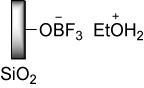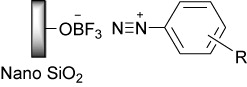Abstract
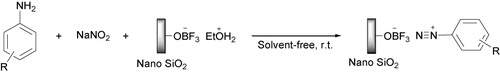
A series of azo dyes were efficiently synthesized by mixing aromatic amines and NaNO2 in the presence of nano silica supported boron trifluoride (nano BF3·SiO2) and then diazo coupling with 1-naphthol under solvent-free conditions at room temperature. The present methodology has proven to be simple, rapid, environmentally benign, green, and cost-effective compared with previous synthetic methods. Also, by using this procedure, aryl diazonium salts supported on nano BF3·SiO2 were very stable to be kept at room temperature for several months without any special conditions.
Introduction
At the beginning of the new century, synthesis of organic compounds using grinding technique under solvent-free conditions (Citation1–Citation8) has received much attention, as it provides manipulative simplicity, greater selectivity, easier workup, lesser reaction times, and higher yields which match with the green chemistry protocol. On the other side, more attention has been directed toward the application of solid acids in organic synthesis because such reagents not only simplify purification processes but also help prevent release of reaction residues into the environment (Citation9, Citation10). Azo dyes are very important compounds in many branches of chemistry and are widely used as colorants in the textile industries (Citation11), colorants for digital printing and photography (Citation12), chiral receptors (Citation13), liquid crystals (Citation14), new glassy materials (Citation15), chiral switches in photochemistry (Citation16), dyes for drug, food, and cosmetic applications, in the biomedical field (Citation17), and for molecular recognition (Citation18). Therefore, due to the importance of the azo dyes, continuous efforts have been made to achieve simple and eco-friendly methodologies for the synthesis of these compounds. Recently, various heterogeneous solid acids have been used for the preparation of azo dyes (Citation19–Citation21). Although satisfactory yields of products have usually obtained, nano structure solid acids have attracted great interest in recent years due to their particular physical and chemical properties. The properties of these materials mainly depend on their shape, size, and structure which are strongly determined by the synthetic processes (Citation22). BF3·SiO2 and its nano structural form are excellent proton sources and convenient and inexpensive catalysts, which can be used for the promotion of many acid-catalyzed organic reactions (Citation23–Citation30). As a continuation of our researches on the application of heterogeneous solid acids in organic transformations (Citation31–Citation40), we herein report an expedient practical method for the synthesis of azo dyes using nano BF3·SiO2 under solvent-free conditions by grinding. In this method, it is not necessary to provide special cold conditions for stabilization of the diazonium salt. The reaction easily takes place at room temperature, and the resulting diazonium salt can remain on the solid substrate for several months.
Results and discussion
Synthesis of azo naphthol dyes is a two-step process. The first step is the formation of aromatic diazonium salt (Ar–N+≡N:), and the second step is coupling reaction between the above-mentioned cation and naphthoxide salt. In the traditional methods, synthesis of azo dyes requires special conditions such as low temperature and liquid acids. Processes involving conventional acids are typically associated with numerous drawbacks such as high toxicity and corrosion, acid waste, difficulty of separation, using large amounts of acid, and poor stability of diazonium salts. To overcome these limitations, we decided to use an efficient solid acid named nano BF3·SiO2 for the synthesis of diazonium salts and their diazo coupling with 1-naphthol in a green and solvent-free medium.
This research was performed in three stages. Initially, nano BF3·SiO2 was prepared and the size and structure of nanoparticles were characterized by transmission electronic microscope (TEM) and X-ray diffraction (XRD). In the second stage, aryl diazonium salts were synthesized in the presence of nano BF3·SiO2 at room temperature, and their structure and stability was investigated. The third stage was diazo coupling of diazonium salts with 1-naphthol under solvent-free and grinding conditions.
Nano BF3·SiO2 characterization
The final structure of BF3·SiO2 () (Citation41) and its nanoparticles morphology determined by scanning electron microscopy images (Citation28) have been previously reported. Herein, the properties of BF3·SiO2 nanoparticles were characterized by two other techniques including TEM and XRD.
