 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We describe a new approach for investigating the control strategies of compartmental disease transmission models. The method rests on the construction of various alternative next-generation matrices, and makes use of the type reproduction number and the target reproduction number. A general metapopulation SIRS (susceptible–infected–recovered–susceptible) model is given to illustrate the application of the method. Such model is useful to study a wide variety of diseases where the population is distributed over geographically separated regions. Considering various control measures such as vaccination, social distancing, and travel restrictions, the procedure allows us to precisely describe in terms of the model parameters, how control methods should be implemented in the SIRS model to ensure disease elimination. In particular, we characterize cases where changing only the travel rates between the regions is sufficient to prevent an outbreak.
AMS SUBJECT CLASSIFICATION:
1. Introduction
In mathematical epidemiology, one of the most important issues is to determine whether an infectious disease can invade a susceptible population. The basic reproduction number (), defined as the expected number of secondary cases generated by a typical infected host introduced into a susceptible population [Citation1, Citation7, Citation17], serves as a threshold quantity for epidemic outbreaks. The next-generation matrix (NGM), initially introduced by Diekmann et al. [Citation7], provides a powerful approach to derive the basic reproduction number. This matrix (often denoted by
) gives the average number of new infections among the susceptible individuals of type i, generated by an infected individual of type j. The NGM is nonnegative, and
is identified as its dominant eigenvalue, that is,
.
If then the disease can persist in the population. For successful disease elimination, it is necessary to decrease
below 1, that may be achieved by implementing intervention strategies. Vaccination targets particular or all individual groups, and decreases the fraction of the population susceptible to the disease, thereby reducing the reproduction number. Another powerful tool in endemic situations is to decrease the probability of transmission, by reducing the interaction between particular groups within the population, or by reducing the contact between infected and susceptible individuals.
When modelling the prevention and control strategies of infectious diseases, the goal is to bring below 1 by controlling various model parameters. However, in many models the reproduction number is often obtained as a complicated expression of the parameters, and it may be difficult to determine how the parameters should be changed to decrease
. Entries of the NGM usually arise by less complicated formulas than that one of the reproduction number. Assume that by controlling model parameters, for each entry of the NGM a proportion more than
of the entry is reduced. Then it follows from the definition
(where
is the NGM) that the dominant eigenvalue of the NGM drops below 1 and the outbreak is prevented. Not only is the basic reproduction number a threshold for epidemic outbreaks, but it also determines the critical effort needed to eliminate infection from the population, provided that all entries of the NGM can be controlled.
In some situations, however, there are limitations in implementing intervention strategies, so there may be some entries of the NGM that are not subject to change. This was noted by Heesterbeek and Roberts [Citation10], Roberts and Heesterbeek [Citation13], and Shuai et al. [Citation15], who developed methods to decrease by reducing only particular elements of the NGM. The procedure of Heesterbeek and Roberts [Citation10] and Roberts and Heesterbeek [Citation13] applies to entire columns or rows of the NGM, and is based on the consideration that control is often aimed at only particular disease compartments, such as specific host types in multi-host models (e.g. vector control) or a particular group of individuals in heterogeneous population models. Shuai et al. [Citation15] extend the ideas of the above works, and address the cases where control targets the interactions between different types of individuals. The method of Shuai et al. [Citation15] reduces individual entries of the NGM, or sets of such entries. In both approaches mentioned above, new quantities are introduced – the type reproduction number in [Citation10,Citation13] and the target reproduction number in [Citation15] – that measure the strength of the effort needed to prevent outbreaks. However, when applied to specific disease transmission models, these procedures do not characterize in terms of the model parameters, how the intervention should be executed. In fact, control strategies are often aimed at particular model parameters rather than entries of the NGM.
In this paper, we address the gap in previous works, and present an approach for the design of control strategies that determines how model parameters should be changed to prevent outbreaks. Our procedure rests on various ‘alternative’ next-generation matrices that one can define for a disease transmission model. Applying this method, we systematically investigate the intervention strategies of a general SIRS (susceptible–infected–recovered–susceptible) model, that is appropriate for the spread of an infectious disease in a geographically dispersed metapopulation of individuals. While the qualitative properties of metapopulation (patchy) epidemic models have been widely studied in the literature, evaluating the intervention strategies in these models has received less attention (see, for instance, [Citation2, Citation3, Citation6, Citation11, Citation14, Citation18, Citation19] and the references therein). It is particularly challenging to understand the dependence of movement between populations on the reproduction number [Citation2, Citation4,Citation5]. Our procedure allows for the design of intervention strategies that target exclusively the movement of particular groups in the metapopulation SIRS model. Making use of the methods proposed in [Citation10, Citation13, Citation15], we identify controllable model parameters, and characterize various control strategies in terms of the targeted parameters. The procedure of how these parameters should be changed to execute control will be precisely described. We give conditions for cases where changing movement rates exclusively is sufficient for disease elimination, and provide recommendation for intervention in both local (patch-wise) and global scale.
The paper is organized as follows. After describing our approach in Section 2, we demonstrate the use of the method on a two-patch SIRS model in Section 3, where feasible control approaches will be systematically investigated. Section 4 is devoted to the intervention strategies of a more general metapopulation SIRS model in r patches. Finally, we discuss our findings in the last section.
