Abstract
Aim: The present study describes benzothiazole derived thiazolidinone based thiadiazole derivatives (1–16) as anti-Alzheimer agents. Materials & methods: Synthesis of benzothiazole derived thiazolidinone based thiadiazole derivatives was achieved using the benzothiazole bearing 2-amine moiety. These synthesized compounds were confirmed via spectroscopic techniques (1H NMR, 13C NMR and HREI-MS). These compounds were biologically evaluated for their anti-Alzheimer potential. Binding interactions with proteins and drug likeness of the analogs were explored through molecular docking and ADMET analysis, respectively. In the novel series, compound-3 emerged as the most potent inhibitor when compared with other derivatives of the series. Conclusion: The present study provides potent anti-Alzheimer’s agents that can be further optimized to discover novel anti-Alzheimer’s drugs.
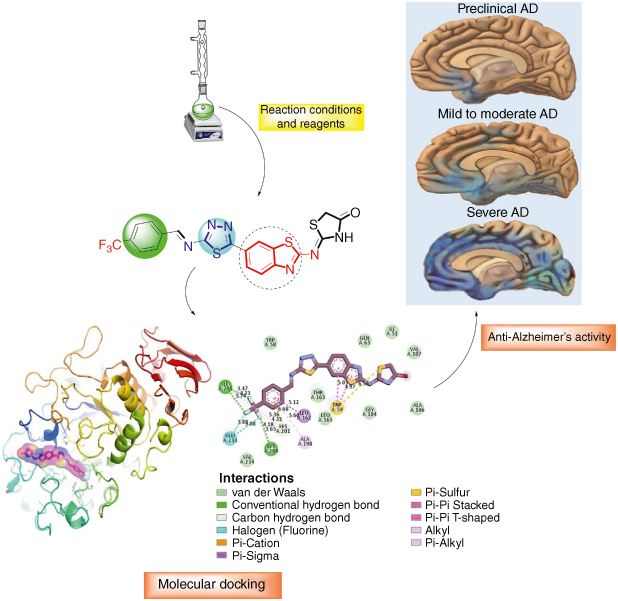
GRAPHICAL ABSTRACT
Background
Alzheimer’s disease is a neurodegenerative cholinergic impairment which is reported as the fourth major death causing disease. Thus, a new selective anti-Alzheimer’s agent is highly demanded across the world.
Synthesis
In order to combat the global health challenge, a novel series of benzothiazole derived thiazolidinone based thiadiazole derivatives was designed and synthesized. Synthetic process is mainly composed of treating benzothiazole bearing 2-amine moiety with ammonium isothiocyanate to obtain benzothiazole derived thiazolidinone moiety. In the subsequent reactions with thiosemicarbazide and varied substituted benzaldehyde were added to afford novel compounds.
Spectroscopic analysis
These derivatives were spectroscopically characterized through 1H-NMR, 13C-NMR and HREI-MS.
Anti-Alzheimer’s potential
Anti-Alzheimer’s potential of the synthesized scaffolds was evaluated in comparison to standard drug donepezil having IC50 = 4.20 ± 0.10 and 5.30 ± 0.20 μM. Compounds 3, 6 and 10 having fluoro and chloro substituents exhibited excellent inhibition profile due to the presence of groups such as -F, -Cl, -OH and -CF3 groups. These functional moieties interact with the target enzyme via number of different interactions. Among these interactions, hydrogen bonding plays a significant role in biological profile of the compound.
Structure–activity relationship
Inhibitory potential of the compounds was demonstrated on the basis of structure–activity relationship interpretation which mainly depends on number, nature (electron-donating/ electron-withdrawing) and position of the substituents around the phenyl ring. It was found that the groups involved in formation of hydrogen bonding have a profound impact on the inhibitory activity, significantly influencing the ability of the compound to bind and interact with targets.
Molecular docking study
Protein–ligand interaction profile of the potent analogs was also explored via molecular docking study. The compounds showing promising inhibition were selected for further investigation through in silico studies to elucidate their binding interactions with enzymes. Detailed 2D and 3D visualizations revealed a range of interactions, including hydrogen bonding and other contacts at various distances, providing valuable insights into their binding mechanisms.
Absorption, distribution, metabolism, excretion & toxicity analysis
Additionally, an absorption, distribution, metabolism, excretion and toxicity (ADMET) analysis was performed on the lead compounds to assess their drug-like properties and evaluate the pharmacokinetic and safety profiles of the most promising analogs.
Supplemental material
Supplementary data for this article can be accessed at https://doi.org/10.1080/17568919.2024.2366159
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSPD2024R812), King Saud University, Riyadh, Saudi Arabia.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