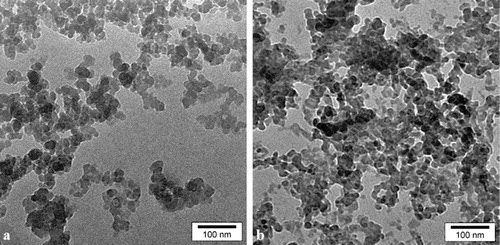
Inspection of the TEM images in indicated involvement of nanoparticles with an average size distribution of 20 nm for nano silica gel and 25 nm for nano BF3·SiO2.
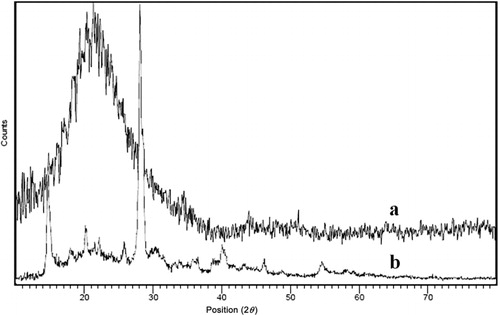
Nano silica gel XRD pattern displayed a broad strong peak in 2θ value of 21.8024 with full width at half maximum (FWHM) equal to 0.1771. Nano BF3·SiO2 XRD pattern showed three main sharp peaks in 2θ of 28.4516 with FWHM of 0.1771, 27.9718 with FWHM of 0.2952, and 14.5404 with FWHM of 0.2362. In addition, there was a short broad peak in 21.9895 with FWHM of 0.1771 related to amorphous nano silica gel. The broadness of the peak in verified the amorphous nature of nano silica gel and the sharpness of the peak in represented the crystallike structure of nano BF3·SiO2.
Synthesis and characterization of aryl diazonium salts
Aryl diazonium salts are usually synthesized in the presence of a liquid acid dissolved in water at low temperatures between 0°C and 5°C because temperatures above 5°C generally promote phenol formation in aqueous media.
An initial optimization of the kind and amount of acid was performed by diazotization of 4-chloro aniline as a model substrate and diazo coupling with 1-naphthol. A range of parameters such as stability of diazonium salt at room temperature, reaction time of diazotization and yield of resulted azo naphthol were screened in . At first, nano silica gel and BF3·Et2O sources were tested individually. There was no reaction in the presence of neither nano silica gel nor BF3·Et2O (, entries 1 and 2), while BF3·SiO2 afforded an improved yield of 80% for azo naphthol (, entry 3). These results clearly indicated that the presence of Brønsted acidic sites on the solid acid surface is essential for promoting diazotization reactions.
Table 1. Optimization of the kind and amount of acid for diazotization reaction.
In another comparative study (, entries 4–7), the effect of nano silica gel loading by BF3·Et2O on the acidic performance of nano BF3·SiO2 was studied. Although the time of diazotization and the stability of diazonium salt supported on nano BF3·SiO2 with different loadings in the same conditions were approximately identical, 15 mol% nano BF3·SiO2 resulted in the highest yield of azo naphthol. In conclusion, 15 mol% nano BF3·SiO2 was selected as the most ideal acid for synthesis of azo naphthols among those listed in .
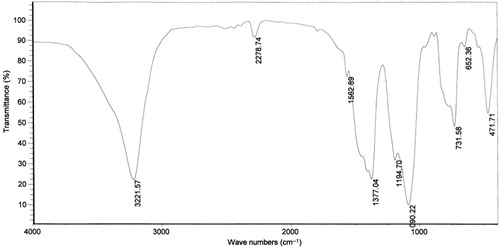
With more investigations, it was found that aryl diazonium salts supported on nano BF3·SiO2 underwent no changes at room temperature for several months. To explore the reason of this stability, FT-IR spectrum of 4-chlorophenyl diazonium salt was studied (). In this spectrum, the ethanolic OH and existing moisture in nano silica gel and BF3·Et2O caused a broad O–H stretching band in wave number of 3221 cm−1. The appearance of the band at 2278 cm−1 clearly demonstrated N≡N stretching vibration and verified the formation of diazonium salt. The absorption bands of B–O and Si–O vibrations were observed in 1377 cm−1 and 1090 cm−1, respectively. The absorption band of B–F was hidden under Si–O band. Aromatic C–H bending vibrations and C–Cl stretching band were revealed in 731 cm−1 and 652 cm−1.