2. Description of the method
First, we recall the main steps of the procedure described by Diekmann et al. [Citation8], for the calculation of the basic reproduction number in compartmental epidemic models. For this approach, the population of infected individuals is divided into discrete categories, and one needs to derive the average number of secondary cases per one infected individual in the various categories, in the initial phase of the epidemic. This way, the NGM is constructed (denoted by ), and
is identified as the dominant eigenvalue of the NGM, that is,
.
To derive the NGM, one identifies the infection subsystem in the compartmental model, that is, the equations that describe the generation of new infections and changes in the epidemiological statuses among infected individuals. The matrix of the linearization of the infection subsystem about the disease-free equilibrium (DFE) gives the Jacobian . Then,
is decomposed as
, where
describes the production of new infections (transmission part in the linear approximation), and
represents changes in status, as recovery or death (transition part in the linear approximation). Under the conditions that are satisfied in epidemic models, the inverse of
exists and
, and the product of
and
gives ‘the NGM with Large domain’ (see [Citation8]). In some cases (e.g. for SLIR-based models with latent period), further steps are required to obtain
(the NGM) from
, since the decomposition relates the expected offspring of individuals of any status (both latent and infected statuses in the SLIR model) and not just new infections. However, these matrices have the same spectral radii, that is,
. In SIR- and SIRS-type models, it holds that
. Nevertheless, it is meaningful to define
as
[Citation8].
The criterion saying that the disease can invade into the population if whereas it cannot if
, follows from the result that the dominant eigenvalue (the spectral radius) of
gives a threshold for the stability of the DFE [Citation8]. This result is shown in terms of M-matrices by van den Driessche and Watmough [Citation17]. We say that a square matrix
has the Z-sign pattern if all entries of
are non-positive except possibly those in the diagonal. If
has the Z-sign pattern and
holds then we say that
is a non-singular M-matrix (several definitions exist for M-matrices, see [Citation9, Theorem 5.1]). In the vast majority of epidemic models – including the ones considered in this paper – these conditions are satisfied for the matrix
. By the definition of
, it also holds that
is a nonnegative matrix.
Now, we discuss how to construct ‘alternative’ next-generation matrices. Besides the matrices for new infections and
for transfer between classes, there may exist different splittings of the Jacobian that satisfy the same conditions as
and
. Consider matrices
and
such that
,
is a nonnegative matrix and
is a non-singular M-matrix. Then, the matrix
, defined by
, serves as an alternative NGM. Albeit the NGM is not necessarily irreducible, here we only consider splittings such that
is irreducible. As
and
have the same properties as
and
, respectively, it follows that
and
agree at the threshold value 1. In fact, we can say more:
Proposition 2.1:
Consider a splitting of the Jacobian of the infected subsystem about the DFE, where
is a nonnegative matrix and
is a non-singular M-matrix. Then for the matrix
it holds that
if and only if
if and only if
and
if and only if
.
Proof:
By similar arguments as in the proof of Theorem 2 in [Citation17], we claim that if and only if
,
if and only if
, and
if and only if
, where
denotes the maximum real part of all eigenvalues of
. Note that this statement holds true for any
and
that satisfy the conditions of the proposition. The matrix for new infections
, and
for the transitions between infected statuses, give special cases of such
and
, respectively. We remind that
and
, that complete the proof.
Next, we give a brief overview of how the methods of Heesterbeek and Roberts [Citation10, Citation13], and Shuai et al. [Citation15] (see also [Citation16] for Erratum) work on the NGM. We follow the terminology of the latter as it generalizes the former. For the NGM , one identifies the set of targeted entries S, that is, the set of entries in
that are subject to change in control. The target matrix
is identified as
if
, and zero otherwise. The target reproduction number
is defined as
provided that
, where
is the identity matrix. The last condition can be referred to as the condition for controllability, since if the spectral radius is greater than 1 then the disease cannot be eliminated by targeting only S (in such case,
is not defined [Citation15]). The controlled NGM
is formulated by replacing the entry
in
by
whenever
.
Theorem 2.1 in [Citation15] states that if is irreducible and the condition for controllability holds, then
if and only if
. According to Shuai et al. [Citation15, Theorem 2.2], the controlled next-generation matrix satisfies
. Similar to the basic reproduction number, the target reproduction number
serves as a quantity to measure the effort needed to eliminate the disease, when control is applied on the set S.
Now, we are ready to describe a procedure that will allow us to design and systematically investigate the intervention strategies of compartmental epidemic models. Assume that and the disease can invade the population; otherwise no control is necessary. First, we identify a set of model parameters
that are subject to change in the control. Then, we decompose the Jacobian of the infected subsystem as
, to construct an alternative NGM
and
in the decomposition must satisfy the conditions of Proposition 2.1, moreover we only consider splittings such that
is irreducible. Next, we select the entries of
that depend on the parameters in Ω, and define the target set
as the set of the indices of the entries. With
the entry
depends on some of the parameters
for
, and otherwise
is independent of each parameter in Ω. Given
, we follow the description above to construct the target matrix
as
and obtain the controllability condition
Provided that the controllability condition holds, the target reproduction number is defined as
and the controlled alternative NGM
is formulated as
The assumption that , implies by [Citation15, Theorem 2.1] that
. The goal is to reduce the proportion
of all entries in
, since this way
is transformed into
and
implies that the disease can be eradicated (see [Citation15, Theorem 2.2]). Thus, our last step is to characterize how each targeted parameter
should be changed such that
is transformed into
. To formalize this, we think of
as a matrix that is dependent of the targeted parameters, and look for
such that
holds, where
is the set of targeted parameters after control. To this end, the functions
need to be identified that transform targeted parameters such that
Different control approaches (that is, different choices of the set of targeted parameters) may require the construction of different alternative next-generation matrices. We will see in the analysis of the proposed models that some splittings of the Jacobian are easier to handle than others. Each alternative NGM provides an alternative threshold quantity for disease elimination (see Proposition 2.1); this number, however, is not equal to the basic reproduction number. Hence, the significance of this alternative threshold quantity is that reducing it to 1 by means of epidemic control ensures disease elimination, but this number is not useful for estimating
.
