Abstract
The purpose of this review is to examine how biochar additions to soil can affect plant diseases caused by soilborne pathogens, with particular attention to mechanisms and knowledge gaps. Until now, biochar soil amendment has been reported to affect the progress of diseases caused by soilborne plant pathogens in six distinct pathosystems. Disease severity frequently exhibits a U-shaped response curve, with a minimum at some intermediate biochar dose. Alteration of plant disease intensity by biochar added to soil may result from its varied influences on the soil–rhizosphere–pathogen–plant system. These influences may involve myriad biochar properties such as nutrient content, water holding capacity, redox activity, adsorption ability, pH and content of toxic or hormone-like compounds. The direct and indirect impacts of biochar on the soil environment, host plant, pathogen and the rhizosphere microbiome can have domino effects on both plant development and disease progress.
This new fertilizing-agent is composed of animal charcoal or bone-black and hydrocarbon oil mixed together, the oil being taken up and retained in the pores of the bone-black … petroleum is an antiseptic, an insecticide, and is destructive to cryptogamic and fungus growths. … [T]he oil, when mixed with bone-black, is not injurious to vegetation. Consequently, I thus obtain a fertilizer, which is also a preservative against those insects, parasites and creatures of fungus origin, which develop beneath the soil and oftentimes destroy the life of the plant – such, for instance, as the potato-rot, the borrowing worm, the ‘philoxera’ or grapevine pest, and a host of others. (US Patent number 152725, by Robert Chesebrough of New York, 1874.)
There were 350 beds sown in the Mont Alto Nursery this year, White pine leading with 250, Norway spruce being sown on 50, and larch, Scotch pine, and Pitch pine occupying a small number each. Constant observation of these beds seems to indicate that where the proportion of charcoal in the bed is large, there is less ‘damping-off’. [Citation1]
Intensive research into agronomic impacts of soil amendment with biochar, the solid co-product of biomass pyrolysis, has revealed that many soil chemical and biological characteristics may be altered when biochar is added to soil: pH, microbial diversity and community structure, GHG emissions, nutrient retention and soil physical structure, among others. In addition, studies have shown that soil amendment with various biochars can affect crop growth and productivity (both positively and negatively), and in some cases, induce systemic plant resistance responses to foliar fungal pathogens [Citation2–4]. Real-time quantitative polymerase chain reaction techniques (qPCR) have provided molecular evidence for the mediation by biochar of systemic strawberry plant defences along with both salicylic acid and jasmonate/ethylene defence pathways, as well as priming for gene expression upon infection by foliar fungal pathogens [Citation3].
| Key terms | ||
| Biochar: | = | Biochar is the solid co-product of biomass pyrolysis – the direct thermal decomposition of biomass in the absence of oxygen to gaseous (syngas), liquid (bio-oil) and solid (biochar) energy-bearing components. Applying biochar to soil, where its turnover is very slow, is considered a means of permanently removing carbon from the atmosphere. |
| Soilborne pathogens: | = | Organisms that live and survive in soil and inflict damage to plants by parasitizing roots and other below-ground plant organs or by growing inside plant inner tissues. Pathogens include bacteria, fungi and fungus-like micro-organisms, viruses, viroids, mycoplasmas, spiroplasmas and nematodes. |
| Foliar pathogens: | = | Pathogens that infect above-ground plant organs are referred to as foliar pathogens. Foliar pathogens include bacteria, fungi and fungus-like micro-organisms, viruses, viroids, mycoplasmas and spiroplasmas. |
| Diseases caused by soilborne pathogens: | = | Common diseases caused by soilborne pathogens are as follows: (i) Seedling blight and damping-off diseases, commonly caused by pathogens that belong to the genera Pythium, Rhizoctonia, Sclerotium or Phytophthora, which infect seedlings during germination, post-emergence or pre-emergence stages. (ii) Vascular wilt diseases, caused by fungi such as Fusarium spp., Verticillium spp. or the bacterium Ralstonia solanacearum, which plug xylem vessels disturbing water movement, especially at the maturation stage. (iii) Root rots, caused by species of Phytophthora, Fusarium, Pythium, Rhizoctonia, etc., which are characterized by decay of the true root system either by attacking juvenile roots or older parts of the root system. (iv) Stem and collar rots, triggered by species of Phytophthora, Sclerotium, Rhizoctonia, Sclerotinia, and Fusarium, causing decay of the stem at ground level leading to symptoms of wilting, death of leaves and death of the plant. |
