Abstract
A classic question in plant ecology is “why is the world green?” That is, if plants are food for animals why do not animals eat all the available food – changing a ‘green world’ into a ‘brown world’. We first reviewed this question in 2009 and now revisit our arguments in the light of new data and new thinking. Here we argue that (1) the top–down bottom–up dichotomy is probably too simple for understanding a complex system – such as vegetation – rich in feedback processes. (2) Nevertheless it appears that bottom–up processes are generally more important for maintaining the presence of some sort of vegetation while top–down control process are generally more important in determining the type of vegetation at a site. (3) Although this review mainly takes a qualitative and experimental approach to the question, we also argue that simple well-known mathematical models from population ecology can be very informative in thinking about the types of explanations for the green world phenomenon, and demonstrating that it is rarely a simple choice between one form of control or another.
Introduction
Viewed from space much of the Earth’s land is coloured green with chlorophyll, leading Beerling (Citation2007) to entitle his book on the role of plants in Earth history The Emerald Planet. This raises an obvious question – why is the world green? One answer is that the climate (often rainfall) allows some parts of the land to be green with plant life, while making other areas arid and brown. However, these greens of extensive plant life are still a puzzle – indeed Crawford (Citation1989) described as “one of the marvels of nature” the fact that plants “while providing the original source of food for all animal and microbial life are not themselves consumed to the point where they are no longer able to support their predators.” Herbivores turning a green world into a brown one are an uncommon occurrence in natural systems (). But why is this so? Why are so many parts of our world green in the face of this threat from herbivores? If, as seems likely, herbivores are the key to our question, then what starts as a question in plant ecology ends up being a question about factors that limit the size of herbivore populations. In effect, we need to understand why herbivore populations do not increase in density to such a level that they destroy all the available plants, giving a land that is coloured brown rather than green. This has the important effect that in terrestrial ecosystems the majority of primary production is not consumed by herbivores but is eventually consumed by microbes and invertebrate detritivores in soils or aquatic systems (Steffan et al. Citation2015).
Figure 1. A general view and a close-up of heavily grazed savannah in the southern part of the Kruger National Park, South Africa (photographed on 14 July 2014, during the dry season). Although this looks close to a ‘brown world’, in fact only 64% of the area shown in the general view photograph was completely bare soil. It is likely that the high grazing levels were due to particularly nutrient-rich soils (and so nutrient-rich vegetation) at this site.

There are many mathematical models of species interactions which allow species coexistence (see Otto and Day Citation2007). For example, if we model plant and herbivore population dynamics using coupled differential equations, it is easy to show that under some parameter values plants and herbivores can coexist, while with other values the plants become extinct along with the herbivores which then starve (). In this review, we attempt to elucidate the reasons for this persistence from a predominately qualitative perspective, although we believe that mathematical models, however simple, have some role to play in formalising and verifying verbal arguments.
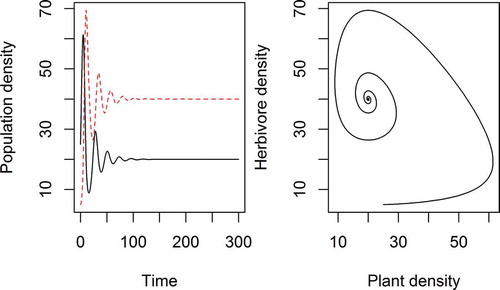
Figure 2. A simple model of a plant population (P) fed on by a herbivore population (H) according to continuous time Lotka–Volterra dynamics where the plant population has a carrying capacity K (for convenience, no such density dependent limitation is assumed for the herbivores). The coupled differential equations representing the rates of change are:
. Model parameters were K = 100, r = 0.5, a = 0.01, f = 0.01, b = 0.2 with P(0) = 25 and H(0) = 5. The left plot shows the population densities of both species (herbivore red broken line, plant black continuous) moving towards an equilibrium over time, the right plot shows the same dynamics presented in phase space. Why is a non-zero equilibrium of herbivores and plants possible despite the fact that herbivores eat plants? Radically altering any of the model parameters (or starting conditions) can result in extinction of the herbivore population or both the plant and herbivore populations, so the important point is that there is no single answer as to what keeps this artificial world green – it depends on a combination of parameters.
In this review, we give our overview of the current state of the answer to “Why is the world Green.” We previously addressed this question in chapter 7 of our book Big Questions in Ecology and Evolution (Sherratt and Wilkinson Citation2009), and this review paper is an extensively revised and updated version of that chapter (with a tighter focus on top–down and bottom–up processes than in our book). As our main expertise in ecology and evolutionary biology lies elsewhere, this paper should be considered as a pair of outsiders’ view of one of the classic questions of vegetation ecology.
Hairston, Smith, Slobodkin and the green world question
The question “Why is the world green?” was famously articulated in a classic short paper published in The American Naturalist by Hairston et al. (Citation1960) After discussing various alternatives, their tentative answer was that herbivores do not consume all of their food supplies simply because predators keep their numbers in check. They supported their arguments by pointing to exceptions where human action or ‘natural events’ removed predators leading to rodent plagues or insect outbreaks destroying the vegetation. Very often in science posing a good question can be as important as providing an answer and the questions raised by Hairston et al. (Citation1960) have become important ones in ecology – even for scientists who did not support their proposed answer. As Power (Citation1992, p. 733) pointed out: “As an irritant, the HSS theory (Hairston et al. Citation1960) has been highly productive. Ecologists challenging the assumption that a green world is an edible one have developed the active field of plant defence theory.”
On first reflection the idea of Hairston et al. (Citation1960), that predators are crucial to answering the green world problem, seems persuasive. Particularly interesting examples are those herbivore species that are well protected from predators and can sometimes completely exploit their plant hosts in the way Hairston et al. (Citation1960) suggested. A good example, familiar to many naturalists living in Europe, are the caterpillars of the cinnabar moth (Tyria jacobaeae); these are particularly relevant to our discussion as there are reasons to suppose that these caterpillars may have less trouble from predators than do many insects. These black-and-orange-striped caterpillars are commonly seen on the ragwort (Senecio jacobaea) and a few related plant species, and will sometimes eat all the leaves and flowers of their food plant before leaving the now-bare stems to crawl away in search of a new plant – often dying during their search (Majerus Citation2002). The striking colours of the caterpillars are a warning to potential predators that these larvae contain poisonous chemicals, which include a range of alkaloids acquired from their food plants (Aplin et al. Citation1968; Majerus Citation2002). Certainly, the caterpillars often feed in full view of potential predators without being eaten (). As we discuss in more detail later, because the cinnabar moth caterpillars only eat a few closely related plant species, they cannot change their whole environment from green to brown. However, if all insect species behaved in a similar way plant biomass could be greatly reduced. Therefore, Hairston et al.’s (Citation1960) original argument, that predators of herbivores may be crucial to keeping the world green, is certainly plausible.
Figure 3. Cinnabar moth Tyria jacobaea caterpillars feeding on ragwort Senecio jacobaea in Lincolnshire, England. Note that they are prominently positioned on the plant relying on their toxins and warning colouration to escape predation.

Such examples can be summarised as a simple food chain, with effects driven by taxa higher in the chain (e.g. predators) being described as top–down effects, while those driven by taxa low in the chain being bottom–up effects. Therefore, Hairston et al.’s (Citation1960) explanation for why the world is green is a top–down one based on the predators of herbivores – indeed, this paper is often cited as introducing the idea of top–down control to ecology (Power Citation1992; Ripple and Beschta Citation2012). The other main class of explanation makes use of bottom–up effects, with plants themselves limiting the effectiveness of herbivores. However, this is still a controversial area of ecology with “a lack of consensus about how top–down and bottom–up forces interact to structure terrestrial ecosystems” (Ripple and Beschta Citation2012, p. 733). We describe both of these ‘forces’ in more detail below in the context of relevant experimental and other evidence, before briefly reviewing some related ideas which do not neatly fit into this top–down vs. bottom–up dichotomy, including recent ideas on the importance of interactions between these two processes. This review is limited to terrestrial vegetation (see Sherratt and Wilkinson Citation2009, chapter 8 for a summary of our view of marine systems). We conclude by suggesting that of these two processes bottom–up is probably more important for explaining the continual presence of extensive terrestrial vegetation, while stressing that the nature of vegetation is controlled by a combination of these two processes (along with other factors, such a climate). A simple ‘either/or’ approach (i.e. top–down or bottom–up) can be a useful starting point for organising one’s ideas but applied too simplistically to a complex system, such as vegetation, it is ultimately likely to confuse as much as it helps.
