ABSTRACT
We have developed a novel lipase-mediated method to realize the Dakin reaction. A wide range of hydroxylated benzaldehydes could be oxidized with high yields (from 90% to 97%) under mild reaction conditions. Moreover, this lipase-mediated reaction could be scaled up easily and Novozym 435 could be reused more than 10 runs without an obvious decrease in enzyme activity. This protocol expands the application of lipase in organic synthesis and offers a complementary route for the Dakin reaction.
GRAPHICAL ABSTRACT
Introduction
Enzyme catalytic promiscuity is the hidden ability of an enzyme to catalyze more than one type of chemical transformation (Citation1, Citation2). Lipase is one of the most popular biocatalysts in this research area due to its excellent catalytic performance in many kinds of reactions (Aldol reaction, Morita-Baylis–Hillman reaction, Mannich reaction, Henry reaction, Markovnikov addition, Michael addition, Hantzsch reaction, etc.) (Citation3–9). Over the past few years, many reports have demonstrated that lipase could mediate the in situ generation of peracids through a perhydrolysis of carboxylic acids or esters (Citation10–12). And the in situ formed peracids have been successfully utilized in the epoxidation of alkenes, Baeyer–Villiger reactions, the oxidation of N-heteroaromatic compounds and sulfanyl compounds (Citation13–18).
The Dakin reaction replaces an aldehyde group with a hydroxyl group in an aromatic aldehyde or ketone that has a hydroxyl group in the ortho- or para- position (Citation19). The conventional procedure for the Dakin reaction utilizes excess hydrogen peroxide and sodium hydroxide at elevated temperatures. Up until now, numerous stoichiometric alkylperoxides and catalytic transition metal complexes have been developed for Dakin oxidations (Citation20–25). However, most of these reported methods suffered from unstable oxidants, the use of transition metals, expensive reagents or extreme reaction conditions. To the best of our knowledge, the enzymatic process for the Dakin reaction has not been reported yet. Herein we report a mild and efficient method for the Dakin reaction mediated by lipase (Scheme 1), which can provide a new case of enzyme catalytic promiscuity.
Results and discussion
As a starting point, we selected salicylaldehyde as the substrate in this lipase-mediated oxidation (). In this study, ethyl acetate (EA) was used as the peracid source (in situ generated by lipase-catalyzed perhydrolysis) and the reaction medium, meanwhile urea hydrogen peroxide (UHP) was used to release the oxidant controllably. We screened some lipases as the catalysts for the enzymatic Dakin reaction. As shown in , the yields of catechol were dramatically influenced by the type and origin of the enzyme. Only ANL, CalB and Novozym 435 (a commercial immobilized CalB) could mediate the Dakin reaction and the highest yield (96%) could be obtained when Novozym 435 was selected as the catalyst. The results of Entry 7 showed that UHP only exhibited a poor catalytic activity in this reaction. And the denatured Novozym 435 could not improve the Dakin reaction (Entry 6), which indicated that the active conformation of enzyme was necessary for this reaction. Moreover, the oxidation mediated by lipase obtained a much higher yield than that catalyzed by mCPBA (meta-chloroperoxybenzoic acid, a commercial organic peracid), which suggested that the lipase-mediated Dakin reaction was worthy of further study.
Table 1. The effect of enzyme source on the lipase-mediated Dakin reactiona.
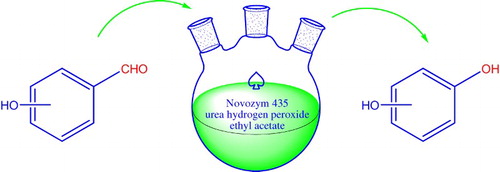
The effect of enzyme dosage on the Dakin reaction has been studied and the results were shown in . The concentrations of salicylaldehyde and UHP were kept constant, while enzyme dosage was changed from 0 to 400 U. The yield of catechol improved when the amount of Novozym 435 was increased. However, the yield could not be increased further when the amount of enzyme was beyond 300 U. By increasing the amount of enzyme, the number of active sites that take part in the generation of peracid would increase. Thus, more in situ-produced peracids would oxidize salicylaldehyde into catechol. Considering the cost of the enzyme, 300 U of Novozym 435 was adopted as the optimal enzyme dosage for further investigation.
