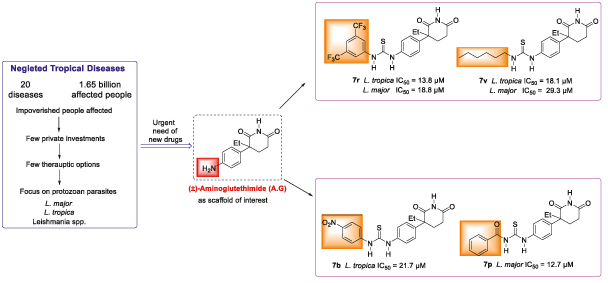
Abstract
Aim: We aim to develop new anti-leishmanial agents against Leishmania major and Leishmania tropica. Materials & methods: A total of 23 thiourea derivatives of (±)-aminoglutethimide were synthesized and evaluated for in vitro activity against promastigotes of L. major and L. tropica. Results & conclusion: The N-benzoyl analogue 7p was found potent (IC50 = 12.7 μM) against L. major and non toxic to normal cells. The docking studies, indicates that these inhibitors may target folate and glycolytic pathways of the parasite. The N-hexyl compound 7v was found strongly active against both species, and lacked cytotoxicity against normal cells, whereas compound 7r, with a 3,5-bis-(tri-fluoro-methyl)phenyl unit, was active against Leishmania, but was cytotoxic in nature. Compound 7v was thus identified as a hit for further studies.
GRAPHICAL ABSTRACT
Leishmaniasis is a protozoal disease exists in three forms, visceral, cutaneous and mucocutaneous. This disease is responsible of thousands of deaths annually.
23 (7a–w) new thiourea derivatives of (±)aminoglutethimide (5) were synthesized by using simple single chemical transformation.
All compounds were purified by column chromatography and characterized by using IR, UV, NMR and MS data.
All synthesized compounds were evaluated for in vitro activity against promastigotes of Leishmania major, and Leishmania tropica as well as BJ human normal cell line.
The mechanism of anti-leishmanial activity of these compounds was predicted by docking studies, indicating that these inhibitors may target folate and glycolytic pathways of the parasite.
The N-benzoyl analogue 7p was found potent (IC50 = 12.7 μM) against L. major, with less activity against L. tropica, and nontoxicity to normal human BJ cells.
The N-hexyl compound 7v was found strongly active against both species, and lacked cytotoxicity against normal cells, whereas compound 7r, which contains a 3,5-bis-(tri-fluoro-methyl)phenyl unit, was active against Leishmania, but was cytotoxic in nature.
Compound 7v was thus identified as a hit for further studies.
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/17568919.2024.2359362
Acknowledgments
We also thank the following researchers of the ICCBS, for the bioassays: U Iqbal and S Ghani (anti-leishmanial), and K Fida (cytotoxicity).
Financial disclosure
We acknowledge the UK Research and Innovation’s Global Challenges Research Fund, under the project agreement ‘A Global Network for Neglected Tropical Diseases’ (grant MR/P027989/1), for part funding of the project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Conflict of interest
The authors have no conflict of interests.
Supporting information summary
A supplementary file provides FTIR, UV/Visible, mass and NMR spectral data in Supplementary Figures S1–S213 & Supplementary Table S1 for molecular docking, Supplementary Figure S214 represent the COSY and HMBC data. Similarly, Supplementary Figures S215–S217 represents the graphical representation of active compounds against L. major, L. tropica and BJ cell line, respectively.
