Abstract
Aim: This study aimed to enhance the aqueous dissolution of SRPK inhibitor N-(2-(piperidin-1-yl)-5-(trifluoromethyl)phenyl)isonicotinamide (SRPIN340). Materials & Methods: A complex with p-sulfonic calix[6]arene (Host) and SRPIN340 (Guest) was prepared, studied via 1H nuclear magnetic resonance (NMR) and theoretical calculations and biologically evaluated on cancer cell lines. Results & conclusion: The 1:1 host (H)/guest (G) complex significantly enhanced the aqueous dissolution of SRPIN340, achieving 64.8% water solubility as determined by 1H NMR quantification analysis. The H/G complex reduced cell viability by 75% for HL60, ∼50% for Nalm6 and Jurkat, and ∼30% for B16F10 cells. It exhibited greater cytotoxicity than free SRPIN340 against Jurkat and B16F10 cells. Theoretical studies indicated hydrogen bond stabilization of the complex, suggesting broader applicability of SRPIN340 across diverse biological systems.
GRAPHICAL ABSTRACT
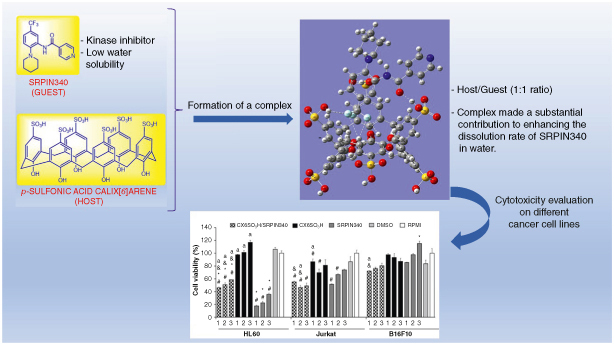
This study aimed to enhance the aqueous dissolution of the serine/arginine-rich protein-specific kinase (SRPK) inhibitor N-(2-(piperidin-1-yl)-5-(trifluoromethyl)phenyl)isonicotinamide (SRPIN340), which has low water solubility.
A complex of p-sulfonic calix[6] arene (CX6SO3H) with SRPIN340 was prepared and analyzed using 1H Nuclear Magnetic Resonance (NMR), showing that the host molecule induced changes in the chemical shifts of SRPIN340 compared to its free form.
The Job plot method indicated a 1:1 stoichiometry for the CX6SO3H/SRPIN340 complex.
The formation of the CX6SO3H/SRPIN340 complex was further investigated using B3LYP theoretical calculations, which revealed stabilization through hydrogen bonds.
The 1:1 host/guest complex significantly enhanced the aqueous dissolution of SRPIN340, with 64.8% of the complex being water-soluble, as determined by 1H NMR quantification.
The CX6SO3H/SRPIN340 complex exhibited a reduction in cell viability of 75% for HL60, approximately 50% for Nalm6 and Jurkat, and around 30% for B16F10 cells.
Notably, the complex showed enhanced cytotoxicity against Jurkat and B16F10 cells compared to free SRPIN340.
The development of the CX6SO3H/SRPIN340 complex represents a significant advancement in improving the aqueous availability and cytotoxic efficacy of SRPIN340.
Supplemental material
Supplementary data for this article can be accessed at https://doi.org/10.1080/17568919.2024.2366690
Financial disclosure
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG – CBB-APQ-02556-15 to GCB; RED-00140-16 to GCB; CBB-APQ-01084-21 to GCB), Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq - grant 420648/2016-0 to GCB) and Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