According to this information, the structure of diazonium salts supported on nano BF3·SiO2 was guessed. reveals that in this probable structure, aryl diazonium cations are located on the surface of negatively charged particles called nano silica trifluroborate. So, the presence of bulky anions and charge–charge interactions between nitrogen and boron atoms are the possible reasons of unusual stability of these salts.
After optimization of the conditions, we ground aniline derivatives including electron-withdrawing and electron-donating substituents with NaNO2 and nano BF3·SiO2 (). Diazonium salts were obtained in a very short time (between 5 seconds and 15 seconds) with an excellent conversion at room temperature. Indeed, due to the high acidic strength of nano BF3·SiO2, the reaction was done so rapid that the substituent type could not effect on the time of diazotization.
In addition, all aryl diazonium salts prepared with this procedure were nonexplosive because their reactivity decreased when they were supported on nano BF3·SiO2 as a solid acid. Therefore, diazotization method was detected to be safe, and the grinding of these aryl diazonium salts was not found to be hazardous. In conclusion, long-term stability, high speed conversion, and explosion proof properties of diazonium salts supported on nano BF3·SiO2 were a part of the valuable results of our work.
Synthesis of azo dyes based on 1-naphthol
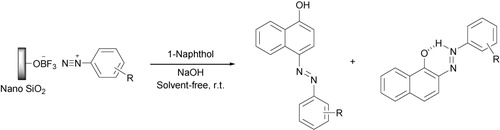
The most common synthetic route to the arylazo-1-naphthol compounds involves coupling of aryl diazonium salts with 1-naphthol in basic solution. Although using water for the preparation of 1-naphthoxide salt seems to be necessary, but like the first step, the presence of water causes the formation of some phenoxide salt after the addition of diazonium salt. In continuation, phenoxide nucleophilic attack to diazonium salt forms phenolic azo dyes as side products. To prevent this problem, the reaction was performed under solvent-free conditions toward green chemistry. Then, we ground solid 1-naphthol with some NaOH in a mortar. The moisture absorbed by the reaction mixture during the grinding seems to be sufficient for the formation of a homogeneous mixture. Moreover, the higher concentration of reactants in the absence of solvent at room temperature usually leads to more favorable kinetics than in solution (Citation42, Citation43).
In this reaction, diazo coupling of aryl diazonium salts with 1-naphtoxide led to two products in which 4-arylazo-1-naphthol derivatives were the major products and 2-arylazo-1-naphthol derivatives were obtained as the minor products ().
The resulting two main products were easily separated by flash column chromatography. However, although no solvent is used in the synthetic steps of azo naphthols and the reaction is fully solvent-free, we certainly need to add some solvents such as n-hexane and ethyl acetate to isolate the two main products. Total yield of the two products is shown in .
Table 2. Solvent-free synthesis of arylazo-1-naphthol derivatives at room temperature.
It is also important that the electronic effects of diazonium salt substituents do not play a significant role in the rate of diazo coupling step. However, coupling reaction occurred rapidly regardless of substituent effects.