The above-described procedure readily allows us to compare control approaches, by means of their properties as the controllability condition and the target reproduction number. We will give examples when the controllability condition (a condition of the model parameters) holds for one control strategy but cannot be satisfied for another. By the transformation of targeted parameters that ensures disease eradication, we can determine the critical control effort needed to prevent an outbreak. Doing so for each feasible intervention strategy, we become capable of evaluating the advantages of one over another. Hence, the analysis is applicable to provide recommendation, when it comes to making decisions about which control strategy is best to implement.
3. Control in a two-patch SIRS model
We consider the classical SIRS model in two patches that are connected by individuals' travel. In patch i (), we denote the total population at time t by
, whereas
,
, and
give the numbers of susceptible, infected, and recovered individuals, respectively, at time t. It holds for any
that
. Recruitment into the susceptible class of patch i is described by
, and
is the constant death rate. Disease transmission in patch i is modelled by the term
(standard incidence), where
is the constant transmission rate. We denote by
the recovery rate of infected individuals, and
is the rate of losing immunity. Note that if
then the model in patch i reduces to the classical SIR model, whereas with
it is assumed that the period of immunity is so short that it can be ignored, and we arrive at a model equivalent to the SIS model. To incorporate movements between the patches, we introduce the parameters
and
for the travel rate from patch 2 to 1, and from patch 1 to 2, respectively. Based on the above assumptions, we give the following system of ODEs to describe the spread of an infectious disease in and between two patches:
(M1)
(M1) For the dynamics of the total population in patch 1 and patch 2, we obtain the system
for which we assume that there exists a unique equilibrium
(if, for instance,
, or if the recruitment is constant, then this assumption is fulfilled). It is easy to see that
gives the unique DFE of the system (Equation1
(M1)
(M1) ).
We let , and define the local reproduction number in patch i (
) as
that gives a threshold for the stability of the DFE
in the absence of travelling. In the SIRS model (Equation1
(M1)
(M1) ), the infected subsystem reads
which we linearize at the DFE to give the
Jacobian matrix
To calculate the NGM, we decompose
into
, with
to separate new infections from transitions between disease classes in the linear approximation. The matrix
is nonnegative, and
has the Z-sign pattern and a nonnegative inverse (
is a non-singular M-matrix). We derive the NGM
and the basic reproduction number
Assuming that implying that the disease can invade into the population, potential control strategies may target transmission rates (
,
), travel rates (
,
), or a combination of those above. It is easy to see that decreasing both
and
will decrease all elements of
, and hence
as well. However, it is difficult to tell from the formulas of
and
if controlling travel rates can contribute to disease elimination. To answer the above question, it is more convenient to decompose the Jacobian in a way different from
. With the splitting
,
the alternative NGM
arises as
It is easy to check that
is nonnegative,
is a non-singular M-matrix, and
is irreducible. By Proposition 2.1 and the assumption that
, it follows that
. We identify three possible approaches for control:
control targets one or both of the transmission rates
and
;
control targets one or both of the travel rates
and
;
a combination of the above two.
3.1. The approach (A)
We begin with investigating the approach (A), which covers intervention strategies that decrease the probability of transmission, like social distancing. We first show conditions when controlling a single transmission rate is sufficient for disease elimination. Assume we want to change . This parameter appears in only one entry of
, hence the target set is
. The target matrix
is defined as
and
otherwise, so the controllability condition
reads
(1)
(1) If the condition (Equation2
(1)
(1) ) holds, then the definition of the target reproduction number – as the dominant eigenvalue of
– is meaningful; this number reads
that is larger than 1 because of
([see 15,]Theorem 2.1]). Control is executed as we replace the targeted entry
by
in the next-generation matrix
; this way, we arrive to the controlled matrix
corresponding to the target set S, and it holds that
. Such transformation on the matrix is achieved as we replace
by
in
, and leave all other parameters intact. By
it is clear that
, that means that the transmission rate needs to be decreased for disease elimination.
Note that if then the condition (Equation2
(1)
(1) ) is never satisfied, otherwise by the computations (equivalent to Equation (Equation2
(1)
(1) ))
we obtain that if
then targeting
alone is sufficient for control. However, if
then controllability depends on the travel rates, and it follows that the above inequality is satisfied if
is sufficiently large, moreover it can also hold for small
if
. These arguments suggest that mutual control of
and
(that is, decreasing
) is always sufficient for disease elimination, moreover the approach (C) that involves the travel rates might also be successful.