Top–down – experimental data
An obvious first step to evaluating top–down explanations is to observe what happens when the number of herbivores is altered. The most direct test of the top–down theory would involve removing all the predators and parasites of the herbivore species in question and simply seeing what happens – if the herbivores reach such high densities that they consume all the available vegetation, then we would have direct proof of the validity of the top–down hypothesis. However, such manipulations are extremely challenging, and most studies have understandably settled for simply evaluating the impact of herbivores on vegetation in a general sense, either by removing them or by increasing their density. This can be done either by experimentally manipulating the number of herbivores, or by finding a location where natural or man-made changes have led to the required ‘experimental’ conditions. To give an idea of the sort of results such studies have produced we describe three examples in some detail (slightly modified from Sherratt and Wilkinson Citation2009), and then try to draw together their main message relating to the validity of top–down explanations in answering this central question of this review.
A common experimental approach to studying the effects of herbivores are exclosure experiments. A good example is a long-running series of experiments in the mountains of North Wales, British Isles. These experiments were set up in 1957 to investigate the effects of excluding domestic sheep (Ovis aries) from patches of montane vegetation (note that small herbivores such as voles and invertebrates, such as slugs, could still access the vegetation). Initially, there was a rather complex experimental design where, by moving the fences during the year, sheep were excluded from some areas all the time but allowed to graze others at certain times of the year. This proved rather time consuming to maintain and from 1982, sheep were permanently excluded from all the exclosures (Hill et al. Citation1992). The results of these experiments are typical of many similar studies (Watts Citation1981; Jacobs and Naiman Citation2008; Wigley et al. Citation2014; Moore et al. Citation2015) in that the vegetation inside the exclosure grew taller than the more heavily grazed surrounding vegetation and, more importantly, developed a different species composition. Several plant species, such as ling heather (Calluna vulgaris), which were kept small and rare by the sheep grazing, developed to dominate parts of the exclosures, so even from a distance the vegetation in some of the exclosures appears different from its surroundings (). It is of note that some of these Welsh exclosures showed greater changes than others – illustrating there is more to what governs the vegetation than just grazing levels. However, it is clear that herbivores do indeed have a measurable impact on the physical structure and species composition of vegetation, yet we still have to explain why the heavily grazed land around these exclosures was still ‘green’, as food for herbivores is apparently still available but not being used.
Figure 4. One of the North Wales, Great Britain exclosures – this one is in Cwm Idwal (‘site 2ʹ of Hill et al. Citation1992). The top photograph (a) was taken in 2002 – note the much shorter grass outside the exclosure where there has been sheep grazing and the dark coloured heather Calluna vulgaris bushes within the exclosure. As part of the management of the Cwm Idwal nature reserve, attempts were made to exclude all sheep from late 1998. In 2001, Britain suffered a major outbreak of foot and mouth disease, which led to a decline in sheep numbers in the area making it easier to exclude sheep from the reserve (although a few still get in). Slowly, the grass outside the exclosure is getting taller, and it is easier to fine small heather plants amongst the grass. Therefore, the 2002 photograph shows about the maximum difference between inside and outside the exclosure. The lower photograph (b) shows the same view taken in 2015; note how the vegetation outside the exclosure is starting to look more similar to that within the exclosure, now that sheep grazing levels are much reduced (a 2008 photograph is reproduced in Sherratt and Wilkinson (Citation2009) and shows amounts of heather outside the exclosure intermediate between the above two photographs).

The above experiment used fences – rather than predators – to alter the density of herbivores, and in this sense it is rather artificial. In addition, the principal herbivore was a domesticated animal whose density was mainly determined by agricultural policy. A more realistic ‘experiment’ has been established in eastern Venezuela by the creation of a new lake for the production of hydroelectricity. This has led to the formation of a large number of forest-covered islands, which have been the subject of detailed studies by Terborgh et al. (Citation2006).
Six of the islands investigated by Terborgh et al. were too small to support viable populations of medium-sized mammals such as armadillos and various primate species, which can be significant predators of invertebrate herbivores – especially leaf cutter ants (Attini). The six small islands were found to have much lower densities of tree saplings than the larger islands, almost certainly because there were no predators to reduce the ant populations that defoliate the trees. Indeed, Terborgh et al. suggested that the small (predator free) islands with higher leaf cutter ant densities were heading towards a future vegetation with very few trees, that would eventually be dominated by ant-resistant lianas. They describe their results as consistent with the top–down green world hypothesis of Hairston et al. (Citation1960) – however, they note that the absence of major predators of the herbivores is not leading to a plant free system (a brown world) but one that is still green, albeit dominated by very different types of plants. In addition, the lianas presumably survive because of bottom–up processes, such as chemical defences, of the kind we discuss below making them poor food for ants.
Top–down – large-scale observational data
Both of the examples, we presented above come from experimental or quasi-experimental approaches. However, although potentially very informative, formal experiments also have some disadvantages, such as being limited to relatively small-scale studies as very large-scale modification of the environment can range between the impractical and the impossible. The alternative is to make detailed observations on relatively natural systems, preferably over many years. This can often allow studies of much larger systems than those that can be experimentally manipulated.
One good example of such an observational study are the effects of the size of the moose (Alces alces) population that feed on young balsam fir trees (Abies balsama) on Isle Royale in Lake Superior, in Michigan, USA, where moose exert a potential top–down control on the plant life. This island is 544 km2 in area, and both moose and wolf (Canis lupus) populations (which prey on moose) have been studied since 1958 (wolves first reached the island in the late 1940s and their population has now declined to close to extinction). This work forms a classic example of the importance of long-term field data in ecology (Nelson et al. Citation2011) and these long-term studies have identified an apparent link between snow depth and moose population size, and also demonstrated a role for wolf predation and even global climate fluctuations in affecting the Isle Royale system (Post and Stenseth Citation1998). At the end of the 1990s, Post et al. (Citation1999) published a stimulating hypothesis about the ways in which different components of the system might be inter-related, which we describe below.
In some winters, the island has much more snowfall than in others due to changes in the behaviour of air masses over the North Atlantic (the North Atlantic Oscillation). During these snowy winters, the wolves tended to form larger packs which made them abler to successfully hunt bigger prey, namely moose. This caused the moose population density to decline and consequently reduced the amount of browsing on fir tree saplings. Post et al. (Citation1999) were able to show connections between fir tree growth and moose numbers by studying the annual growth rings in the firs – trees that survived the attentions of moose showed periods of low growth when they were saplings during years with higher moose numbers. This provides another example of top–down effects on vegetation, at a larger scale than the other studies we have described, but we again note that it is affecting the type of vegetation (reducing the success of young trees and so potentially favouring less woody vegetation), not the presence or absence of vegetation. It also illustrates a new complication, which was the main focus of the paper by Post et al. (Citation1999), that vegetation changes were being driven not by changes in the number of predators but by changes in predator behaviour, which, in turn, was caused by changes in the climate Indeed, the role of the behaviour of predators and/or prey, rather than just their population size, is a growing area of research in relation to its effects on vegetation.
What are the main implications of the studies discussed so far (which appear typical of many others we have not had space to describe) for explaining why parts of the world manage to remain green? They suggest that while top–down processes controlling herbivore numbers can have big effects on the type of vegetation, they often do not exercise sufficient control over herbivore numbers to turn a green world into a brown one, even when the majority of predators of herbivores are removed from the system. In some instances, for example, as the numbers of a particular herbivore increase through control of their predators, then vegetation that is more resistant to the herbivore is competitively favoured. The species traits that are well suited to high levels of herbivory include thorns, toxins or other defences (see later). So, while herbivores may select for the types of vegetation that are competitively successful, it is the defences of these more herbivore-resistant species that help keep these otherwise vulnerable populations viable. Paine (Citation2000) has summarised the position well, writing that while the world may stay ‘green’ in the presence of abundant herbivores “the dynamics are substantially altered” – that is, even if herbivores cannot (for whatever reason) completely exploit their green resources, they can control the type of vegetation present (as in the earlier examples).