Figure 1. The effect of enzyme dosage on the lipase-mediated Dakin reaction.
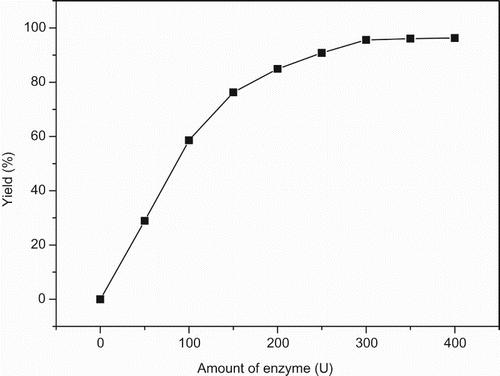
Generally, the amount of oxidant might affect the yield of Dakin reaction. In this study, the effect of the amount of UHP was investigated (). The yield of Dakin reaction increased when the amount of UHP was increased from 0.4 to 1.2 equiv.; after that, the yield did not improve markedly. Therefore, 1.2 equiv. of UHP was selected for this lipase-mediated Dakin reaction.
Figure 2. The effect of UHP on the lipase-mediated Dakin reaction.
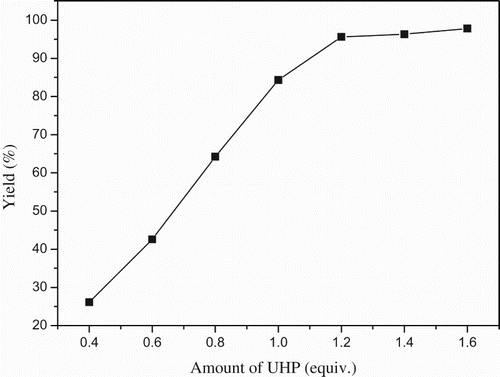
Under the optimized reaction conditions, we scaled up this lipase-mediated process to 50-fold (salicylaldehyde (50 mmol), EA (50 mL), Novozym 435 (15,000 U) and UHP (60 mmol)) and the time course was illustrated in . The yield of catechol was up to 97% after 0.5 h, which demonstrated that this green and efficient method had high potential for industrial application.
Figure 3. The time course of the lipase-mediated Dakin reaction.
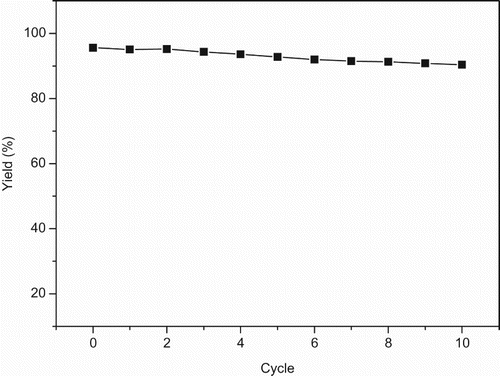
It is known that the immobilized enzyme is easily recovered and reused, which can drive down the product cost and make the enzymatic process economically viable (Citation26–28). Thus, we have studied the reusability of Novozym 435 for this lipase-mediated reaction. Novozym 435 can be easily recovered after each run by filtration. The recovered Novozym 435 was then reused directly for the oxidation of salicylaldehyde under the same conditions. As shown in , Novozym 435 exhibited an excellent reusability, and up to 90% oxidation yield could be obtained even after 10 reaction cycles. The slight decrease of enzyme activity might be attributed to the deactivation of enzyme by the oxidants or the leakage of enzyme from the support.
Figure 4. The reusability of Novozym 435 in the Dakin reaction.
