Abstract
Aim: A new series of 1,2,3-triazole-hydrazone derivatives were developed to evaluate their anti-Alzheimer's activity. Materials & methods: All compounds were screened toward cholinesterases via the modified Ellman's method. The toxicity assay on SH-SY5Y cells was performed using the MTT assay, and the expression levels of GSK-3α, GSK-3β, DYRK1 and CDK5 were assessed in the presence of compounds 6m and 6p. Results: 6m and 6p; acting as mixed-type inhibitors, exhibited promising acetylcholinesterase and butyrylcholinesterase inhibitory activity, respectively. 6m demonstrated no toxicity under tested concentrations on the SH-SY5Y cells and positively impacted neurodegenerative pathways. Notably, 6m displayed a significant downregulation in mRNA levels of GSK-3α, GSK-3β and CDK5. Conclusion: The target compounds could be considered in developing anti-Alzheimer's disease agents.
GRAPHICAL ABSTRACT
A new series of 1,2,3-triazole-hydrazone hybrids were synthesized and evaluated for their anti- Alzheimer's activity.
Synthesized compounds were screened for cholinesterase inhibitory activity to select potent compounds for further biological assessment.
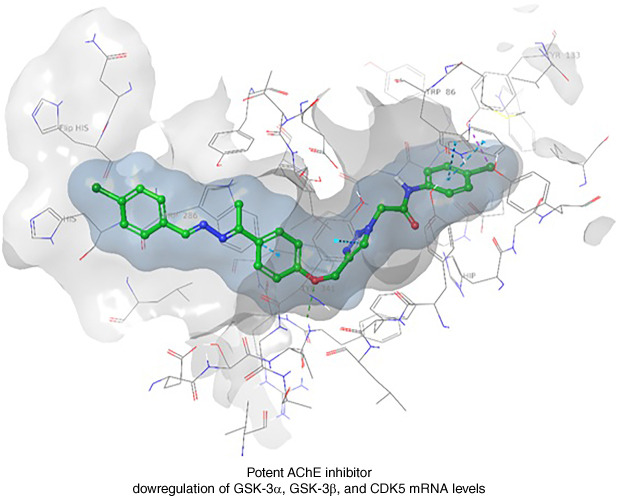
Compounds 6m and 6p exhibited promising inhibitory activity, with IC50 values of 5.23 and 3.39 μM against AChE and BChE, respectively, as mixed-type inhibitors.
Compounds 6m demonstrated no toxicity under tested concentrations on the SH-SY5Y cell line and positively impacted neurodegenerative pathways.
Compound 6m displayed a significant downregulation in mRNA levels of GSK-3α, GSK-3β and CDK5, suggesting a potential indirect role in mitigating toxic neurofibrillary tangle and amyloid β (Aβ) aggregate formation.
Supplemental material
Supplementary data for this article can be accessed at https://doi.org/10.1080/17568919.2024.2359894
Author contributions
D Shareghi-Boroujeni synthesized compounds. A Iraji performed docking studies, wrote the manuscript and supervised biological assays. M Dara studied and analyzed the gene expression. MH Hashempur contributed to the biological assay. S Zare performed MTT assay. R Hariri conducted ChE inhibitory assay. T Akbarzadeh supervised ChE inhibitory assay. M Saeedi designed the project, wrote the manuscript, and supervised all phases.
Financial disclosure
The authors acknowledged the support from the National Institute for Medical Research Development with project No. 996147. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