Over time, the planned or incidental removal of top predators (e.g. wolves) has created a range of relevant natural experiments. For example, Ripple and Beschta (Citation2012) analysed 42 published studies across North America and Eurasia and found that deer numbers averaged around six times greater in areas without wolves compared to those where wolves were present. However, while high deer densities in areas, such as in the British Isles (in which the wolf is extinct) are greatly altering the type of vegetation in many woodlands, and other habitats, they are not leading to a brown world just a different green one (e.g. Rackham Citation2003; Kirby and Morecroft Citation2010).
Bottom–up?
If top–down processes cannot provide the full answer to our green world question what about bottom–up processes? The naïve view of plants is that they just sit there, rooted to the spot, waiting for animals to eat them. This is an easy assumption to hold, as with the exception of thorns, spines and stings, the anti-herbivore adaptations of plants are less obvious that those of most animals, who can fight or run away and hide from danger. However, plants may be difficult to eat because they contain poisonous chemicals (such as the alkaloids in ragwort). Indeed, 40 years ago Janzen (Citation1975, p. 39) described the herbivores’ view of plants thus: “The world is not coloured green to the herbivore’s eyes, but rather is painted morphine, l-DOPA, calcium oxalate, cannabinol, caffeine … etc.” In addition to these chemicals, plants may also be of poor nutrient quality, for example, being low in nitrogen by the standards required by an animal. There are two complementary ways in which bottom–up explanations could work, namely that (i) individual herbivores will poison themselves (or cease eating) in the short term if they consume too much plant material thereby leaving the rest of the plant, and (ii) there is a long-term effect of such chemicals on herbivore population size, such that plants with secondary compounds and low nutrients cannot support high herbivore densities (as this second option can be hard to phrase in a form that does not appear implausibly group selectionist – presumably (i) is the main factor driving selection with (ii) being a unintentional but beneficial consequence). Not all commentators have distinguished clearly between these two inter-related phenomena, but both may help to explain our green world. Ultimately, however, it will be the population size(s) of an herbivore species (or a range of species) that determines whether their food source is exploited to destruction, not the appetites of individual organisms.
The bottom–up explanation for why the world is green was mainly developed during the 1970s (Southwood Citation1973; Janzen Citation1975: Feeny Citation1976; Lawton and McNeill Citation1979); these concepts built on the idea developed during the 1950s that many secondary plant compounds were responsible for preventing invertebrate attack. Our modern understanding of the functions of these compounds is generally credited to Frankel (Citation1959) although the idea can be traced back to the nineteenth century. For example, the German Botanist E. Stahl wrote in 1888 that “the great differences in the nature of chemical products … [produced by plants] … are brought nearer to our understanding, if we regard these compounds as means of protection, acquired in the struggle with the animal world” (translation from Frankel Citation1959).
We have already used the cinnabar moth to illustrate how poisons taken from an insect’s food plant can be used to protect the insect itself. However, these chemicals make it difficult for most herbivores, without the cinnabar’s special adaptations, to eat large quantities of ragwort. To see evidence of the effect of plant chemical protection on insects one only needs to look at a field guide to butterflies where one will see that the caterpillars of most species specialise in eating a limited number of plant species. One obvious potential explanation for this is that caterpillars have to specialise in only a few food plants because these are the only ones to which they have evolved mechanisms for dealing with potentially poisonous chemicals in the plant tissue. There is also a potentially important asymmetry here – with the plants needing only one effective toxin to restrict an insect’s ability to eat it, while the insect needs to be able to detoxify all toxic chemicals produced by a plant it wants to eat (Speed et al. Citation2015).
The idea that plant secondary compounds can cause dietary specialisation does not apply only to insects; similar patterns are seen in some herbivorous mammals where dietary specialisation allows them to deal with only a subset of all potential toxic plant chemicals. This detoxification may rely on enzymes produced by the mammals themselves or on the properties of the microorganisms that live in their guts (Freeland and Janzen Citation1974).
The diversity of plant secondary compounds is impressive with a wide range of defensive chemicals used by plants including alkaloids, cyanogenic glycosides and various toxic proteins. All of the above-mentioned chemicals have probably evolved to reduce the consumption of plants by herbivores (but see the caveats later). Some of them are continually present in plants and others are synthesised when the plant is attacked – e.g. many alkaloids (Janzen Citation1988). Such induced defences, which are switched on when a plant is attacked, make it harder for herbivores, such as caterpillars, to defoliate whole plants so turning a green world into a brown one. The mechanisms and ecological effects of induced plant defences have been recently reviewed in some detail by Kant et al. (Citation2015).
To recap, the most obvious bottom–up explanation for our green world stresses poisons, and we have good evidence that such chemicals can provide some protection from herbivores (although there are complications – described later). All the same, as the example of ragwort and the cinnabar moth showed, poisons can be circumvented by specialist predators. So, as Janzen (Citation1988) has pointed out, there are really two questions here, while chemical poisons may explain why most herbivores cannot eat most plants, they do not explain why specialist herbivores do not consume all their food supply.
Bottom up – not only poisons
The second proposed bottom–up mechanism keeping the world green suggests that plants make poor food for animals even if they are non-toxic. From the perspective of a typical animal, there is a stoichiometric problem with eating plants. Nitrogen is a particular problem as a typical animal may contain in its constituent chemicals 10 times more nitrogen than the leaf it is eating, and if it feeds on especially nitrogen-poor plant material, such as wood, the ratio is even higher (Lawton and McNeill Citation1979). Therefore, to obtain enough nitrogen an animal may need to eat very large amounts of plant material, much more than they require for energy, in order to acquire enough nitrogen to manufacture proteins and nucleic acids. There is also a connection with the previous toxic chemical explanation, because in eating this large quantity of plant material the animal may ingest a high dose of any poisonous chemicals which are present. There is, however, a potential problem – if a great deal of plant material has to be eaten for an individual herbivore to survive and reproduce then why would this lead to a greener world and not a browner world (Moran and Hamilton Citation1980)? One answer is that if a potential herbivore is struggling to acquire nutrients and energy, then it is unlikely to leave as many offspring. It, therefore, seems reasonable to assume that the long-term average population size of an herbivore species faced with a shortage of high quality food would be lower than one with an abundance of quality food resources. Again, such verbal arguments are best formalised and verified using mathematical models.
Plants can also produce chemicals, such as tannins, which, while not directly poisonous, reduce the palatability of plants to herbivores and may also be broken down into potentially toxic compounds in the animal’s gut (Crawford Citation1989; McArthur and Sanson Citation1993). A tannin-rich diet can cause herbivores to alter the amount of time they spend feeding in a given vegetation type (Mkhize et al. Citation2015). In addition, plants can have tough leaves and contain varying amounts of structural compounds such as lignin and cellulose that are very difficult for animals to break down (Hartley and Jones Citation1997). Many of these compounds are probably not primarily adaptations to protect plants from herbivores; their defensive properties being pleiotropic by-products of other selection pressures on plants. For example, lignins and cellulose, which both contribute to making plants difficult to eat, are key in providing structural support that allows plants to grow tall, and hence compete for light with other plants. The low nitrogen levels may also not have directly evolved as adaptations against herbivores, since nitrogen is often scarce in soils so it is not surprising that it is also often scarce in plant tissues as well (Vitousek Citation2004). In addition to the low nitrogen problem, the relative shortage of other nutrients may force herbivores to eat a range of plant species reducing the consumption of any given species. For example, although the mopane tree (Colophospermum mopane) is a preferred food of elephants Loxodonta africana in the Kruger National Park, South Africa they appear to seek out other less abundant (and apparently less nutritious) plants, such as dry season grasses to supplement their diet, presumably with nutrients scarce in their preferred foods plants – rather than just eating the abundant and usually preferred mopane (Codron et al. Citation2011).