| Rhizosphere: | = | The narrow region of soil that is directly influenced by root secretions. The rhizosphere nourishes many micro-organisms that feed on nutrients, such as proteins and sugars released by roots, and on plant cell discharges and remains. |
| Rhizosphere microbiome: | = | The rhizosphere microbiome is the population of micro-organisms in the rhizosphere which differs substantially from the bulk soil microbial population in terms of member species distribution, population levels and diversity. Plants rely on the rhizosphere microbiome for specific functions and traits, and, in return, nurture and influence the microbiome by exuding photosynthetically fixed carbon. The rhizosphere microbiome has profound effects on seed germination, seedling vigour, plant growth and development, plant nutrition and plant diseases. |
| Induced resistance: | = | Plants are induced to activate resistance systems to infections caused by foliar or soilborne pathogens through treatment by various biotic or abiotic agents. Biotic inducers include plant growth-promoting micro-organisms, non-pathogenic microbes, pathogenic microbes and cell wall fragments. Abiotic inducers include chemicals of varying natures. The elicitors act by inducing signalling pathways that are involved in disease resistance. |
There is also some evidence that biochar can alter the intensity of diseases caused by soilborne plant pathogens [Citation5–9]. In contrast to systems involving diseases caused by above-ground pathogens, the placement of biochar in the soil environment means it can have both direct and indirect effects on the pathogen and its development. The purpose of this review is to examine plant diseases caused by soilborne pathogens in the context of biochar addition, with particular attention to mechanisms and knowledge gaps.
Plant disease – background
Plant pathogens include species of fungi, chromalveolata (oomycetes, fungus-like organisms), bacteria, viruses, viroids, phytoplasmas, spiroplasmas and nematodes. These pathogens fall into two broad groups: obligate parasites, which depend entirely on living host plant tissue for their nutrition and reproduction, and facultative parasites, which can cause considerable damage to plants, but can also live as saprophytes on plant residues and organic material. Pathogens that infect above-ground plant organs are referred to as foliar pathogens, while those that infect the root system and reside mainly in the soil are referred to as soilborne pathogens.
Soilborne plant pathogens survive in the soil matrix and in residues on the soil surface for extended periods (soil inhabitants) or short periods (soil invaders or soil transients) [Citation10]. They can affect a wide variety of plants, including fruits, vegetables, ornamental crops, annual plants, trees and shrubs, and can significantly reduce yield and quality. Soilborne diseases have a severe impact on nursery and greenhouse production, where plants are grown typically under monoculture [Citation11–13]. Soilborne plant pathogens can survive actively on hosts, as saprophytes on plant residues and organic material, or in resting structures such as melanized mycelium, chlamydospores, oospores and sclerotia until triggered for germination [Citation10,Citation14]. In most cases, underground plant organs are directly affected by soilborne pathogens; however, above-ground plants parts are also affected indirectly [Citation14]. Economic losses due to soilborne pathogens are estimated at 10–20% of the attainable yield for many crops [Citation15]. Acute diseases such as vascular wilts and take-all of cereals may be even more severe and occasionally destroy entire yields. In the USA alone, soilborne diseases are estimated to cause crop losses that exceed US$4 billion/year [Citation16,17].
Relative to methods employed against diseases affecting aerial parts of plants, there are few effective disease control options for the management of soilborne diseases, and those in use do not usually result in comprehensive disease control. In addition, certain practices employed for the control of soilborne diseases can have significant impacts on society and the environment that far exceed the direct costs of the disease to the grower and consumers. For example, although effective disease control may be achieved with a soil fumigant (e.g., methyl bromide), it results in major ecological disturbances to the production system as a whole.
Growth of soilborne pathogens is generally stimulated by root exudates such as amino acids, carbohydrates, organic acids and phenols [Citation18]. Pathogens frequently produce enzymes that degrade host plant polymers during early stages of infection, playing a crucial role in host penetration [Citation19]. Besides production of extracellular enzymes, pathogens can produce non-enzymatic phytotoxins such as phenylacetic acid and its meta- and para-hydroxy derivatives, succinic acid, lactic acid, β-furoic acid and oxalic acid [Citation20–22].