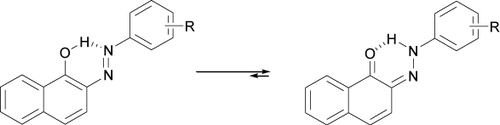
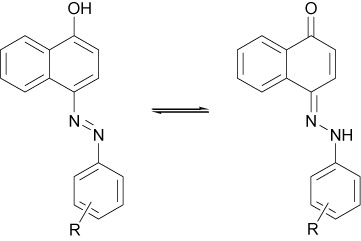
As reported earlier (Citation44, Citation45), in 2-arylazo-1-naphthols, but not in 4-arylazo-1-naphthols, there is a strong intramolecular hydrogen bonding both in the hydroxyazo and ketohydrazone forms (). In many cases, a factor which contributes to the explanation of the predominance of the hydrazone isomer is that in this form the intramolecular hydrogen bonding is significantly stronger than the azo form. This may provide an explanation for the observations that in the case of 2-arylazo-1-naphthol, the hydrazone form predominates while in the case of the isomeric 4-arylazo-1-naphthol, in which there is no intramolecular hydrogen bonding, the two possible tautomers have comparable stability (). However, in all cases, proton nuclear magnetic resonance (1H-NMR) spectra of 1-hydroxy-2-azo compounds represented a signal with a chemical shift in the range 15–17 ppm in CDCl3, corresponding to the N–H…O proton involved in the relevant intramolecular hydrogen bond, while in 1-hydroxy-4-azo compounds, chemical shift was observed in the range 10–12 ppm in deuterated dimethyl solfoxide (DMSO-d6). Also, the molar ratios of these two dyes were determined from integral intensities of OH/NH signals in 1H-NMR spectrum (ratio 5:1 for 4-arylazo-1-naphthol: 2-arylazo-1-naphthol).
Totally, we investigated the application of nano BF3·SiO2 as a strong and useful solid acid for the synthesis of azo dyes based on 1-naphthol by a green procedure.
Experimental procedure
Chemicals and solvents were purchased from Merck Company. Commercial nano silica gel was prepared from Sigma-Aldrich Company. Fourier Transform Infrared (FT-IR) spectra were run on a Nicolet Magna 550 spectrometer. 1H-NMR (400 MHz) spectra were recorded on a Bruker DRX-400 Avance spectrometer. Tetramethylsilane was used as an internal reference and CDCl3 and DMSO-d6 used as solvents. TEM micrographs of nano silica gel and nano BF3·SiO2 were taken using LEO 912 AB OMEGA instrument (Germany) with an LaB6 cathode and accelerating voltage of 100 kV. XRD patterns were acquired using a Philips Xpert MPD diffractometer equipped with a Cu Kα anode (λ = 1.54 Å) in the 2θ range from 10° to 80°.
Preparation of 15 mol% nano BF3·SiO2
Nano BF3·SiO2 was easily prepared from nano silica gel and BF3·Et2O according to the literature (Citation28). In short, BF3·Et2O (2.65 mL) was added dropwise to a slurry containing nano silica gel (7 g) and ethanol (30 mL, instead of chloroform). This reaction must be carried out under a strong hood. The mixture was stirred for 1 hour at room temperature. The resulting suspension was filtered to obtain the white solid named nano BF3·SiO2 (15 mol%).
Typical procedure for synthesis of azo-1-naphthol dyes
For synthesis of azo-1-naphthol derivatives, we mixed aromatic amines (2 mmol) with sodium nitrite (3 mmol) and nano BF3·SiO2 (0.4 g) in a mortar with a pestle by rapid grinding. The progress of reaction was monitored by thin layer chromatography (TLC) (Ethyl acetate/n-Hexane). On the other hand, for the preparation of 1-naphtoxide salt, we ground 2 mmol of 1-naphthol and 10 mmol of NaOH in the other mortar. Then, aryl diazonium salt was added to 1-naphthoxide salt and mixing and grinding resumed for a short time (about 3 minutes). After the completion of reaction and formation of two main products (2-arylazo-1-naphthol and 4-arylazo-1-naphthol), the mixture was washed by distilled water (3 × 10 mL) and then by acetone (4 × 5 mL). Two final products were separated by flash column chromatography with silica mesh of 230–400 (40–63 µm). Final products were identified by 1H-NMR and FT-IR spectroscopic methods.
Spectroscopic data for some selected 2-arylazo-1-naphthol and 4-arylazo-1-naphthol dyes
2-(3-Methylphenylazo)-1-naphthol
FT-IR (KBr) cm−1: 3454 (O–H), 1614 (C=O), 1503 (C=N), 1467 (N=N); 1H-NMR (400 MHz, CDCl3) δ (ppm): 16.24 (1H, OH), 8.43 (1H, CH), 7.59 (2H, CH), 7.47 (3H, CH), 7.33 (2H, CH), 7.07 (1H, CH), 7.01 (1H, CH), 2.42 (3H, CH3).