To evaluate the scope and limitation of this method, a wide variety of hydroxylated benzaldehydes were used for this lipase-mediated Dakin reaction. As depicted in , all the listed hydroxylated benzaldehydes have been oxidized efficiently to provide the corresponding products. The results also indicated that the electronic nature of substituents of the hydroxylated benzaldehyde has no distinct effect on the lipase-mediated Dakin reaction. Furthermore, some other aromatic aldehydes (such as benzaldehyde, 2-chlorobenzaldehyde, 4-chlorobenzaldehyde, 4-nitrobenzaldehyde and 4-methoxybenzaldehyde) have also been investigated as the substrates at the same reaction conditions. However, only the corresponding aromatic acids were obtained instead of the corresponding phenols. Therefore, the valid substrates for this lipase-mediated Dakin reaction should be the benzaldehydes with ortho- or para- hydroxyl group.
Table 2. Lipase-mediated Dakin reaction with various hydroxylated benzaldehydes
Experimental
Materials
Lipase from Aspergillus niger (ANL), Candida antarctica lipase B (CalB) and Porcine pancreatic lipase (PPL) were purchased from Sigma (Beijing, China). Lipase from Pseudomonas sp. (PSL) was purchased from Amano Pharmaceutical Co., Ltd (Japan). Hydroxylated benzaldehydes and UHP were purchased from J&K Scientific (Beijing, China). All the other chemical reagents were purchased from Shanghai Chemical Reagent Company (Shanghai, China). All the commercially available reagents and solvents were used without further purification. NMR spectra were recorded on an Inova 500 (500 MHz) spectrometer.
General procedure of the lipase-mediated Dakin reaction
A mixture of hydroxylated benzaldehyde (1 mmol), UHP (1.5 mmol), Novozym 435 (300 U) in EA (5 mL) was stirred at room temperature in a round-bottom flask for 0.5 h. The reaction was monitored by thin-layer chromatography. Then, the mixture was filtered and the residue was washed with EA. The combined organic phases were concentrated under vacuum, and the resulting residue was purified by column chromatography on silica gel with EA/hexane (1/4) to afford the desired phenol. All the isolated products were well characterized by their 1H-NMR spectral analysis. Each experiment was performed triplicate, and all the data were obtained based on the average values.
Conclusion
In this study, a mild and efficient method for the Dakin reaction mediated by lipase was reported. After thorough optimization of the reaction conditions, all the products could be obtained in high yields (from 90% to 97%). This lipase-mediated method could be easily scaled up and the catalyst (Novozym 435) presented an excellent reusability. The notable features of this new synthetic route are atom economy, environmental friendliness and simple operational process. More importantly, this work provides a new case of enzyme catalytic promiscuity which can contribute to the development of novel synthetic approach and green technology.
Supplement_Material.docx
Download MS Word (754.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributor
Zhi Wang was born in 1973. He was obtained his Ph. D degree in 2003. Recently, her research has focused on the enzymatic resolution of chiral compounds.
Xiao Chen is studying as a master candidate in School of Life Sciences, Jilin University. Her research has focused on the synthesis and development of new drugs.
Chunyu Wang is working at State Key Laborarory of Supramolecular Structure and Materials, Jilin University. His research has focused on the synthesis of bioactive compounds.
Liu Zhang is studying as a master candidate in School of Life Sciences, Jilin University. Her research has focused on the biocatalysis and biotransformation.
Fengxi Li is studying as a master candidate in School of Life Sciences, Jilin University. His research has focused on the biocatalysis and biotransformation.
Weian Zhang is studying as a master candidate in School of Life Sciences, Jilin University. His research has focused on the synthesis of bioactive compounds.
Peng Chen is working at department of Pediatrics, the Second Hospital of Jilin University. Now, she is mainly investigating the biological activity of chiral drugs.
Lei Wang is working in Key Laboratory of Molecular Enzymology and Engineering of Ministry of Education (Jilin University, Changchun, China) as a professor. Recently, his research has focused on the new synthetic methodologies to synthesize bioactive compounds.