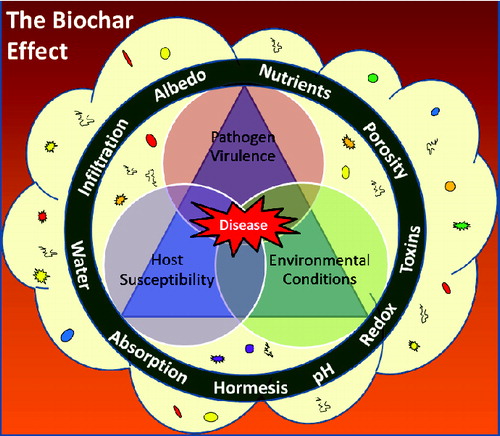
The severity of diseases caused by plant pathogens has traditionally been considered a function of the interplay between the factors at the three vertices of the ‘disease triangle’ [Citation19]: host susceptibility, pathogen virulence and environmental conditions (). In order for disease to occur, all three of these factors need to be suitable. For example, mostly very young seedlings are susceptible to damping-off caused by Pythium, while older seedlings and plants are more resistant. The traditional disease triangle paradigm lacks an essential component: the rhizosphere microbiome (depicted as a cloud in ). The plant, which can be considered a ‘super-organism’ [Citation23], relies on the rhizosphere microbiome for specific functions and traits and, in return, deposits photosynthetically fixed carbon into the rhizosphere, thereby nurturing the microbial community and influencing its composition and activities [Citation23]. The rhizosphere microbiome has profound effects on seed germination, seedling vigour, plant growth and development, plant nutrition and plant diseases [Citation23–27]. Rhizosphere organisms such as nitrogen-fixing bacteria, mycorrhizal fungi, plant growth-promoting rhizobacteria and fungi, biocontrol micro-organisms, and protozoa all can have beneficial effects on plant growth and health and may contribute to the translocation of nutrients and minerals from soil to the plant, improve soil physical structure and provide front-line defences against soil pathogens. The rhizosphere microbiome also contributes to the ability of some plant species to survive under extreme environmental conditions such as drought, flooding and high salinity [Citation28]. Other environmental stressors, including pH and high concentrations of toxic compounds, are also moderated by rhizosphere bacteria [Citation28].
Figure 1. Our conceptual model for the interplay between biochar, the rhizosphere microbiome and the disease triangle. The vertices of the disease triangle include host susceptibility, pathogen virulence and environmental conditions. These are all influenced by the rhizosphere microbiome (depicted as a cloud with microbes). The ring represents the effects of biochar on this system. The various aspects related to biochar (adsorption, soil physical properties, toxins, redox, hormesis, nutrient supply, availability, etc.) can all influence factors of the disease triangle directly (depicted as the ring impinging on the vertices of the triangle) as well as indirectly through impacts on the rhizosphere microbiome (depicted as the ring impinging on the cloud representing the rhizosphere microbiome).
Biochar influences on diseases caused by pathogens
When a biochar is added to the soil, we speculate that it may profoundly influence the complex rhizosphere–root–soil–pathogen system by virtue of a number of physical and chemical properties such as nutrient content, water holding capacity, redox activity, adsorption ability, pH and content of toxic and hormone-like compounds (depicted as ring in ). In our conceptual model, we anticipate that it can affect the disease triangle factors directly as well as indirectly (via its influence on the rhizosphere microbiome). In turn, the direct and indirect impacts of biochar on the environment, host plant, pathogen and rhizosphere microbiome can have domino effects on both plant development and disease progress. Some of these aspects are explored in this section.