Indeed, let for the mutual control of
and
, so we have
and obtain the condition for the controllability
(2)
(2) that is satisfied for any travel rates. The target reproduction number
is defined as
and
implies by (see [Citation15, Theorem 2.1]) that
. The controlled matrix
corresponding to the target set U, arises as we replace
by
,
. It follows that the diagonal elements of
decrease, that is achieved by reducing
and
to
and
, respectively.
3.2. The approach (C)
The approach (A) might be insufficient for disease elimination in situations when it is not possible to control both transmission rates. If is targeted through
but
cannot be controlled, then based on the arguments above, intervention strategies must be extended to travel rates (unless
and
are already such that
and
hold, in which case the condition (Equation2
(1)
(1) ) is satisfied).
Assume that we can control the transmission rate and the travel rate of individuals in patch 1, that is, and
are subject to change. Such intervention affects the two entries
and
, so the target set is defined as
, and the target matrix
is defined as
,
,
,
. We assume that the controllability condition
(3)
(3) holds, and give the target reproduction number
Again,
follows from
and [Citation15, Theorem 2.1], that implies that the targeted entries of
need to be decreased. In the controlled matrix
corresponding to W, we have
,
.
The entry is zero at
, and monotonically increasing in
. Thus for every
there exists a unique
such that
is equal to
. Once we found
, we need
such that
and
are equal. From the linearity of
in
it is clear that there exists such
, that is unique and smaller than
.
Summarizing, controlling the epidemic by decreasing the transmission rate of region 1 () and the rate of travel outflow from region 1 (
) is possible; in fact, the controlled parameters are given as
Our results for the control approaches (A) and (C) are illustrated in Figure . In the numerical simulations, we let , so the total population of the two patches (denoted here by
) is constant. In the DFE it must hold that
, that is ensured with
,
. We let
,
,
for the initial conditions, and choose parameter values as
,
years,
days,
(
),
,
,
,
, that makes
. Figure (a) shows that reducing
is sufficient for disease elimination if the condition (Equation2
(1)
(1) ) is satisfied. If, however, a higher outflow rate
from the patch 1 is considered, then the condition (Equation2
(1)
(1) ) does not hold, yet
and a different approach is necessary. As illustrated in Figure (b), the condition (Equation4
(3)
(3) ) is satisfied and the approach (C) can be applied, that includes the control of
and
.
Figure 1. Morbidity curves of patch 1 (red) and patch 2 (blue), without control (solid curves) and with control (dashed curves). We let (
),
,
, and
for (a) and
for (b). Other parameters are as described in the text. Figure (a): When
, then
(solid curves), the condition (Equation2
(1)
(1) ) is satisfied (
), so we calculate
and
. Choosing
(dashed curves), the reproduction number drops below 1 (see in the bracket) and the outbreak is prevented. Figure (b): When
, then
(solid curves), the condition (Equation4
(3)
(3) ) is satisfied (
), so we calculate
and
,
. Choosing
and
(dashed curves), the reproduction number drops below 1 (see in the bracket) and the outbreak is prevented.
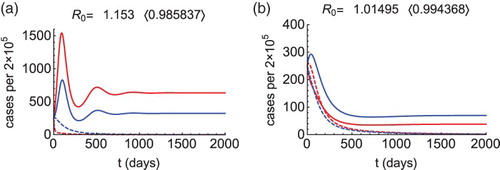
Despite the fact that in some cases changing only is sufficient for disease elimination, it is beneficial to include further parameters in the intervention strategy because it requires less effort. Following the terminology of Shuai et al. [Citation15], the strategies defined by the sets W and U are stronger than S since
and
. Then, by [Citation15, Theorem 4.3] it holds that
and
, provided that the target reproduction numbers are well defined (that is, the conditions for the controllability are satisfied). For each strategy, the controlled transmission rate
is defined as we divide
by the target reproduction number. Hence, the relationship between
,
, and
implies that in the strategy S that changes only
, the transmission rate needs to be decreased more compared to when other parameters are also involved (
in the strategy U, and
in the strategy W). Moreover, the conditions for controllability (Equation3
(2)
(2) ) and (Equation4
(3)
(3) ) in the strategies U and W, respectively, are less restrictive than the condition (Equation2
(1)
(1) ) in the strategy S, that means that stronger strategies can be applied more widely.
3.3. The approach (B)
We investigate the approach (B) for the control of the epidemic with changing the travel rates exclusively. We first show two situations when movement has no effect on whether an outbreak occurs. A standard result for nonnegative matrices (see, e.g. [Citation12, Theorem 1.1]) says that the dominant eigenvalue of a nonnegative matrix is bounded below and above by the minimum and maximum of its column sums. Using basic calculus, we derive bounds for the column sums of as
and
Thus, if
and
then the dominant eigenvalue of
is larger than 1, that also implies
; with other words, if both local reproduction numbers are greater than 1 then so is
, and no travel rates can reduce it below 1. On the other hand, when both
and
are less than 1 then it holds for every
that
which is equivalent to
, so the DFE is locally asymptotically stable and movement is unable to destabilize the situation.
If, however, but
then
, and epidemic control might be necessary. In fact, with the approach (C) we are unable to apply the method of the target reproduction number on the alternative NGM
. The approach (C) targets one or both of the travel rates, so assume without loss of generality that
is subject to change. For those two entries of
that depend on this parameter, we note that the monotonicity of
in
is opposite of that of
. This means that the procedure of reducing related entries of
cannot be successful without controlling
and/or
.