Additional information
Funding
References
- Bornscheuer, U.T.; Kazlauskas, R.J. Catalytic Promiscuity in Biocatalysis: Using Old Enzymes to form New Bonds and Follow New Pathways. Angew. Chem. Int. Ed. 2004, 43, 6032–6040. doi: 10.1002/anie.200460416
- Devamani, T.; Rauwerdink, A.M.; Lunzer, M.; Jones, B.J.; Mooney, J.L.; Tan, M.A.O.; Zhang, Z.J.; Xu, J.H.; Dean, A.M.; Kazlauskas, R.J. Catalytic Promiscuity of Ancestral Esterases and Hydroxynitrile Lyases. J. Am. Chem. Soc. 2016, 138, 1046–1056. doi: 10.1021/jacs.5b12209
- Li, C.; Feng, X.W.; Wang, N.; Zhou, Y.J.; Yu, X.Q. Biocatalytic Promiscuity: The First Lipase-catalysed Asymmetric Aldol Reaction. Green Chem. 2008, 10, 616–618. doi: 10.1039/b803406k
- Reetz, M.T.; Mondière, R.; Carballeira, J.D. Enzyme Promiscuity: First Protein-catalyzed Morita-baylis-hillman Reaction. Tetrahedron Lett. 2007, 48, 1679–1681. doi: 10.1016/j.tetlet.2007.01.063
- Li, K.; He, T.; Li, C.; Feng, X.W.; Wang, N.; Yu, X.Q. Lipase-catalysed Direct Mannich Reaction in Water: Utilization of Biocatalytic Promiscuity for C-C Bond Formation in A “One-pot” Synthesis. Green Chem. 2009, 11, 777–779. doi: 10.1039/b817524a
- Wang, J.L.; Li, X.; Xie, H.Y.; Liu, B.K.; Lin, X.F. Hydrolase-Catalyzed Fast Henry Reaction of Nitroalkanes and Aldehydes in Organic Media. J. Biotechnol. 2010, 145, 240–243. doi: 10.1016/j.jbiotec.2009.11.022
- Du, L.H.; Cheng, B.Z.; Yang, W.J.; Xu, L.L.; Luo, X.P. Markovnikov Addition of Imidazole Derivatives with Vinyl esters Catalyzed by Lipase TL IM from Thermomyces lanuginosus/K2CO3 in A Continuous-flow Microreactor. RSC Adv. 2016, 6, 59100–59103. doi: 10.1039/C6RA05983J
- Svedendahl, M.; Hult, K.; Berglund, P. Fast Carbon-Carbon Bond Formation by a Promiscuous Lipase. J. Am. Chem. Soc. 2005, 127, 17988–17989. doi: 10.1021/ja056660r
- Wang, J.L.; Liu, B.K.; Yin, C.; Wu, Q.; Lin, X.F. Candida antarctica Lipase B-catalyzed the Unprecedented Three-Component Hantzsch-Type Reaction of Aldehyde with Acetamide and 1, 3-Dicarbonyl Compounds in Non-aqueous Solvent. Tetrahedron 2011, 67, 2689–2692. doi: 10.1016/j.tet.2011.01.045
- Yin, D.L.T.; Kazlauskas, R.J. Revised Molecular Basis of the Promiscuous Carboxylic Acid Perhydrolase Activity in Serine Hydrolases. Chem. Eur. J. 2012, 18, 8130–8139. doi: 10.1002/chem.201200052
- Yin, D.L.T.; Bernhardt, P.; Morley, K.L.; Jiang, Y.; Cheeseman, J.D.; Purpero, V.; Schrag, J.D.; Kazlauskas, R.J. Switching Catalysis from Hydrolysis to Perhydrolysis in Pseudomonas Fluorescens Esterase. Biochemistry 2010, 49, 1931–1942. doi: 10.1021/bi9021268
- Bernhardt, P.; Hult, K.; Kazlauskas, R.J. Molecular Basis of Perhydrolase Activity in Serine Hydrolases. Angew. Chem. 2005, 117, 2802–2806. doi: 10.1002/ange.200463006