2-(2,4-Dimethylphenylazo)-1-naphthol
FT-IR (KBr) cm−1: 3437 (O–H), 1612 (C=O), 1537 (C=N), 1463 (N=N); 1H-NMR (400 MHz, CDCl3) δ (ppm): 16.51 (1H, OH), 8.47 (1H, CH), 7.87 (1H, CH), 7.61 (2H, CH), 7.47 (1H, CH), 7.31 (1H, CH), 7.14 (1H, CH), 7.09 (1H, CH), 7.05 (1H, CH), 2.54 (3H, CH3), 2.37 (3H, CH3).
2-(3-Chlorophenylazo)-1-naphthol
FT-IR (KBr) cm−1: 3434 (O–H), 1619 (C=O), 1507 (C=N), 1467 (N=N), 677 (C–Cl); 1H-NMR (400 MHz, CDCl3) δ (ppm): 15.98 (1H, OH), 8.41 (1H, CH), 7.69 (1H, CH), 7.64 (1H, CH), 7.56 (1H, CH), 7.47 (1H, CH), 7.42 (1H, CH), 7.36 (1H, CH), 7.20 (1H, CH), 7.17 (1H, CH), 7.01 (1H, CH).
4-(4-Methoxyphenylazo)-1-naphthol
FT-IR (KBr) cm−1: 3430 (O–H), 3239 (N–H), 1593 (C=O), 1541 (C=N), 1449 (N=N), 1017-1239 (C–O); 1H-NMR (400 MHz, DMSO-d6 ) δ (ppm): 11.01 (1H, OH), 8.85 (1H, CH), 8.20 (1H, CH), 7.93 (2H, CH), 7.79 (1H, CH), 7.65 (1H, CH), 7.56 (1H, CH), 7.11 (2H, CH), 6.98 (1H, CH), 3.82 (3H, OCH3).
4-(2-Chlorophenylazo)-1-naphthol
FT-IR (KBr) cm−1: 3422 (O–H), 3347 (N–H), 1626 (C=O), 1581 (C=N), 1449 (N=N), 695 (C–Cl); 1H-NMR (400 MHz, DMSO-d6 ) δ (ppm): 11.34 (1H, OH), 8.91 (1H, CH), 8.23 (1H, CH), 7.90 (1H, CH), 7.82 (1H, CH), 7.69 (3H, CH), 7.57 (1H, CH), 7.47 (1H, CH), 7.04 (1H, CH).
4-(2-Nitrophenylazo)-1-naphthol
FT-IR (KBr) cm−1: 3433 (O–H), 3294 (NH), 1642 (C=O), 1604 (C=N), 1494, 1318 (NO2), 1463 (N=N), 546 (C–Br); 1H-NMR (400 MHz, DMSO-d6 ) δ (ppm): 11.60 (1H, OH), 8.76 (1H, CH), 8.18 (1H, CH), 8.05 (1H, CH), 7.94 (1H, CH), 7.83 (3H, CH), 7.70 (1H, CH), 7.59 (1H, CH), 6.99 (1H, CH).
Conclusions
Finally, some brilliant azo dyes were successfully synthesized by standard methods of diazotization and diazo coupling with 1-naphthol in the presence of nano BF3·SiO2 as an eco-friendly, inexpensive, and efficient solid acid at room temperature. This green protocol avoids the use of toxic liquid acids and solvents, prevents the generation of side products, and has a distinct advantage by stabilizing diazonium salts supported on nano BF3·SiO2, having a short reaction time and high conversion.
Acknowledgment
The authors thank the University of Kashan for supporting this work [grant number 159189/31].
References
- Vibhute, A.; Mokle, S.; Karamunge, K.; Gurav, V.; Vibhute, Y. A Simple and Efficient Method for Solvent-Free Iodination of Hydroxylated Aromatic Aldehydes and Ketones Using Iodine and Iodic Acid by Grinding Method. Chin. Chem. Lett. 2010, 21, 914–918.