We can, however, use another alternative NGM, that has the same properties as and
. Define
that satisfy
, and
is a nonnegative matrix by
. If there is no travel outflow from the patch 2 then it is clear from
that the outbreak cannot be prevented. Otherwise,
and
is a non-singular M-matrix, with nonnegative inverse. Thus,
gives an alternative NGM, which is also irreducible.
Our target set is
, the target matrix
is given by
,
,
,
, and the controllability condition reads
(4)
(4) that holds since
. The target reproduction number is calculated as
and by Proposition 2.1,
is equivalent to
, hence
(see [Citation15, Theoreom 2.1]).
The controlled matrix corresponding to the strategy Z, is defined by
,
, while control does not affect the first row of
. To determine how this transformation of
is achieved in terms of the targeted parameters, we need to derive
and
that satisfy
,
. To this end, we solve the system
that reduces to
It follows that
and
, which means that the travel inflow rate into patch 1 with
(that rate is also the travel outflow rate of patch 2 with
) needs to be increased, and the other travel rate must remain unchanged.
We close this section with some concluding remarks. Three control approaches were investigated for the SIRS model with individuals' travel between two patches. Intervention strategies that target transmissibility are powerful tools in epidemic control; as shown in this section, preventing outbreaks by reducing the transmission rates and
, is possible for any movement rates and for any value of the basic reproduction number
. We also described cases in the approach (A) when changing (reducing) only one of the transmission rates is sufficient, and showed that allowing the additional control of travel rates requires less effort. In particular, if
then
and no control is necessary, but if
and
then bringing the basic reproduction number below 1 is possible by targeting
and
if
holds. Hence, the approach (C) is successful if
and
(since
), but more interestingly, the strategy might also be feasible even when
, if
is such that
. Biologically, the case when
,
, and
means that if the travel rate from an endemic area (patch 2) is large enough, then disease control is feasible by decreasing the transmission rate in the non-endemic patch (patch 1) and reducing the travel inflow to the endemic area. See Figure (a) that illustrates this phenomenon. We let
,
,
,
, and other parameters are as described for Figure .
Figure 2. Morbidity curves of patch 1 (red) and patch 2 (blue), without control (solid curves) and with control (dashed curves). We let (
),
,
,
. Other parameters are as described in the text. These parameters make
(solid curves). Figure (a): The condition (Equation4
(3)
(3) ) is satisfied (
), so we calculate
, and
,
. Choosing
and
(dashed curves), the reproduction number drops below 1 (see in the bracket) and the outbreak is prevented. Figure (b): The condition (Equation5
(4)
(4) ) is satisfied (
), so we calculate
, and
. Choosing
(dashed curves), the reproduction number drops below 1 (see in the bracket) and the outbreak is prevented.
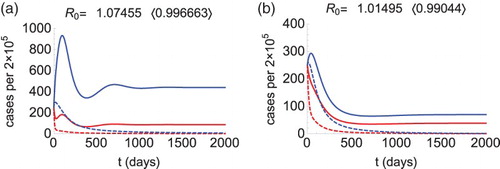
Lastly, we investigated for the approach (B) whether epidemic control is possible without changing any of the transmission rates. If both local reproduction numbers are greater than 1 then it is impossible for movement to prevent the outbreak, since is greater than 1 for any travel rates. On the other hand, we learned that
can be reduced to 1 by increasing the inflow rate to a patch where the local reproduction number is less than 1. Figure (b) illustrates such a case, where
,
, and
, so we increase
to eliminate the disease. We point out that if both local reproduction numbers are below 1 then movement cannot destabilize the DFE, hence no outbreak will occur.
4. A generalized SIRS model for r patches
In this section, control strategies are investigated in a general demographic SIRS model with individuals' travel between r patches, where is positive integer. Understanding the dynamics of such high-dimensional models remains a challenging problem in mathematical epidemiology. We give the system of
ODEs
(M2)
(M2) The parameter
is the travel rate in the class X, from region i to j (
,
,
), and we define
for
,
. Besides that we allow different movement rates of the three disease classes, it is also incorporated that the disease increases mortality by rate
. All other parameters, model variables and functions have been introduced in Section 2. Following the arguments made for a similar model in [Citation6], we assume that there is a unique DFE
in the model (Equation6
(M2)
(M2) ). With
, we define the local reproduction number of patch i as
. With
, the infected subsystem is obtained as
We define
as the movement matrix of infected individuals, and
as the total outflow of infected individuals from region i,
:
In the sequel, we will simply say ‘movement matrix’ for
and ‘total outflow from region i’ for
, and the reference to infected individuals will be omitted. It is reasonable to assume that
is irreducible. Otherwise, the patches are not strongly connected with respect to the disease, so a subsets of the patches can be constructed to where the epidemic cannot spread from other patches. Linearization of the infected subsystem about the DFE gives the Jacobian
, as
The basic reproduction number is defined as we follow the usual procedure of decomposing the Jacobian as
, where
is the matrix representing new infections and
represents transitions between and out of infected classes. It is easy to see that
and
has the Z-sign pattern. As
is diagonally dominant, the equivalence of the properties 3 and 11 in [Citation9, Theorem 5.1] implies that
exists and it is nonnegative. Following van den Driessche and Watmough [Citation17], the basic reproduction number
is defined as the dominant eigenvalue of the NGM
, that is,
. Note that the entries of
and
arise by complicated expressions, and hence no closed formula is derived for
.