These bottom–up ideas are very plausible explanations for why the land is green, but ideally we would like experimental tests that show that they actually work – rather like the exclosure experiments we described for evaluating the role of herbivores. The difficulty is that it is usually much easier to alter the number of herbivores, or their predators, than it is to modify the chemical make-up of plants. One of the more interesting tests of these bottom–up ideas comes from a ‘natural experiment’ caused by air pollution in north-west Europe. Heathlands (plant communities dominated by ericaceous dwarf shrubs) are greatly prized by many European conservationists because several rare species are associated with them. Many of these heathlands have tended to lose heather species during the twentieth century and purple moor grass (Molinia caerulea) has expanded in population size to take its place. These declines in heather abundance are usually blamed on air pollution raising the nutrient status of the heathland, although there is evidence from plant remains preserved in peat cores that such changes could also have happened before the rise of industrial air pollution (Chambers et al. Citation1999). Given that pollution-related nutrient levels are heavily implicated, the question is “how do increased nutrients cause these changes?” There is evidence from heaths in Holland that bottom–up processes are involved. In experiments conducted by Brunsting and Heil (Citation1985), fertilizer was added to Calluna-dominated heath. They were able to show that this raised the nutrient levels in the plants and so allowed a build-up of heather beetle (Lochmaea suturalis) populations due to this improvement in their food supply. They suggested that these beetles reduced the dominance of the heather and so allowed the purple moor grass to invade the plant community. Although not a perfect experiment – for example, it would have been helpful if they had been able to keep beetles out of a sample of nutrient-enriched sites to prove that it was not just that the fertilizer directly benefited the grass, although such a manipulation would be logistically difficult – it strongly suggests a role for bottom–up processes. Notice also that as with the top–down experiments a brown world has not been created due to an increase in plant palatability, only a different type of green one. This is a recurring theme throughout this review.
More bottom–up – spatial processes and hiding places
Besides poisons and nutritionally poor ingredients there is another possibility – perhaps plants can hide from their enemies? We are used to the idea that many animals are camouflaged to help escape their predators and some plants could use the same trick. An example may be the stone plants of southern Africa () where many species closely match the colours of the stones in its particular desert habitat (Culham Citation2007), presumably making it hard for any visually hunting herbivores to recognise them, although we are not aware of any experiments that show this. However, visually camouflaged plants appear unusual. An intriguing possibility is that many more plants may show olfactory camouflage – as many herbivores rely on smell and taste to find food rather than mainly using vision. Perhaps the most common way that plants evade detection by herbivores is simply through their wide spatial distribution. Essentially, this suggests a view of the world as a shifting mosaic of plants getting to grow relatively unmolested in a given area, until herbivores find the resource, reproduce and begin consuming. The herbivores then die or disperse, and plants can recover from seeds, rhizomes or woody tissue that are protected from attack (Owen Smith Citation2008). As this explanation depends on the distribution of plants, it is a bottom–up explanation (although it does not directly involve plant chemicals). Such spatial ideas, and in particular concepts of refugia, have been very influential in population ecology in the last few decades since they can clearly facilitate coexistence (see, for example, Hassell et al. Citation1994; ) and they have recently started to be considered in the specific context of the ‘green world’ problem (Gripenberg and Roslin Citation2007).
Figure 5. Two living Transvaal Stone Plants in the Namibian Desert (one is at the centre of the photograph with the other to its left). The various species of stone plants are one of the few plant groups that appear to be visually camouflaged in the same way many animals are.

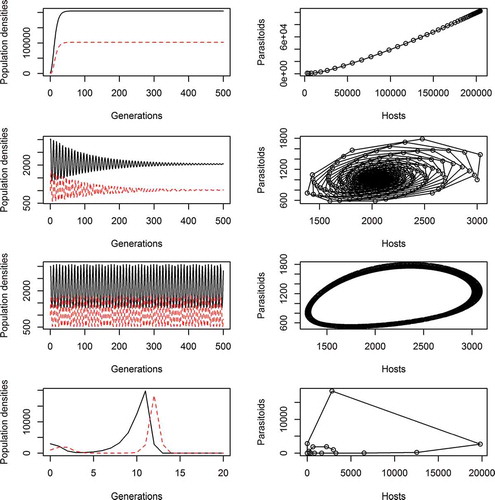
Figure 6. The role of spatial refugia in promoting population coexistence. Here, we depict the dynamics of hosts (H) and parasitoids (P) using the classical Nicholson–Bailey difference equations: Ht+1 = λHt f(Pt), Pt+1 = cHt(1 – f(Pt)), where f(Pt) = 1/{1+(aPt/k)}–k and λ = 2, a = 0.001, c = 1, H0 = 3000, P0 = 1000, and k is the clumping parameter of the negative binomial (low k represents a clumped distribution of parasitoid eggs over hosts, and infinite k represents a random distribution of eggs over hosts). In the case of insect herbivores, such as caterpillars, it is easy to see the plants as ‘hosts’ and the herbivores as egg-laying ‘parasites’ – suggesting such classical models may be informative in the context of the green world problem. The first column shows how the densities of hosts (black, continuous line) and parasitoids (red, broken line) change over time, while the second column shows the parallel plot of the density of hosts against the density of parasitoids at any given time (the phase portrait). Rows 1–4 from the top depict k = 0.1, 0.95, 1, 1000, respectively. For high k (close to random distribution of parasites to hosts, bottom row), the coexistence of hosts and parasitoids is not possible under these dynamical rules (see Hassall Citation1978 for more details). However, for low k (top three rows) then coexistence is readily achieved – parasitoids at no stage kill all available hosts.
Explicit consideration of the challenges of finding plants to eat also raises other interesting issues. During the 1960s and 1970s, several scientists realised that some species of plants would be more easily found by herbivores than others, and that this has potentially important implications for anti-herbivore adaptations of plants. The idea is known as ‘apparency’ and proposes that large long-lived plants, such as trees may be more easily found by herbivores than small short-lived plants, such as annuals (Feeny Citation1976; Hartley and Jones Citation1997). It suggests that the more apparent plants (e.g. most trees) should invest in chemicals that reduce digestibility – such as tannins or lignin – while less apparent plants should use the kinds of toxins we have already described. The logic behind this is that apparent plants are likely to be found by specialist herbivores which will have evolved ways of neutralising a plant’s toxic secondary compounds, so the plants are better off using other means of defence. There are problems with this idea; for example, trees produce many of their indigestible compounds for structural reasons so their presence may have little to do with anti-herbivore adaptations. In addition, it has been very difficult to establish a convincing way of scoring a plant’s apparency – which makes testing the idea difficult (Hartley and Jones Citation1997). Because of such problems, the idea has tended to fall out of favour with many plant ecologists. However, Peter Grubb (Citation1992) – for whom this review series is named – considered the apparency idea in the context of plant spines, rather than chemicals, and suggested that despite the problems we have just outlined he considered it a useful idea, albeit a bit too simplistic. So, although the idea of apparency has not been very fashionable with plant ecologists in recent years, it is probably capturing something of importance for bottom–up explanations of the green world. Collectively, these spatial processes provide a rather mixed explanation for why specialist herbivores do not destroy all plant life – herbivores simply cannot find all available plants, and when they do, the plant is not entirely palatable.
What about microbes?
In our review, we have so far largely considered insects and mammals as herbivores but we have been forced to ignore microbes as there is little in the way of relevant experiments to draw on. Although not usually described as herbivores, many microorganisms can consume and destroy fresh plant material as well as leaf litter.
Despite this relative lack of study, microbes may play a role in keeping the world green and some fascinating relationships are starting to be discovered. For example, one study showed that antibiotic-producing bacteria in several species of southern beech trees (Nothofagus spp.) and other trees, apparently provide partial protection from fungal attack (Castillo et al. Citation2007). Similarly, it is well known that some species of fungi that live within grasses can produce alkaloids that can help protect both fungi and grass from attack by herbivores (Wilson Citation1993; Spooner and Roberts Citation2005). So, microbes may feature in bottom–up processes. Micro-parasites are also probably very important in many top–down processes, by potentially controlling the population size of many herbivores. Of course, it is a two-way street because microbes can facilitate as well as prevent defoliation – for example, gut-living microbes are also important in allowing many animals to detoxify plant secondary compounds (Freeland and Janzen Citation1974) and can also directly destroy plant material. Indeed, why microbes – given their potential for rapid population growth, do not destroy the green world is an interesting question. Presumably bottom–up processes such as the innate immune system of plants (Jones and Dangl Citation2006) play a major role in this. On occasions, top–down process may also have a role – for example one of us has recently speculated that heterotrophic protists may play a role in keeping bacterial populations in lichens under control (Wilkinson et al. Citation2015). The role of microbial processes in each of these contexts is understudied, and we may find out a lot more over the next few decades.