Nutrient content/supply
Nutrients supplied by biochar [Citation29] or nutrients which are made more available [Citation30] by the presence of biochar could improve plant vigour and hence influence the ability of the pathogen to infect roots or grow in soil. Improved nutrient supply improves morphological, histological and functional properties of plant tissues and also maintains a high level of inhibitory compounds in tissues or enables quick plant responses to pathogen attack [Citation31]. For instance, high calcium (Ca2+) content in tissues suppresses macerating diseases caused by soilborne plant pathogens by increasing the structural integrity and resistance of the middle lamella, cell wall components and cell membranes to the extracellular macerating enzymes secreted by the pathogens [Citation32,33]. Increased potassium (K+) levels have been seen to increase cotton resistance to Verticillium wilt, probably because K+ acts as a mobile charge carrier and is involved in enzyme activity, membrane transport activity and cell extension [Citation34]. Also, when nutrition is improved under biochar addition, alterations in root architecture such as reduced root hair development may ensue [Citation35]; this can have consequences for host susceptibility since finer root systems offer a greater surface area for attack by soil-borne pathogens [Citation36]. Nutritional impacts are not all unidirectional, as overly fertilized plants are sometimes more susceptible to pathogen attack than are poorly nourished plants. For instance, excessive nitrogen (N) increases the damping-off of broad-leaf tree seedlings caused by Rhizoctonia solani and Pythium spp. [Citation37] and root rot caused by R. solani of spring wheat [Citation38]. Limited root growth and extended shoot growth under high N was suggested as a possible mechanism [Citation38]. Moreover, biochar additions sometimes have deleterious impacts on nutrient availability, leading in particular to N immobilization and suboptimal plant nutrition [Citation39].
pH/Eh
Biochar, which is commonly alkaline, has been frequently observed to increase the pH of soil. In addition, both solid and water soluble parts of biochar are redox active [Citation30,Citation40] and can alter the soil redox potential (Eh). Since many soil pathogens thrive under narrow Eh–pH ranges [Citation41], biochar-induced changes in pH or coupled changes in Eh–pH [Citation41,42] in the rhizosphere could strongly alter pathogen viability. In general, pH and Eh are important drivers of microbial community development, diversity, structure and even pathogen virulence [Citation41]. Given its redox activity, biochar may participate in a wide range of chemical and biological electron transfer reactions in the rhizosphere [Citation30]. These include microbial processes that depend on electron transfer [Citation43], particularly those involved in N cycling [Citation44], as well as chemical processes involving reduction and solubilization of nutrients such as Fe and Mn [Citation30]. The high pH and buffer capacity of many biochars could also reduce the influence of toxic acids near plant roots.
Adsorption
Biochars are well-known adsorbents of large and small organic compounds, with adsorption affinities and capacities that can exceed those of typical soil components by several orders of magnitude [Citation45–48]. Biochars can also interact with many transition metals and alkaline earth elements [Citation49,50] as well as with cationic species such as ammonium. Addition of a strong adsorbent to the soil environment can have far-reaching impacts on the chemistry and biochemistry of the rhizosphere and bulk soil, which can have cascading effects on plant susceptibility to disease. For instance, the adsorption of allelopathic compounds by biochar may improve plant germination and development [Citation51], plant vigour [Citation52] and root colonization by mycorrhizal fungi [Citation5]. Moreover, biochar may adsorb extracellular enzymes [Citation53,54] and other toxins produced by soil disease-causing pathogens. Sorption immobilization of cellulolytic, pectinolytic and other cell wall degrading enzymes by biochars could reduce their contact with root cell walls, thus protecting the plant to some extent. Biochar could change the chemistry of plant root exudates in the rhizosphere by differential adsorption of exudates such as oxalic, malonic and succinic acids [Citation55]. Since root exudates act as chemoattractants for soilborne pathogens by inducing germination of their propagules, attracting motile propagules (e.g., zoospores and bacteria), stimulating pathogen growth and inducing formation of infection structures [Citation12], changes in the exudate profile could influence the suitability of the rhizosphere for development of pathogenic and other micro-organisms. Due to selective adsorption of root exudates, hyphal growth, spore germination and ultimately, pathogen abundance, could actually decrease.