- Xu, Y.; Khaw, N.R.B.J.; Li, Z. Efficient Epoxidation of Alkenes with Hydrogen Peroxide, Lactone, and Lipase. Green Chem. 2009, 11, 2047–2051. doi: 10.1039/b913077b
- Kotlewska, A.J.; van Rantwijk, F.; Sheldon, R.A.; Arends, I.W.C.E. Epoxidation and Baeyer-Villiger Oxidation Using Hydrogen Peroxide and A Lipase Dissolved in Ionic Liquids. Green Chem. 2011, 13, 2154–2160. doi: 10.1039/c1gc15255f
- Yang, F.J.; Wang, Z.; Zhang, X.W.; Jiang, L.Y.; Li, Y.Z.; Wang, L. A Green Chemoenzymatic Process for the Synthesis of Azoxybenzenes. Chemcatchem 2015, 7, 3450–3453. doi: 10.1002/cctc.201500720
- Yang, F.J.; Zhang, X.W.; Li, F.X.; Wang, Z.; Wang, L. Chemoenzymatic Synthesis of Α-Cyano Epoxides by A Tandem-Knoevenagel-Epoxidation Reaction. Eur. J. Org. Chem. 2016, 7, 1251–1254.
- Yang, F.J.; Zhang, X.W.; Li, F.X.; Wang, Z.; Wang, L. A Lipase-Glucose Oxidase System for the Efficient Oxidation of N-Heteroaromatic Compounds and Tertiary Amines. Green Chem. 2016, 18, 3518–3521. doi: 10.1039/C6GC00780E
- Bjǒrkling, F.; Frykman, H.; Godtfredsen, S.E.; Kirk, O. Lipase Catalyzed Synthesis of Peroxycarboxylic Acids and Lipase Mediated Oxidations. Tetrahedron 1992, 48, 4587–4592. doi: 10.1016/S0040-4020(01)81232-1
- Wang, Z.D.Z. Comprehensive Organic Name Reactions and Reagents, John Wiley: Hoboken, NJ, USA, 2009.
- Chen, S.; Hossain, M.S.; Foss, Jr.F.W. Organocatalytic Dakin Oxidation by Nucleophilic Flavin Catalysts. Org. Lett. 2012, 14, 2806–2809. doi: 10.1021/ol3010326
- Varma, R.S.; Naicker, K.P. The Urea−Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles. Org. Lett. 1999, 1, 189–192. doi: 10.1021/ol990522n
- Silva, E.T.; Camara, C.A.; Antunes, O.A.C.; Barreiro, E.J.; Fraga, C.A.M. Improved Solvent-free Dakin Oxidation Protocol. Synth. Commun. 2008, 38, 784–788. doi: 10.1080/00397910701820673
- Zambrano, J.L.; Dorta, R. Improving the Dakin Reaction by Using an Ionic Liquid Solvent. Synlett 2003, 10, 1545–1546. doi: 10.1055/s-2003-40848
- ChandraáBarua, N. H2O2 in WEB: A Highly Efficient Catalyst System for the Dakin Reaction. Green Chem. 2015, 17, 4533–4536. doi: 10.1039/C5GC01404B
- Saikia, B.; Borah, P. A New Avenue to the Dakin reaction in H2O2-WERSA. RSC Adv. 2015, 5, 105583–105586. doi: 10.1039/C5RA20133K
- Rueda, N.; Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Chemical Modification in the Design of Immobilized Enzyme Biocatalysts: Drawbacks and Opportunities. Chem. Rec. 2016, 16, 1436–1455. doi: 10.1002/tcr.201600007
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. doi: 10.1039/C2CS35231A
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the One-step Immobilization-Purification of Enzymes as Industrial Biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. doi: 10.1016/j.biotechadv.2015.03.006