Bottom–up processes appear to be a large part of the explanation, but there are some problems
If, as we suggested earlier, top–down mechanisms do not provide a full explanation for why the world is green, then what about bottom–up explanations? Do these provide an adequate answer? They certainly look more promising than most top–down explanations. As we have previously outlined, the bottom–up explanations fall into two main categories. First, either plants are poor sources of the nutrients needed by animals, or plants make poor food because they contain poisons and other defences. Second, spatial processes may play a role in reducing the accessibility of plants to herbivores.
There is a wide range of ways in which plants can provide poor ingredients by the standards of what is required to sustain most animals – for example, we have already mentioned that plants are low in nitrogen compared to what is required by animals. However, there may be a small complication in the specific argument about low nitrogen reducing the density of herbivores if we consider nitrogen’s role in a geological context. This is because there are good reasons for thinking that the average nitrogen content of plants is higher now than it has been in the geological past (Midgley Citation2005). For example, nitrogen concentrations are known to be higher in modern flowering plants than in ferns or cycads – which have a much longer geological history than flowering plants. In addition, the nitrogen concentration in contemporary leaves is related to carbon dioxide levels in the atmosphere, with high concentrations of atmospheric carbon dioxide being correlated with low leaf nitrogen – as higher CO2 levels lead to lower levels of nitrogen-rich Rubisco in leaves (Korner Citation2004).
It is well established that carbon dioxide has declined during geological history. For example, there was a particularly large drop in atmospheric carbon dioxide during the Permian and Carboniferous periods (ca. 250–350 million years ago). This long-term decline is partly because of the effects of land plants on the weathering of various types of minerals, which leads to the removal of carbon dioxide from the atmosphere (Berner Citation2004; Sherratt and Wilkinson Citation2009), and implies that forests of the past had leaves of rather lower nitrogen content. As well as these changes in plant nitrogen, early forests were dominated by huge ferns (Leptosporangiate ferns) and club mosses (Lycopodiaceae) which were much richer in lignin than modern trees and so had less nitrogen in their tissues (Robinson Citation1990).
Collectively, the above observations cause us to be a little more cautious in suggesting a strong role for nitrogen limitation today – as herbivores have apparently coped with even lower levels in the past. For example, in detailed studies of fossil leaves from rocks around 200 million years old in South Africa (which matches another ‘peak’ in carbon dioxide levels (Berner Citation2004)), Scott et al. (Citation2004) found that some of the plant species had up to 50% of their leaves showing signs of insect damage. In addition, the largest land herbivores known were various species of dinosaur, and they apparently had no problem feeding on vegetation – although their large size may have helped them process poor quality plant food, for example by allowing them to retain food in the gut for longer so giving more time for the gut microbes to do their work. Indeed, these potential problems with C:N ratio have been suggested as a possible selection pressure leading to very large size in these dinosaurs (Midgley et al. Citation2002; Wilkinson and Ruxton Citation2013).
One apparent prediction of the bottom–up approach is that plants with more diverse or unusual secondary plant compounds may be expected to have fewer problems with herbivores. However, this ‘common sense’ prediction has not been upheld in all studies. For example, Jones and Lawton (Citation1991) compared the biochemical make-up of British plants in the Apiaceae family and could not find any evidence that biochemically diverse or unusual plant species supported a less (or more) species-rich insect assemblage. Recently, Carmona et al. (Citation2011) conducted a meta-analysis of genetic studies of the relationship between plant traits and herbivore susceptibility. They found no overall relationship between concentrations of plant secondary metabolites and herbivore susceptibility – with other traits (such as life history traits such as flowering time or growth rate) being most strongly linked to herbivore susceptibility. Does this mean that plant secondary metabolites (often cited as the ‘textbook’ example of bottom up anti-herbivore defences) just do not work? Not entirely, as such an analysis is looking for statistical patterns across a large number of examples and this does not rule out there being some convincing examples hidden within this big picture. For example, there are well-known cases where plant chemical defences have been shown to work. Good examples are cyanogenic glycosides, which can liberate hydrogen cyanide to help protect plants under herbivore attack. Although the anti-herbivore effects of such compounds was suggested over a hundred years ago, many scientists remained sceptical because such plants were not perfectly protected from attack (Crawford Citation1989). However, by the 1970s several studies had provided good evidence of its anti-herbivore effects – e.g. a lack of cyanogenic glycosides in populations with little herbivory and its presence where herbivores were a greater selection pressure (Ellis et al. Citation1977; Crawford Citation1989). So, clearly, there are examples of secondary compounds working as anti-predator defences.
Indeed, Carmona et al. (Citation2011) also suggested that such secondary compounds do have a role but that it is ‘more complex than often appreciated’. As illustrated by the example of the cinnabar moth caterpillars discussed above, herbivores can evolve to side-step plant chemical defences. Such co-evolution may lead to many of these chemicals having little function (but if they are not too costly to produce they may be slow to be lost). In models of the co-evolution of plant toxins and the evolution of anti-toxin mechanisms in insects the effectiveness of any given toxin can cycle from effective to relatively useless but then back to effective again over evolutionary time (Speed et al. Citation2015). Carmona et al. (Citation2011) suggested that “secondary metabolites could have evolved to be important defensive mechanisms not because they have the largest effect on herbivores, but because the constraints on their evolution are the weakest.” So, although life history traits may be more important in avoiding herbivores because they have many other functions, the scope for them to be optimised as anti-herbivore defences is limited. In the context of this review it is of note that these life history traits are also examples of bottom–up effects – even if secondary metabolites are not so important as often assumed it does not necessarily undermine the importance of bottom–up processes. In addition, we suggest that the possibility that many secondary metabolites may have evolved as anti-microbial, rather than anti-herbivore, defences.
Top–down and bottom–up; the story so far
Where does this leave us when it comes to explaining why the world is green? Like many questions in ecology there is no single clear answer. The current consensus (which we broadly support) seems to be that bottom–up processes are the most important in maintaining a green world. So, herbivore population densities tend to be limited by their food supply more often than by their predators – but with the important caveat that in natural vegetation there are many feedbacks between these various processes. Indeed, taking an evolutionary perspective, members of a plant species with few defences and low capacity to reproduce would probably go rapidly extinct – so almost by definition we will be left with populations of plant species that can persist despite the ravages of herbivores, even without the help of predators to keep herbivore numbers low. So, while top–down processes can often determine the type of vegetation they are much less likely to explain the persistence of vegetation in the face of herbivory.
Bottom–up processes involving secondary plant compounds probably explain why herbivores do not consume all plant species – many herbivores being required to specialise, or alternatively eat small amounts of many different plants to avoid poisoning. However, some caution is required given the failure of many studies to find a clear relationship between the presence of such secondary compounds and the incidence of herbivore damage. Nevertheless, it is quite possible that top–down processes can play a role in controlling herbivore numbers in some contexts, where predators happen to be at particularly high local densities. In our 2009 review (Sherratt and Wilkinson Citation2009), we suggested that the dual role of these processes was nicely illustrated by recent work in tropical forests in Panama, where predatory control of herbivores appeared to be most important in clearings that allowed intense plant growth, but bottom–up processes dominated in deep shade where plants grew slowly (and had lower nitrogen levels) and food for herbivores was in shorter supply (Richards and Coley Citation2007). In the last few years more studies teasing apart the interactions between top–down and bottom–up processes have appeared. For example, Ford et al. (Citation2014) showed that at a study site in East Africa Acacia species well defended with thorns were found in areas with large numbers of the herbivore impala (Aepyceros melampus) but in areas with fewer impala – because of more cover that might hide predators – less well-defended Acacia species dominated.