Phyto/biotoxins and hormone-like compounds
Organic compounds associated with the labile fraction of biochar [Citation30,Citation56–58] may either suppress or promote disease micro-organisms in the soil. A number of compounds that are known to adversely affect microbial growth and survival have been identified in biochars, including ethylene glycol and propylene glycol, hydroxypropionic and hydroxybutyric acids, benzoic acid and o-cresol, quinones (resorcinol and hydroquinone), and 2-phenoxyethanol [Citation57]. Other studies have confirmed the presence of various volatile organic compounds in wood vinegar (condensates of wood smoke from pyrolysis), which have been traditionally used as pesticides [Citation59,60]. Methoxyphenols and phenols are formed during pyrolysis of hemicelluloses and lignin [Citation61–63]. These compounds, along with carboxylic acids, furans and ketones are known to inhibit microbial activity [Citation64–66]. Toxic compounds in biochar could also damage plant roots and predispose them to pathogen attack [Citation67,68]. The presence of such chemicals in biochar may be one of the causes of frequently observed U-shaped biochar dose–disease severity responses under biochar addition [Citation4,Citation6–9]. In addition, some types of biochars emit ethylene, which at high concentrations is associated with plant growth inhibition and at low doses with growth-promoting effects [Citation69–71]. In general, hormone and hormone-like compounds give such U-shaped dose/response effects, whereby at low doses they have positive physiological impacts (e.g., disease suppression or growth promotion), but at high doses, negative physiological impacts. This dose-response effect is termed ‘hormesis’ [Citation57].
Soil physical characteristics/temperature
Water holding changes [Citation72] or changes in soil structure [Citation73,74], aggregation, water infiltration [Citation75] or temperature [Citation76] due to biochar additions can alter environmental conditions in the rhizosphere, thus altering niches favoured by pathogens. Additionally, biochar may impact water uptake by plants by increasing the size of the soil water reservoir [Citation77,78], increasing the amount of plant-available water [Citation79–81] or by changing water movement and delivery [Citation82–84]. Biochar-mediated increases in water-holding capacity could potentially favour pathogens that produce zoospores such as Pythium and Phytophthora spp. or bacterial species that are able to move through water [Citation85]. By reducing albedo of bare soils [Citation86], biochar can facilitate quicker soil warming in spring, impacting root growth, development and morphology. Several fungal and oomycetes pathogens perform better in specific conditions of both water and temperature, such that biochar-induced changes in environmental conditions can affect their establishment and survival.
Soil microbial communities and functioning
Microbial community structure and diversity is often altered by biochar additions [Citation57,Citation87–91] and such changes can affect activity and abundance of soil pathogens. Generally, susceptibility of the rhizosphere to invasion by soil pathogens is inversely related to the diversity of the rhizosphere microbiome [Citation92,93], whereby increased diversity can result in decreased pathogen virulence. Moreover, promoted microbes can compete with pathogens for resources, produce compounds that are inhibitory to pathogens or parasitize pathogens. For example, Kolton et al. [Citation87] reported a significant increase of the Bacteroidetes-affiliated Flavobacterium genus in biochar-amended soil. Certain Flavobacterium isolates are highly antagonistic towards the fungal pathogens Sclerotium rolfsii, Lasiodiplodia theobromae, Colletotrichum musae, and Phytophthora cactorum [Citation94–96]. Indeed, most rhizosphere bacteria and fungi are prolific producers of metabolites that inhibit the growth or activity of competing micro-organisms [Citation26]. They also produce a variety of volatile organic compounds that may participate in long-distance communication in the rhizosphere, interfere with quorum sensing of phylogenetically different microbes [Citation97] and induce systemic resistance in plants while simultaneously promoting plant growth. Biochar may disrupt communication between microbes by adsorbing compounds used in intercellular signalling and gene expression regulation [Citation98], and in this way possibly interfere with the intercellular signalling needed to trigger root infections by soil pathogens.
Induced resistance
Biochar can induce systemic plant defences [Citation2–4], with elicitors being biochar-borne chemicals, biochar-induced micro-organisms or both [Citation57]. Induction of the plant's innate defence system can decrease its susceptibility to disease-causing soil pathogens. Induced resistance (IR) in plants is effective against a broad range of pathogens and parasites. IR is defined as a physiological state of enhanced defensive capacity elicited by specific stimuli, whereby the plant's innate defences are potentiated against subsequent challenges [Citation99]. Two general forms of IR have been described in plants: systemic acquired resistance (SAR) and induced systemic resistance (ISR). SAR, which can be triggered by both chemical and biological elicitors, involves synthesis of pathogenesis-related proteins and is mediated by the phytohormone salicylic acid [Citation99,100]. Chemical inducers of SAR include the synthetic SA-analogues 2,6-dichloroisoniciotinic acid (INA) and acibenzolar-S-methyl (BTH) [Citation101,102], methyl jasmonate [Citation103], chitin [Citation104] and chitosan [Citation105], laminarin [Citation106] and β-aminobutyric acid (BABA) [Citation107]. Phosphate salts, silicon, amino acids, fatty acids and cell wall fragments can also stimulate SAR [Citation108–110], as can environmental agents such as osmotic, moisture and proton stresses, mechanical wounding and temperature extremes [Citation111,112]. Induced systemic resistance, commonly triggered by plant growth-promoting rhizobacteria and fungi [Citation14], depends on the phytohormones ethylene, jasmonic acid and methyl jasmonate. In addition to being the possible source of chemical inducers, biochars could themselves cause environmental stresses (such as salinity or pH) and/or change the community of plant growth-promoting rhizobacteria and fungi and in the rhizosphere, thus mediating a variety of systemic IR responses [Citation57].
