 1937 . Der Einfluss einer Pflanze auf die andere – Allelopathie , Gustav Fischer Verlag Jena .
- Mukhopadhyay , A. , Chattopadhyay , J. and Tapaswi , P. K. 1998 . A delay differential equations model of plankton allelopathy . Math. Biosci. , 149 : 167 – 189 .
- Mukhopadhyay , W. , Tapaswi , P. and Chattopadhyay , J. 2003 . A space-time state-space model of phytoplankton allelopathy . Nonlinear Anal. , 4 : 437 – 456 .
- S. Myklestad, B. Ramlo, and S. Hestmann, Demonstration of strong interaction between the flagellate chrysolchromulina polylepis (prymnesiophyceae) and a marine diatom, Technical Report, Harmful Marine Algal Blooms, Workshop Report, 1995
- Nielsen , T. , Kiorboe , T. and Bjornsen , P. 1990 . Effects of a Chrysocromulina polylepis subsurface bloom on the planktonic community . Mar. Ecol. Prog. Ser. , 62 : 21 – 35 .
- Pintar , J. and Starmer , W. 2003 . The costs and benefits of killer toxin production by the yeast Pichia kluyveri . Antonie van Leeuwenhoek , 83 : 89 – 98 .
- Raffa , K. F. and Berryman , A. A. 1987 . Interacting selective pressures in coniferbark beetle systems: A basis for reciprocal adaptations . Am. Nat. , 129 : 234 – 262 .
- Rice , E. 1984 . Allelopathy , London : Academic Press, Inc .
- Schmidt , L. and Hansen , P. 2001 . Allelopathy in the prymnesiophyte Chrysocromulina polylepis: Effect of cell concentration, growth phase and pH . Mar. Ecol. Prog. Ser. , 216 : 67 – 81 .
- Liu , Z. and Chen , L. 2007 . Periodic solution of a two-species competitive system with toxicant and birth pulse . Chaos Solitons Fractals , 32 : 1703 – 1712 .
- Skulberg , O. M. , Carmichael , W. W. , Codd , G. A. and Skulberg , R. 1993 . “ Taxonomy of toxic Cyanophyceae (cyanobacteria) ” . In Algal Toxins in Seafood and Drinking Water , Edited by: Falconer , R. 145 – 164 . London : Academic Press Ltd .
- Sole , J. , Garcia-Ladona , E. , Ruardij , P. and Estrada , M. 2005 . Modelling allelopathy among marine algae . Ecol. Modell. , 183 : 373 – 384 .
- Tallamy , D. W. and McCloud , E. S. 1991 . “ Squash beetles, cucumber beetles, and inducible cucurbit responses ” . In Phytochemical Induction by Herbivores , Edited by: Tallamy , D. W. and Raupp , M. J. 155 – 181 . New York, NY : John Wiley & Sons .
- Tapaswi , P. and Mukhopadhyay , A. 1999 . Effects of environmental fluctuation on planktopn allelpathy . J. Math. Biol. , 39 : 39 – 58 .
- Tillmann , U. and John , U. 2002 . Toxic effects of alexandrium spp. on heterotrophic dinoflagellates: An allelochemical defence mechanism independent of psp-toxin content . Mar. Ecol. Prog. Ser. , 230 : 47 – 58 .
- Uchida , T. , Toda , S. , Matsuyama , Y. , Yamaguchi , M. , Kotani , Y. and Honjo , T. 1999 . Interactions between the red tide dinoflagellates Heterocapsa ciruclarisquama and Gymnodinium mikimotoi in laboratory culture . J. Exp. Mar. Biol. Ecol. , 241 : 285 – 299 .
- Van Donk , E. 1997 . Defenses in phytoplankton against grazing induced by nutrient limitation, UV-B stress and infochemicals . Aquatic Ecol. , 31 : 53 – 58 .
- Vardi , A. , Schatz , D. , Beeri , K. , Motro , U. , Sukenik , A. , Levine , A. and Kaplan , A. 2002 . Dinoflagellate-cyanobacteria communication may determine the composition of phytoplankton assemblage in a mesotrophic lake . Curr. Biol. , 12 : 1767 – 1772 .
- Whitham , T. G. 1983 . “ Host manipulation of parasites: Withinplant variation as a defense against rapidly evolving pests ” . In Variable Plants and Herbivores in Natural and Managed Systems , Edited by: Denno , R. F. and McClure , M. S. 15 – 41 . New York : Academic Press .
- Wloch-Salamon , D. , Gerla , D. , Hoekstra , R. and Visser , J. A. 2008 . Effect of dispersal and nutrient availability on the competitive ability of toxin-producing yeast . Proc. R. Soc. B , 275 : 535 – 541 .
Appendix
First of all, we state a lemma related to the characteristic polynomial of a matrix Citation21 and then study the stability properties of our system.
Lemma A1
Let A be an n×n matrix which is symmetrically partitioned into upper or lower triangular-block matrices labelled
Proof of Theorem 2
The Jacobian matrix for the system (2) about E 1 is the following
The above Jacobian matrix can be written as
Similarly, we can show that the necessary and sufficient condition for LAS of the system Equation(2) around E
2 is
Proof of Theorem 3
To determine the local stability character, we compute the Jacobian matrix of the system Equation(2) about E*, which is given below