In the late 1970s, Lawton and McNeill (Citation1979) neatly encapsulated this diversity of explanations for the green world by describing herbivorous insects as caught between the devil (of top–down processes) and the deep blue sea (of bottom–up processes). They extended this metaphor to the dilemmas facing scientists trying to make sense of this area of ecology, describing them as also caught between “the devil of oversimplification on the one hand and a deep blue sea of endless unrelated facts on the other.” This tension between unrealistic simplification and a bewildering array of detail often faces ecologists and it leads us to an important point – clearly a simple binary division between top–down processes or bottom–up process while a useful starting point is far too simple to be a final answer. As Scholes et al. (Citation2003, p. 258) pointed out in the context of the vegetation of the Kruger National Park in South Africa “In a coupled system, it is illogical to say that control of structure and function is exclusively top–down or bottom–up.” Yet in the green world case there are even more complications! Several ecologists (including Scholes et al. Citation2003) have been arguing that to fully understand this problem we have to add yet another factor to the top–down and bottom–up processes we have so far been describing – namely fire (especially, but not exclusively, in Mediterranean biomes).
A sideways look – fire and other disturbance events
There are several approaches to explaining “Why the world is green?” that do not neatly fit into the classic top–down/bottom–up classification; fire is one of these that has received increasing attention in recent years. Plant ecologists usually define the effects of fire as a type of disturbance, that is, a process “associated with the partial or total destruction of the plant biomass” (Grime Citation2001). As well as fire, disturbance includes the effects of herbivores but also things such as storm damage, trampling and flood damage. For example, in a coastal marine system wave disturbance may be more important than either top–down or bottom–up processes (Reed et al. Citation2011), so these shallow water kelp forests may have more in common with terrestrial vegetation ecology than do the planktonic systems which dominate the oceans (and are not considered in this review).
As William Bond and colleagues have pointed out (Bond Citation2005; Bond and Keeley Citation2005), herbivory and fire have a lot in common that differentiates them from other types of disturbance. In an attempt to draw ecologists’ attention to the importance of fire they have described it as effectively a ‘global herbivore’ because it consumes large amounts of plant material in many parts of the world. Fire has the potential to be a particularly successfully ‘herbivore’ as it is unconstrained by plant poisons, woody tissue or low nitrogen; plants with all or without any of these anti-herbivore mechanisms will burn well if reasonably dry. As such, fire is an herbivore substitute that is unaffected by most of the bottom–up (and top–down) processes described in this review (although some vegetation may be more combustible than others).
In many cases, fire has been given relatively little prominence in ecology textbooks (for example the well-known textbook by Krebs (Citation2009) indexes only one page for ‘fire’), unless they were on particularly fire prone systems, such as Mediterranean-type vegetation. One reason for ecologists underplaying the importance of fire in the past may be the assumption that it is a largely modern phenomenon associated with human activity. While it is true that our actions have greatly increased fire frequency in many parts of the world, while decreasing it in others, geological evidence from charcoal preserved in rocks shows that fire (caused by lightning strikes and volcanic activity, amongst other processes) has been a regular occurrence as long as there has been widespread terrestrial vegetation – that is, for at least 420 million years (Scott and Glasspool Citation2006). As such, fire is clearly potentially important for any explanations of why the world is green because of the additional challenges it poses – it seems to have the potential to turn everything brown or black and yet has not done so.
Fire may be particularly important in savannah habitats. In a global analysis of savannah/forest transitions, Staver et al. (Citation2011) found that at intermediate rainfall levels the presence or absence of forest was best explained by fire – so that in the majority of studies excluding fire caused a switch from savannah to forest (Murphy and Bowman Citation2012). It is important to note that once again, fire is apparently not making a green world brown (or black), but affecting the type of green vegetation we see growing at a particular place. As such, it is behaving more like the sheep of North Wales or the leaf cutter ants of Venezuela, described earlier. There are several reasons why fire does not destroy the green world. First and foremost, spatial heterogeneity can prevent it spreading just as we have seen in the case of herbivores. Second, paralleling another bottom–up control, some types of vegetation will not burn even when dry and that most plant life will not burn when wet. Indeed, the oxygen levels required for wet plant material to easily burn (something over 30%, the current atmospheric level being 21%) are thought to set a limit to oxygen levels in the geological past – as global vegetation was never destroyed in a great global conflagration (reviewed in Wilkinson Citation2006). So, like herbivores, fire usually only influences the type of vegetation, not its long-term presence or absence.
Population biology and the sideways perspective
As we pointed out in the Introduction to this review, although the question “Why is the world green” sounds like a plant ecology question, much of the answer must involve animal population ecology. Consideration of population ecology also contributes additional explanations at various levels, or at very least new ways of looking at the same phenomena. For example, the long-known reduction in biomass along food chains because of loss of energy at each stage (Elton Citation1927) can be thought of as reflecting constraints driven by bottom–up processes. The time taken to find plants, and the defences of plants even when they discovered, all contribute to this reduction in efficiency of energy transfer between levels, and may help explain why herbivore populations are not high enough to completely exploit their resource.
Other factors influencing herbivore population size may also be relevant. It may be that herbivore density is sometimes kept in check by density independent mechanisms, thereby indirectly controlling the extent of herbivory. In an influential series of studies by Davidson and Andrewartha in the 1930s and 1940s (e.g. Davidson and Andrewartha Citation1948) showed that population sizes of apple blossom thrips (Thrips imaginis), in Australia, were largely determined by year-to-year climatic variation. Andrewartha was particularly impressed by examples such as these, and when he came to draw together his ideas on animal ecology in the early 1960s for an introductory textbook, he gave major emphasis to the role of weather in influencing animal population size (Andrewartha Citation1961).
So, clearly one possibility is that herbivores do not achieve the numbers required to turn a green world brown because of environmental (often climatic) constraints prevent herbivores from gaining sufficiently high densities to cause complete exploitation. In addition, these climate effects can potentially interact with top–down processes, most notably control of herbivores by predators. Insectivorous birds may provide a good example, in reviewing the effects of birds as predators of insects, Şekercioğlu (Citation2006) suggested that their ability to control insect population sizes may be limited in temperate latitudes by the vicissitudes of inter-annual climate variation, but that they may be more important in the tropics – although as he pointed out, we are short of good tropical studies from which to generalise.
An additional sideways (i.e. neither top–down or bottom–up) population mechanism is density-dependent competition between herbivores themselves, both within the same species and between species, which again may limit their population sizes to levels that cannot destroy the green world. For example, analysis of 40 years’ worth of data on blue wildebeest (Connochaetes taurinus) populations on the Serengeti, in East Africa, showed that levels of rainfall in the dry season was a key factor in their mortality rates, but this effect only operated when wildebeest numbers were high and so competition for limited food in dry conditions was also high (Mduma et al. Citation1999). Similarly, in the Isle Royale moose population, there was a large crash in numbers during late winter/early spring in 1996, apparently due to a combination of high moose numbers (and a coincident reduction in the wolf population) increasing competition and extreme winter weather (Peterson Citation1999). So, the population effects of both environmental fluctuations and competition between herbivores could in theory be important ‘sideways’ processes as well as the effects of fire described earlier.
So why is the world green?