An alternative way to decompose the Jacobian is , where
is nonnegative and
has the Z-sign pattern, so with
an alternative NGM arises. By Proposition 2.1,
gives another threshold quantity for the stability of the DFE; more precisely,
if and only if
,
if and only if
, and
if and only if
. The alternative NGM
is obtained as
that is irreducible since
is irreducible.
Assume that the disease can invade into the population and , that is equivalent to
(see Proposition 2.1). Intervention strategies can potentially target:
various transmission rates;
various movement rates;
the combination of the above, in frames of local control.
For the model (Equation1(M1)
(M1) ) in Section 3 for two patches, the control approaches (A), (B), and (C) have been thoroughly investigated. We derived precise conditions for controllability and described in details the procedures that lead to the decrease of
to 1 (that is, we gave the formulas for the targeted parameters in the various strategies). In this section, we present theorems that generalize to r regions our results obtained for the 2-patch SIRS model (Equation1
(M1)
(M1) ). We also derive novel conclusions.
Proposition 4.1:
If then there is at least one patch with local reproduction number greater than 1. If the local reproduction number is greater than 1 in all patches then it holds that
that is equivalent to
.
Proof:
Indeed, we look at the column sums of to give upper and lower bounds on the dominant eigenvalue. As the column sum in column j is
, we derive by the result of Minc [Citation12, Theorem 1.1] that
The expression
is increasing in x if
, and it is bounded above by 1, hence if
for every
then
follows. The last inequality is equivalent to
that contradicts our assumption that
, hence there must be an i such that
.
On the other hand, decreases in x if
, and it is bounded below by 1. Summarizing, we have
thus 1 gives the lower bound of
if all local reproduction numbers are greater than 1. The last statement implies that if
in all patches then
is greater than 1 for any travel rates. This completes the proof.
Theorem 4.2 (For the approach (A))
The epidemic can be controlled by decreasing the transmission rate in some regions, if the local reproduction number is less than 1 in all other patches. This implies that decreasing the transmission rate in all regions with local reproduction numbers greater than 1, can be sufficient for epidemic control. In particular, the intervention strategy where all transmission rates are subject to change, leads to disease elimination.
Proof:
Since by assumption, there is an
such that
. Without loss of generality, we can assume that
for
whereas
for
(
).
First, consider that are targeted and
are not subject to change. The target set is
, the target matrix is
, and the controllability condition reads
(5)
(5) Column sums of the matrix
are calculated as
Obviously,
for
, and it is easy to check that
if
, that implies that
for
. It is known that the dominant eigenvalue of a nonnegative matrix is bounded above by the maximum of the column sums (see [Citation12, Theorem 1.1]), so applying this result to
we obtain that the condition (Equation7
(5)
(5) ) for controllability holds.
The target reproduction number for the strategy S is given by
and we define the controlled transmission rates as
,
. For
, we replace
by
in
and arrive to the controlled matrix
, that satisfies
(see [Citation15, Theorem 2.2]). The assumption that
implies by Proposition 2.1 and [Citation15, Theorem 2.1] that
, hence targeted transmission rates need to be reduced for successful control.
Theorem 4.3 in [Citation15] says that extending the control strategy to a wider set of entries of requires less effort for disease elimination. If, in addition to
, we also control the transmission rates
(
), then some regions with local reproduction numbers less than 1 are also targeted. However, it remains true that
for all
, that is, for all j such that
is not subject to change. The target set is
, and the control strategy U is stronger than S because of
. Since
, it holds that
, and by a basic result on nonnegative matrices (see, for instance, [Citation9, Lemma 4.6]) we obtain
, that implies that the strategy U is also feasible for control. Theorem 4.3 in [Citation15] says
, thus, stronger control strategies require less effort. Note that these conclusions are valid for the case when
, that is, when all transmission rates are targeted.
Theorem 4.3 (For the approach (C))
Local control in some regions, that involves the control of transmission rates and travel outflow of those regions, can be sufficient if in all other regions. This implies that if all patches with
are under control then the outbreak can be prevented. In particular, the intervention strategy where all transmission rates and travel rates are subject to change, leads to disease elimination.
Proof:
We have seen that for it is necessary that
for some i. As
holds for any
if
, we can assume without loss of generality that there is a
such that
for
, and
for
.
If the patches are under local control, then the parameters
and
are subject to change, where
,
,
, so we introduce
for the set of targeted parameters. The target set (of entries in the NGM
) is
with
, the target matrix
is defined as
if
and 0 otherwise, and the controllability condition reads
(6)
(6) The matrix
is lower triangular with a zero-block in the diagonal and another diagonal block of size
, that we denote by
:
Due to the special structure of
, the dominant eigenvalue arises as the dominant eigenvalue of the square matrix
. Again, by [Citation12, Theorem 1.1] and the assumption that
for
, we obtain that
that implies that the controllability condition (Equation8
(6)
(6) ) holds. We can thus define the target reproduction number
for the strategy W, that is greater than 1 because of
. For successful control, each parameter in the set
needs to be changed such that
for
, where
is the set of targeted parameters after the control. This way,
is equal to the controlled next-generation matrix
, and
follows from
(see [Citation15, Theorem 2.2]).