- Tretyakov, A.N.; Krasnokutskaya, E.A.; Gorlushko, D.A.; Ogorodnikov, V.D.; Filimonov, V.D. A New One-Pot Solvent-Free Synthesis of Pyridinyl Tosylates via Diazotization of Aminopyridines. Tetrahedron Lett. 2011, 52, 85–87.
- Carlier, L.; Baron, M.; Chamayou, A.; Couarraze, G. Use of Co-grinding as a Solvent-Free Solid State Method to Synthesize Dibenzophenazines. Tetrahedron Lett. 2011, 52, 4686–4689.
- Rani, R.; Arya, S.; Kilaru, P.; Sondhi, S.M. An Expeditious, Highly Efficient, Catalyst and Solvent-Free Synthesis of 9,10-Dihydro-anthracene-9,10-α,β-succiniimide Derivatives. Green Chem. Lett. Rev. 2012, 5, 545–575.
- Sato, K.; Ozu, T.; Takenaga, N. Solvent-Free Synthesis of Azulene Derivatives via Passerini Reaction by Grinding. Tetrahedron Lett. 2013, 54, 661–664.
- Talukdar, D.; Thakur, A.J. A Green Synthesis of Symmetrical Bis(indol-3-yl)methanes Using Phosphate-Impregnated Titania Catalyst under Solvent Free Grinding Conditions. Green Chem. Lett. Rev. 2013, 6, 55–61.
- Kumar, S. An Improved One-Pot and Eco-Friendly Synthesis of Aurones under Solvent-Free Conditions. Green Chem. Lett. Rev. 2014, 7, 95–99.
- Brahmachari, G.; Das, S. L-Proline Catalyzed Multicomponent One-Pot Synthesis of Gem-Diheteroarylmethane Derivatives Using Facile Grinding Operation under Solvent-Free Conditions at Room Temperature. RSC Adv. 2014, 4, 7380–7388.
- Clark, J.H. Catalysis of Organic Reactions by Supported Inorganic Reagents; VCH: New York, 1994.
- Okuhara, T. Water-Tolerant Solid Acid Catalysts. Chem. Rev. 2002, 102, 3641–3666.
- Freeman, H.S.; Peters, A.T. Colorants for Non-Textile Applications; Elsevier Science B.V.: Amsterdam, 2000.
- Gregory, P. Ink Jet Printing. In High Technology Applications of Organic Colorants; Plenum: New York, 1991; Chapter 9.
- Kubo, Y.; Maeda, S.; Tokita, S.; Kubo, M. Colorimetric Chiral Recognition by a Molecular Sensor. Nature. 1996, 382, 522–524.
- Steinsträsser, R.; Pohl, L. Chemistry and Applications of Liquid Crystals. Angew. Chem., Int. Ed. 1973, 12, 617–630.
- He, Y.; Gu, X.; Guo, M.; Wang, X. Dendritic Azo Compounds as a New Type Amorphous Molecular Material with Quick Photoinduced Surface-Relief-Grating Formation Ability. Opt. Mater. 2008, 31, 18–27.
- Pieraccini, S.; Masiero, S.; Spada, G.P.; Gottarelli, G.A. A New Axially-Chiral Photochemical Switch. Chem. Commun. 2003, 9, 598–599.
- Tatsuta, M.; Kitao, T. Reagent for Detecting and Diagnosing Cancer, Publication No. JP 01–207247 A, 1989.
- Aszalos, A.; Weaver, J.L.; Pine, P.S. Methods of Using Azo Dyes and Their Derivatives. US Patent 5,468,469, November 21, 1995.
- Bahulayan, D.; John, L.; Lalithambika, M. Modified Clays as Efficient Acid–Base Catalyst Systems for Diazotization and Diazo Coupling Reactions. Synth. Commun. 2003, 33, 863–869.
- Dabbagh, A.H.; Teimouri, A.; Najafi Chermahini, A. Green and Efficient Diazotization and Diazo Coupling Reactions on Clays. Dyes Pigm. 2007, 73, 239–244.