Our question has been described as “one of the most basic yet astonishingly complex questions in ecological research” (Naeem Citation2008). Indeed, the question can be usefully rephrased in population modelling terms to ask why we have coexistence in a system where one species (or group of species) consumes another – as we showed above () this coexistence is not inevitable but can depend on the exact value of various population parameters. The apparently simple question raised by Hairston et al. (Citation1960) illustrates the complexity of the real ecological world with both bottom–up and top–down control of herbivores contributing to the explanation for the type of persistence of vegetation, along with other (‘sideways’) processes such as fire and several aspects of population ecology which do not neatly fit into this simple classification. Clearly, the dichotomy between top–down and bottom–up is a simplification of the complexities of the real world. However, the studies we have discussed above suggest that there is an imbalance in the relative importance of these processes, with top–down explanations only appearing convincing in a few special cases (e.g. the artificial ‘overgrazing’ of arid areas by livestock maintained at high density). However, there is an increasing emphasis on interactions between bottom–up and top–down processes. The most important way in which this review differs from our earlier 2009 attempt is in an increased stress on the role of these interactions. Indeed, we have already cited with approval the comments of Scholes et al. (Citation2003) pointing out that in a coupled system it does not really make sense to focus on an either/or, top–down or bottom–up view of the key mechanisms. In trying to answer Hairston et al. (Citation1960) simple question scientists have also produced important data on how herbivores and fire contribute to determining the type of ‘green’ found at a particular location. In reality, many of the research papers that describe themselves in their Introductions as investigating the green world problem are really studying the factors that determine the type of green (not why green rather than brown). This is clearly also relevant to topics of current applied nature conservation interest – for example the effects of high elephant numbers on African vegetation (Owen-Smith et al. Citation2006; Kuiper and Parker Citation2014) or the deliberate use of grazing animals to manage vegetation of conservation interest (Small Citation2015).
Having pointed out these complexities and the multiple processes involved in a full explanation, it appears to us that bottom–up processes are probably the most widely applicable explanation for why herbivores do not destroy all vegetation and so they provide an important part of the answer to the green world question. Hunter and Price (Citation1992) stated the point in simple but almost unarguable terms, “the removal of higher trophic levels leaves lower levels intact (if perhaps greatly modified), whereas the removal of primary producers leaves no system at all.” At the end of the 1990s, Polis (Citation1999) summarised this bottom–up view in a widely cited review paper and we think his main conclusions still stand (albeit with caveats about the complexity of coupled complex systems), he wrote “The implication is clear: even in a world full of green energy, many/most herbivores cannot obtain enough requisite resources to grow, survive, or reproduce at high rates. Nutritional shortages regulate herbivore numbers, often limit their effects on plant biomass, and form one important reason why much of the world is green.”
Acknowledgements
We thank the editors Laszlo Nagy and Victor Resco de Dios for inviting this review and three anonymous referees for their encouragement and minor suggestions, which helped in improving the final version of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
David M. Wilkinson
David M. Wilkinson is a Reader in environmental science. His research interests include ecology, evolutionary biology, archaeology and the history of science.
Thomas N. Sherratt
Thomas N. Sherratt is a Professor of evolutionary ecology. His laboratory combines experiment with theory and studies a variety of topics including the evolution of anti-predator defences, and the evolution of territorial behaviour.
References
- Andrewartha HG. 1961. Introduction to the study of animal populations. London: Methuen and Co.
- Aplin RT, Benn MH, Rothschild M. 1968. Poisonous alkaloids in the body tissue of the cinnabar moth (Callimorpha jacobaeae L.). Nature 219:747–748.
- Beerling D. 2007. The emerald planet. Oxford: Oxford University Press.
- Berner RA. 2004. The phanerozoic carbon cycle. Oxford: Oxford University Press.
- Bond WJ. 2005. Large parts of the world are brown and black: a different view on the ‘green world’ hypothesis. Journal of Vegetation Science 16:261–266.
- Bond WJ, Keeley JE. 2005. Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends in Ecology & Evolution 20:387–394.
- Brunsting AMH, Heil GW. 1985. The role of nutrients in the interaction between a herbivorous beetle and some competing plant species in heathlands. Oikos 44:23–26.
- Carmona D, Lajeunesse MJ, Johnson MTJ. 2011. Plant traits that predict resistance to herbivores. Functional Ecology 25:358–367.
- Castillo UF, Browne L, Strobel G, Hess WM, Ezra S, Pacheco G, Ezra D. 2007. Biologically active endophytic steptomycetes from Nothofagus spp and other plants in Patagonia. Microbial Ecology 53:12–19.
- Chambers FM, Mauquoy D, Todd PA. 1999. Recent rise to dominance of Molinia caerulea in environmentally sensitive areas: new perspectives from palaeoecological data. Journal of Applied Ecology 36:719–733.
- Codron J, Codron D, Lee-Thorp JA, Sponheimer M, Kirkman K, Duffy KJ, Sealy J. 2011. Landscape-scale feeding patterns of African elephant inferred from carbon isotope analysis of feces. Oecologia 165:89–99.
- Crawford RMM. 1989. Studies in plant survival. Oxford: Blackwell Scientific Publications.
- Culham A. 2007. Mesembryanthemaceae. In: Heyword VH, Brummitt RK, Culham A, Seberg O, editors. Flowering plant families of the world. Buffalo, NY: Firefly.
- Davidson J, Andrewartha HG. 1948. The influence of rainfall, evaporation and atmospheric temperature on fluectuations in the size of a natural population of Thrips imaginis (Thysanoptera). Journal of Animal Ecology 17:200–222.
- Ellis WM, Keymer RJ, Jones DA. 1977. On the polymorphism of cyanogenesis in Lotus VIII Ecological studies in Anglesey. Heredity 39:45–65.
- Elton CS. 1927. Animal ecology. London: Sidgwick and Jackson.
- Feeny P. 1976. Plant apparency and chemical defense. Recent Advances in Phytochemistry 10:1–40.
- Ford AT, Goheen JR, Otieno TO, Bidner L, Isbell LA, Palmer TM, Ward D, Woodroffe R, Pringle RM. 2014. Large carnivores make savannah tree communities less thorny. Science 346:346–349.
- Frankel GS. 1959. The raison d’être of secondary plant substances. Science 129:1466–1570.
- Freeland WJ, Janzen DH. 1974. Strategies in herbivory by mammals: the role of plant secondary compounds. The American Naturalist 108:269–289.
- Grime JP. 2001. Plant strategies, vegetation processes, and ecosystem properties. 2nd ed. London: John Wiley.
- Gripenberg S, Roslin T. 2007. Up or down in space? Uniting the bottom-up versus top-down paradigm and spatial ecology. Oikos 116:181–188.
- Grubb PJ. 1992. A positive distrust in simplicity – lessons from plant defences and from competition amongst plants and animals. Journal of Ecology 80:585–610.
- Hairston NG, Smith FE, Slobodkin LB. 1960. Community structure, population control, and competition. The American Naturalist 94:421–425.
- Hartley SE, Jones CG. 1997. Plant chemistry and herbivory, or why is the world green? In: Crawley MJ, editor. Plant ecology. 2nd ed. Oxford: Blackwell Science. p. 284–324.
- Hassell MP. 1978. The dynamics of arthropod predator-prey systems. Princeton: Princeton University Press.
- Hassell MP, Comins HN, May RM. 1994. Species coexistence and self-organizing spatial dynamics. Nature 370:290–292.
- Hill MO, Evans DF, Bell SA. 1992. Long-term effects of excluding sheep from hill pastures in North Wales. The Journal of Ecology 80:1–13.
- Hunter MD, Price PW. 1992. Playing chutes and ladders: bottom-up and top-down forces in natural communities. Ecology 73:724–732.
- Jacobs SM, Naiman RJ. 2008. Large African herbivores decrease herbaceous plant biomass while increasing plant species richness in a semi-arid savanna toposequence. Journal of Arid Environments 72:891–903.
- Janzen DH. 1975. Ecology of plants in the tropics. London: Edward Arnold.
- Janzen DH. 1988. On the broadening of insect-plant research. Ecology 69:905.
- Jones CG, Lawton JH. 1991. Plant chemistry and insect species richness of British umbellifers. The Journal of Animal Ecology 60:767–777.
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444:323–329.
- Kant MR, Jonckheere W, Knegt B, Lemos F, Liu J, Schimmel BCJ, Villarroel CA, Ataide LMS, Dermauw W, Glas JJ, et al. 2015. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Annals of Botany 115:1015–1051.
- Kirby KJ, Morecroft MD. 2010. The flowers of the forest. In: Savill PS, Perrins CM, Kirby KJ, Fisher N, editors. Wytham Woods; Oxfords ecological laboratory. Oxford: Oxford University Press. p. 75–89.
- Korner C. 2004. Through enhanced tree dynamics carbon dioxide enrichment may cause tropical forests to lose carbon. Philosophical Transactions of the Royal Society B: Biological Sciences 359:493–498.