Controlled parameters need to satisfy the systems
that are pairwise independent so it is sufficient to solve one of them (e.g. the first one), and then generalize. To find the controlled parameters
, we first solve the system
where
. We obtain that
whenever
,
, thus there is a
such that
for every j such that
. If
for some j then define
. It follows that
, and
has to be satisfied, so
is given by
. It is easy to see that
, which means that travel outflow rates from patch 1 need to be decreased for disease elimination. However, the transmission rate of patch 1 needs to be changed such that
is satisfied. Using
we derive the controlled transmission rate
, that is smaller than
since
. The constant
gives the general reduction parameter for patch 1, and one can similarly define
for the rest of the patches that undergo local control.
Similarly as for Theorem 4.2, one can show that less effort is needed for local control if more patches contribute to the intervention (including when all transmission rates and movement rates are subject to change).
Note that the conditions of Theorem 4.3 allow successful disease prevention when in some regions that are not part of the intervention strategy. This is in contrast to the findings of Theorem 4.2, that say that all patches with local reproduction number greater than 1 must be targeted. There are, although, further conditions in Theorem 4.3 that need to hold true, but they are weaker than those in Theorem 4.2, meaning that the results of Theorem 4.3 can be applied more widely than the results of Theorem 4.2. In the same time,
holds for the sets of targeted entries in Theorems 4.2 and 4.3, that again explains why the controllability condition is weaker and the target reproduction number is smaller in the latter than in the former one.
Theorem 4.4 (For the approach (B))
Assume that there are some patches where
and
holds for the patches
. Assume that from each patch
there is a single outflow link. Then, the outbreak can be prevented by increasing the travel outflow of the patches
.
Proof:
From the assumption that for
, it follows that
. Define
It is easy to see that is a nonnegative matrix and
is a non-singular M-matrix, moreover
yields a splitting of the Jacobian. Hence
gives another alternative NGM
,
that is irreducible because
is assumed irreducible. It follows by the properties of
and
that
and
agree at 1, and
implies
(see Proposition 2.1).
By assumption, for every there is a
such that
while all other travel rates from patch i are zero. This is equivalent to
while all other non-diagonal elements in the column are 0. Moreover, by the irreducibility assumption on
, there is an
such that
. With words, for each patch
there only is a single way out, and at least one of these patches connects to a patch with index
. The last assumption guarantees that the block
is not identically zero (otherwise
would be reducible).
When are targeted then only the entries
are subject to change. Indeed, all non-diagonal elements in the columns
are either 1 or 0, thus constants. Similarly as in Theorem 4.2 for the alternative NGM
, we choose
for the target set, hence the target matrix is
, and the controllability condition reads
(7)
(7) Similarly as in Theorem 4.2, we argue that the dominant eigenvalue of
is bounded above by the maximum of the column sums. Note that the column sum equals 1 in columns
, and is less than 1 in columns
, as
for
. Thus, the dominant eigenvalue of
is less than or equal to 1, and now we show that 1 is not an eigenvalue of
; then, these statements yield that the condition (Equation9
(7)
(7) ) holds.
Assume that 1 is an eigenvalue of . Then, there is a positive left eigenvector
associated to 1, and the equality
(8)
(8) is satisfied. Again, the column sums of
are less than 1 in the columns
, so we derive
for
, hence it follows that
(9)
(9) For each patch
, there is a unique outflow link
. By the irreducibility assumption, there is no closed loop of links within
, so every patch i is linked (possibly via other patches) to a patch outside of
. Without loss of generality, we can assume that the structure of the movement network is
where
and
,
. The sets
,
are disjoint and the union gives
. Recall that for each
the column i of
contains a single non-zero element,
. Hence, using Equation (Equation10
(8)
(8) ), we derive that
for
, and with the movement network given above, we obtain the following equalities:
From the above equations, we derive that for every
there is a
such that
. However, this contradicts (Equation11
(9)
(9) ). Summarizing, we showed that the condition (Equation9
(7)
(7) ) for controllability holds.
The target reproduction number can be defined in the usual way
and the strategy to decrease the targeted entries of the NGM
is executed as one replaces
by
in
, for
(note that
appear in the denominators of the targeted entries). Each
that is subject to change, is a single travel rate
. The procedure yields the controlled matrix
that satisfies
(see [Citation15, Theorem 2.2]).
Theorem 4.4 describes a way to apply the intervention approach (B) (changing movements rates only) on a special movement network. The question, whether the approach of controlling movement rates exclusively, is possible on more complex movement networks (that is, when the restriction on the travel outflows is lifted), remains open. However, the results of Theorem 4.4 enable us to give recommendation for designing intervention strategies. We have seen that, with changing movements only, the outbreak cannot be prevented if all local reproduction numbers are greater than 1; however, if there are patches with then the regions with
can potentially reallocate their travel outflow volumes in a way such that the conditions of Theorem 4.4 hold. In this case, the procedure described in the proof of Theorem 4.4 provides instructions for control such that the reproduction number
is decreased to 1. Note that the approaches (B) and (C) that include the control of movement, only aim at the travel rates of infected individuals, and such interventions do not require any restriction on the movement of non-infecteds. Increasing the travel outflow of an infected class is equivalent to shortening the period of stay in that class; such control measure is applied upon entry screening at airports, when infected individuals are denied entrance and after spending only a few hours at the airport, they fly back to their original location.