- Zarei, A.; Hajipour, A.R.; Khazdooz, L.; Mirjalili, B.F.; Najafi Chermahini, A. Rapid and Efficient Diazotization and Diazo Coupling Reactions on Silica Sulfuric Acid under Solvent-Free Conditions. Dyes Pigm. 2009, 81, 240–244.
- Jortner, J.; Rao, C.N.R. Nanostructured Advanced Materials. Perspectives and Directions. Pure Appl. Chem. 2002, 74, 1491–1506.
- Sadeghi, B.; Mirjalili, B.F.; Hashemi, M.M. BF3·SiO2: An Efficient Heterogeneous Alternative for Regio-Chemo and Stereoselective Claisen-Schmidt Condensation. J. Iran. Chem. Soc. 2008, 5, 694–698.
- Mirjalili, B.F.; Bamoniri, A.; Akbari, A. BF3-SiO2: An Efficient Alternative for the Synthesis of 14-Aryl or Alkyl-14H-dibenzo[a,j]xanthenes. Tetrahedron Lett. 2008, 49, 6454–6456.
- Sadeghi, B.; Mirjalili, B.F.; Hashemi, M.M. BF3-SiO2: An Efficient Reagent System for the One-Pot Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles. Tetrahedron Lett. 2008, 49, 2575–2577.
- Reddy, M.V.; Dindulkar, S.D.; Jeong, Y.T. BF3·SiO2-Catalyzed One-Pot Synthesis of α-Aminophosphonates in Ionic Liquid and Neat Conditions. Tetrahedron Lett. 2011, 52, 4764–4767.
- Dindulkar, S.D.; Parthiban, P.; Jeong, Y.T. BF3·SiO2 Is a Simple and Efficient Lewis Acid Catalyst for the One-Pot Synthesis of Polyfunctionalized Piperidin-4-ones. Monatsh. Chem. 2012, 143, 113–118.
- Mirjalili, B.F.; Bamoniri, A.; Akbari, A. One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones (Thiones) Promoted by Nano-BF3·SiO2. J. Iran. Chem. Soc. 2011, 8, 135–140.
- Mirjalili, B.F.; Bamoniri, A.; Akbari, A. Nano-BF3·SiO2: A Reusable and Eco-Friendly Catalyst for Synthesis of Quinoxalines. Chem. Heterocycl. Compd. 2011, 47, 487–491.
- Rajasekhar, D.; Rao, D.S.; Srinivasulu, D.; Raju, C.N.; Balaji, M. Microwave Assisted Synthesis of Biologically Active α-Aminophosphonates Catalyzed by Nano-BF3·SiO2 under Solvent-Free Conditions. Phosphorus, Sulfur Silicon Relat. Elem. 2013, 188, 1017–1025.
- Mirjalili, B.F.; Bamoniri, A.; Karimi Zarchi, M.A.; Emtiazi, H. Zr(HSO4)4/SiO2: An Effective Heterogeneous Alternative for One-Pot Synthesis of β-Acetamido Ketones. J. Iran. Chem. Soc. 2010, 7, 95–99.
- Sadeghi, B.; Mirjalili, B.F.; Bidaki, S.; Ghasemkhani, M. SbCl5.SiO2: An Efficient Alternative for One-Pot Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles in Solvent or under Solvent-Free Condition. J. Iran. Chem. Soc. 2011, 8, 648–652.
- Mirjalili, B.F.; Bamoniri, A.; Akbari, A.; Taghavinia, N. Nano-TiO2: An Eco-Friendly and Re-Usable Catalyst for the Synthesis of 14-Aryl or Alkyl-14H-dibenzo[a,j]xanthenes. J. Iran. Chem. Soc. 2011, 8, 129–134.
- Bamoniri, A.; Mirjalili, B.F.; Jafari, A.A.; Abasaltian, F. Synthesis of 1,3,5-Tri-Substituted Pyrazoles Promoted by P2O5·SiO2. Iran. J. Catal. 2012, 2, 73–76.