- Krebs CJ. 2009. Ecology. 6th ed. San Francisco (CA): Benjamin Cummings.
- Kuiper TR, Parker DM. 2014. Elephants in Africa: Big, grey biodiversity thieves? South African Journal of Science 110:1–3. Art. #a0058. doi:10.1590/sajs.2014/a0058
- Lawton JH, McNeill S. 1979. Between the devil and the deep blue sea: on the problem of being a herbivore. In: Anderson RM, Turner BD, Taylor LR, editors. Population dynamics. Oxford: Blackwell Scientific Publications. p. 223–244.
- Majerus MEN. 2002. Moths. London: Harper Collins.
- McArthur C, Sanson GD. 1993. Nutritional effects and costs of a tannin in a grazing and a browsing macropodid marsupial herbivore. Functional Ecology 7:690–696.
- Mduma SAR, Sinclair ARE, Hilborn R. 1999. Food regulates the Serengeti wildebeest: a 40-year record. Journal of Animal Ecology 68:1101–1122.
- Midgley JJ. 2005. Why don’t leaf-eating animals prevent the formation of vegetation? Relative vs absolute dietary requirements. New Phytologist 168:271–273.
- Midgley JJ, Midgley G, Bond WJ. 2002. Why were dinosaurs so large? A food quality hypothesis. Evolutionary Ecology Research 4:1093–1095.
- Mkhiz NR, Heitkönig IMA, Scogings PF, Dziba LE, Prins HHT, De Boer WR. 2015. Condensed tannins reduce browsing and increase grazing time of free-ranging goats in semi-arid Savannas. Applied Animal Behaviour Science 169:33–37.
- Moore BD, Foley WJ, Wallis IR, Cowling A, Handasyde KA. 2005. Eucalyptus foliar chemistry explains selective feeding by koalas. Biology Letters 1:64–67.
- Moore O, Standen L, Crawley MJ. 2015. The impact of red deer management on liverworts associated with the mixed hepatic mat community and other terrestrial cryptogams. Plant Ecology & Diversity 8:139–145.
- Moran N, Hamilton WD. 1980. Low nutritive quality as defense against herbivores. Journal of Theoretical Biology 86:247–254.
- Murphy BP, Bowman DMJS. 2012. What controls the distribution of tropical forest and savanna? Ecology Letters 15:748–758.
- Naeem S. 2008. Ecology: green with complexity. Science 319:913–914.
- Nelson MP, Vucetich JA, Peterson RO, Vucetich LM. 2011. The Isle Royal wolf-moose project (1958-present) and the wonder of long-term ecological research. Endeavour 35:31–39.
- Owen Smith N. 2008. The refuge concept extends to plants as well: storage, buffers and regrowth in variable environments. Oikos 117:481–483.
- Owen-Smith N, Kerley GIH, Page B, Slotow R, van Arde RJ. 2006. A scientific perspective on the management of elephants in the Kruger National Park and elsewhere. South African Journal of Science 102:389–394.
- Paine RT. 2000. Phycology for the mammologist: marine rocky shores and mammal-dominated communities. Journal of Mammology 81:637–648.
- Peterson RO. 1999. Wolf-moose interaction on Isle Royale: the end of natural regulation? Ecological Applications 9:10–16.
- Polis GA. 1999. Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86:3–15.
- Post E, Peterson RO, Stenseth NCHR, McLaren BE. 1999. Ecosystem consequences of wolf behavioural response to climate. Nature 401:905–907.
- Post E, Stenseth NCHR. 1998. Large-scale climatic fluctuation and population dynamics of moose and white-tailed deer. Journal of Animal Ecology 67:537–543.
- Power ME. 1992. Top-down and bottom-up forces in food webs: do plants have primacy? Ecology 73:733–746.
- Rackham O. 2003. Ancient woodland; its history, vegetation and uses in England. 2nd ed. Dalbeattie (UK): Castlepoint Press.
- Reed DC, Rassweiler A, Carr MH, Cavanaugh KC, Malone DP, Siegel DA. 2011. Wave disturbance overwhelms top-down and bottom-up control of primary production in California kelp forests. Ecology 92:2108–2116.
- Richards LA, Coley PD. 2007. Seasonal and habitat differences affect the impact of food and predation on herbivores: a comparison between gaps and understory of a tropical forest. Oikos 116:31–40.
- Ripple WJ, Beschta RL. 2012. Large predators limit herbivore densities in northern forest ecosystems. European Journal of Wildlife Research 58:733–742.
- Robinson JM. 1990. Lignin, land plants, and fungi: biological evolution affecting phanerozoic oxygen balance. Geology 18:607–610.
- Scholes RJ, Bond WJ, Eckhardt HC. 2003. Vegetation dynamics in the Kruger Ecosystem. In: Du Toit JT, Rogers KH, Biggs HC, editors. The Kruger experience. Washington (DC): Island Press. p. 242–262.
- Scott AC, Anderson JM, Anderson HM. 2004. Evidence of plant-insect interactions in the Upper Triassic Molteno Formation of South Africa. Journal of the Geological Society of London 161:401–410.
- Scott AC, Glasspool IJ. 2006. The diversification of Paleozoic fire systems and fluctuations in atmospheric oxygen concentrations. Proceedings of the National Academy of Sciences USA 103:10861–10865.
- Şekercioğlu CH. 2006. Ecological significance of bird populations. In: del Hoyo J, Elliot A, Christie D, editors. Handbook of the birds of the world. Vol. 11. Barcelona (Spain): Lynx. p. 15–51.
- Sherratt TN, Wilkinson DM. 2009. Big questions in ecology and evolution. Oxford: Oxford University Press.
- Small R. 2015. Livestock biodiversity: coming of age? British Wildlife 27:17–24.
- Southwood TRE. 1973. The insect/plant relationship – an evolutionary perspective. In: van Emden HF, editor. Insect/plant relationships. Oxford: Blackwell Scientific Publications. p. 3–30.
- Sp O, Day T. 2007. A biologists guide to mathematical modelling in ecology and evolution. Princeton: Princeton University Press.
- Speed MP, Fenton A, Jones MG, Ruxton GD, Brockhurst MA. 2015. Coevolution can explain defensive secondary metabolite diversity in plants. New Phytologist 208:1251–1263.
- Spooner B, Roberts P. 2005. Fungi. London (UK): Harper Collins.
- Staver AS, Archibold S, Levin SA. 2011. The global extent and determinants of savanna and forest as alternative biome states. Science 334:230–232.
- Steffan SA, Chikaraishi Y, Currie CR, Horn H, Gaines-Day HR, Pauli JN, Zalapa JE, Ohkouchi N. 2015. Microbes are trophic analogs of animals. Proceedings of the National Academy of Sciences USA 112:15119–15124.
- Terborgh J, Feeley K, Silman M, Nunez P, Balukjian B. 2006. Vegetation dynamics of predator-free land-bridge islands. Journal of Ecology 94:253–263.
- Vitousek P. 2004. Nutrient cycling and limitation; Hawai’I as a model system. Princeton: Princeton University Press.
- Watts AS. 1981. Further observations on the effects of excluding rabbits from Grassland A in East Anglian Breckland: the pattern of change and factors affecting it (1936–73). The Journal of Ecology 69:509–536.
- Wigley BJ, Fritz H, Coetsee C, Bond WJ. 2014. Herbivores shape woody plant communities in the Kruger National Park: lessons from three long-term exclosures. Koedoe 56. Art. #1165. doi:10.4102/koedoe.v56i1.1165
- Wilkinson DM. 2006. Fundamental processes in ecology; an Earth systems approach. Oxford: Oxford University Press.
- Wilkinson DM, Creevy AL, Kalu CL, Schwartzman DW. 2015. Are heterotrophic and silica-rich eukaryotic microbes an important part of the lichen symbiosis? Mycology 6:4–7.
- Wilkinson DM, Ruxton GD. 2013. High C/N ratio (not low-energy content) of vegetation may have driven gigantism in sauropod dinosaurs and perhaps omnivory and/or endothermy in their juveniles. Functional Ecology 27:131–135.
- Wilson D. 1993. Fungal endophytes: out of site but should not be out of mind. Oikos 68:379–384.