Summarizing, Theorems 4.2–4.4 provide various strategies for successful intervention. Control of transmission rates and movement rates (potential cancellation of some travel routes) are powerful tools in epidemic prevention and intervention.
5. Discussion
We illustrated with a demographic metapopulation SIRS model, how our method described in Section 2 can be used to design intervention strategies for disease transmission models. Considering public health measures like social distancing (reducing the likelihood of transmission) and travel restrictions between distant locations, we determined the critical efforts required for disease elimination, and compared these intervention approaches to provide recommendation for more effective control strategies. In particular, we demonstrated that controlling only the movement of infected individuals may be sufficient for preventing an outbreak.
The SIRS model in Section 4 is applicable to an array of communicable diseases that spread in spatially heterogeneous populations. However, the methodology described in Section 2 can be readily used to investigate the control strategies of compartmental models more general than the SIRS model. Based on the dynamical properties of the infection classes in the initial phase of an epidemic, the procedure in Section 2 allows for the construction of alternative next-generation matrices, each designed for a particular control strategy. This way, we are better able to understand the dependence of the dynamics on targeted model parameters, even in high-dimensional models in which these relations are rather complex. Such knowledge greatly contributes to the design of more successful intervention strategies.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- R.M. Anderson and R.M. May, Infectious Diseases of Humans, Oxford University, Oxford, 1991.
- J. Arino, Diseases in metapopulations, in Modeling and Dynamics of Infectious Diseases, Series of Contemporary Applied Mathematics. Vol. 11, Higher Education Press, Beijing, 2009, pp. 65–123.
- J. Arino, J.R. Davis, D. Hartley, R. Jordan, J.M. Miller, and P. van den Driessche, A multi-species epidemic model with spatial dynamics, Math. Med. Biol. 22(2) (2005), pp. 129–142. Available at http://dx.doi.org/10.1093/imammb/dqi003.
- J. Arino, R. Jordan, and P. van den Driessche, Quarantine in a multi-species epidemic model with spatial dynamics, Math. Biosci. 206(1) (2007), pp. 46–60. Available at http://dx.doi.org/10.1016/j.mbs.2005.09.002.
- J. Arino and P. van den Driessche, A multi-city epidemic model, Math. Popul. Stud. 10(3) (2003), pp. 175–193. Available at http://dx.doi.org/10.1080/08898480306720.
- J. Arino and P. van den Driessche, Disease spread in metapopulations, Nonlinear Dyn. Evol. Equ. 48 (2006), pp. 1–13.
- O. Diekmann, J.A.P. Heesterbeek, and J.A.J. Metz, On the definition and computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations, J. Math. Biol. 28 (1990), pp. 365–382. doi: 10.1007/BF00178324
- O. Diekmann, J.A.P. Heesterbeek, and M.G. Robert, The construction of next-generation matrices for compartmental epidemic models, J. R. Soc. Interface 7 (2010), pp. 873–885. doi: 10.1098/rsif.2009.0386
- M. Fiedler, Special Matrices and Their Applications in Numerical Mathematics, Martinus Nijhoff Publishers, Dodrecht, The Netherlands, 1986.
- J.A.P. Heesterbeek and M.G. Roberts, The type-reproduction number T in models for infectious disease control, Math. Biosci. 206(1) (2007), pp. 3–10. Available at http://dx.doi.org/10.1016/j.mbs.2004.10.013.
- Y. Jin and W. Wang, The effect of population dispersal on the spread of a disease, J. Math. Anal. Appl. 308(1) (2005), pp. 343–364. Available at http://dx.doi.org/10.1016/j.jmaa.2005.01.034.
- H. Minc, Nonnegative Matrices, John Wiley and Sons, New York, 1988.
- M.G. Roberts and J.A.P. Heesterbeek, A new method to estimate the effort required to control an infectious disease, Proc. R. Soc. London, Ser. B 270 (2003), pp. 1359–1364. doi: 10.1098/rspb.2003.2339
- M. Salmani and P. van den Driessche, A model for disease transmission in a patchy environment, Discret. Contin. Dyn. Sys. Ser. B 6(1) (2006), pp. 185–202.
- Z. Shuai, J.A.P. Heesterbeek, and P. van den Driessche, Extending the type reproduction number to infectious disease control targeting contacts between types, J. Math. Biol. 67(5) (2013), pp. 1067–1082. Available at http://dx.doi.org/10.1007/s00285-012-0579-9.
- Z. Shuai, J.A.P. Heesterbeek, and P. van den Driessche, Erratum to: Extending the type reproduction number to infectious disease control targeting contacts between types, J. Math. Biol. 71(1) (2015), pp. 1–3. Available at http://dx.doi.org/10.1007/s00285-015-0858-3.
- P. van den Driessche and J. Watmough, Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission, Math. Biosci. 180(1) (2002), pp. 29–48. Available at http://dx.doi.org/10.1016/S0025-5564(02)00108-6.
- W. Wang and G. Mulone, Threshold of disease transmission in a patch environment, J. Math. Anal. Appl. 285(1) (2003), pp. 321–335. Available at http://dx.doi.org/10.1016/S0022-247X(03)00428-1.
- W. Wang and X.Q. Zhao, An epidemic model in a patchy environment, Math. Biosci. 190(1) (2004), pp. 97–112. Available at http://dx.doi.org/10.1016/j.mbs.2002.11.001.