- Mirjalili, B.F.; Bamoniri, A.; Mirhoseini, M.A. Nano-SnCl4·SiO2 – A Versatile and Efficient Catalyst for Synthesis of 14-Aryl- or 14-Alkyl-14H-dibenzo [a,j]xanthenes. Chem. Heterocycl. Compd. 2012, 48, 856–860.
- Mirjalili, B.F.; Bamoniri, A.; Zamani, L. Nano-TiCl4/SiO2: An Efficient and Reusable Catalyst for the Synthesis of Tetrahydrobenzo[a]xanthenes-11-ones. Lett. Org. Chem. 2012, 9, 338–343.
- Mirjalili, B.F.; Bamoniri, A.; Zamani, L. One-Pot Synthesis of 1, 2, 4, 5-Tetrasubstitutedimidazoles Promoted by Nano-TiCl4.SiO2. Sci. Iran. 2012, 19, 565–568.
- Bamoniri, A.; Mirjalili, B.F.; Nazemian, S. Nano Silica Phosphoric Acid: An Efficient Catalyst for the One-Pot Synthesis of 1, 2, 4, 5-Tetrasubstituted Imidazoles. J. Nanostruct. 2012, 2, 101–105.
- Bamoniri, A.; Mirjalili, B.F.; Nazemian, S. Nano-Silica Phosphoric Acid: An Efficient Catalyst for One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones (Thiones) under Solvent-Free or Sonication Conditions. Iran. J. Catal. 2012, 2, 17–21.
- Bamoniri, A.; Mirjalili, B.F.; Nazemian, S. Microwave-Assisted Solvent-Free Synthesis of 14-Aryl/Alkyl-14H-dibenzo[a,j]xanthenes and Tetrahydrobenzo[a] xanthen-11-ones Catalyzed by Nano Silica Phosphoric Acid. Curr. Chem. Lett. 2013, 2, 27–34.
- Wilson, K.; Clark, J.H. Synthesis of a Novel Supported Solid Acid BF3 Catalyst. Chem. Commun. 1998, (19), 2135–2136.
- Toda, F.; Tanaka, K. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074.
- Tanaka, K. Solvent-Free Organic Synthesis; Wiley: New York, 2003.
- Hihara, T.; Okada, Y.; Morita, Z. Azo-Hydrazone Tautomerism of Phenylazonaphthol Sulfunates and Their Analysis Using the Semiempirical Molecular Orbital PM5 Method. Dyes Pigm. 2003, 59, 25–41.
- Alarcon, S.H.; Olivieri, A.C.; Sanz, D. Claramunt, R.M.; Elguero, J. Substituent and Solvent Effects on the Proton Transfer Equilibrium in Anils and Azo Derivatives of Naphthol. Multinuclear NMR Study and Theoretical Calculations. J. Mol. Struct. 2004, 705, 1–9.
- Ogoleva, L.N.; Stepanov, B.I. The Isomer Ratio in Azo Coupling. Ž. Org. Chim. 1965, 1, 2083–2086.
- Morgan, K.J.; Infrared Spectra and Structure of Arylazonaphthols. J. Chem. Soc. 1961, 2151–2159.
- Charrier, G.; Ferreri, G. Gazz. Chim. Ital. 1914, 44, 234.
- Bamberger, E.; Meimberg, F. Einige Weitere Beobachtungen über Azofarbstoffe. Chem. Ber. 1895, 28, 1887–1897.
- Brode, W.R.; Herdle, L.E. The Relation Between the Absorption Spectra and the Chemical Constitution of Dyes. XIX. Mono-and Poly-Azo Dyes with a Single Auxochrome. J. Org. Chem. 1941, 6, 713–721.
- Bamberger, E. Ueber Die Einwirkung von Alphylhydrazinen auf β-Naphtochinon. Chem. Ber. 1897, 30, 513–516.
- Kropacova, H.; Panchartek, J.; Sterba V.; Valter, K. Kinetics and Mechanism of Diazo Coupling. XVII. Coupling Kinetics of Substituted Benzenediazonium Cations with 1-Naphthol and 1-Naphthol-4-sulfonic Acid. Collect. Czech. Chem. Commun. 1970, 35, 3287–3295.