In short, we propose that the ‘biochar effect’ as related to plant infection caused by soilborne pathogens is composed of many various mechanisms that may be inter-related (), making for highly complex systems that may not behave in a linear fashion. Moreover, mechanisms that are dominant in a given soil–biochar–crop–soilborne pathogen system may be quite distinct from those that are important in another system. (This is discussed in the next section, which is a review of the sparse literature regarding biochar impact on the progression and severity of diseases caused by soilborne pathogens).
Current knowledge
Until now, biochar has been reported to affect the progress of soilborne diseases in six distinct pathosystems: Fusarium oxysporum f. sp. asparagi–asparagus [Citation5,Citation7], Ralstonia solanacearum (bacterial wilt)–tomato [Citation9], Phytophthora spp.–red oak [Citation8], Phytophthora spp.–red maple [Citation8], R. solani–cucumber [Citation6] and R. solani–common bean [Citation113] (). An observation common to most of these studies is that disease severity exhibits a U-shaped curve of response versus biochar dose [Citation6–9,Citation113]. In other words, a minimum in disease severity is frequently observed at some intermediate biochar dose, and disease severity is greater at lower and higher doses. U-shaped biochar dose–response curves were also reported against the foliar fungal pathogen, Botrytis cinerea, in several different biochar–tomato systems [Citation4]. U-shaped dose–response curves illustrate the inherent difficulty involved in deciphering the mechanisms responsible for biochar impacts on severity of diseases. Some of the possible mechanisms, if acting alone, would not be expected to produce a U-shaped dose–response curve. For example, if adsorption of pathogen-produced toxins was the dominant factor in disease suppression, severity of the disease would be expected to decrease monotonically to a minimum asymptotic value with increasing biochar dose. However, as discussed above, any given effect does not operate in an isolated vacuum; rather it generally results in a cascade of further impacts on the chemistry and biology of the rhizosphere, which plays a pivotal role in plant functioning and health.
Table 1. Summary of studies investigating the effect of biochar on diseases caused by soilborne pathogens.
One of the first reports that biochar additions to soil affected the progress of diseases caused by soilborne pathogens was by Matsubara et al. [Citation7], who studied the ascomycete fungus F. oxysporum f. sp. asparagi in asparagus with and without inoculation of arbuscular mycorrhizal (AM) fungi. They found that disease indices were strongly reduced in AM fungi inoculated plants and further reduced when biochar was added. Root colonization by the AM fungi increased in treatments with biochar, which led to the suggestion that biochar may have enhanced the ability of the AM fungi to help the plants resist fungal pathogen infection. Such biochar-induced increases in mycorrhizal competence have also been reported elsewhere [Citation114,115]. A means by which AM fungal growth could be enhanced under biochar addition was put forth in a study of the same F. oxysporum–asparagus pathosystem [Citation5]. In that study, biochar additions enhanced the colonization of asparagus roots by AM fungi despite the addition of allelopathic agents known to reduce AM fungal colonization in asparagus [Citation5]. On this basis, it was suggested that addition of biochar aided root colonization by AM fungi by adsorbing and deactivating the allelopathic compounds that are antagonistic to the AM fungi. Indeed, adsorption of allelopathic compounds by charcoal was suggested as one means by which charcoal additions to soil can improve plant vigour [Citation52]. Yet, evidence shows that a positive effect of biochar additions on AM fungal colonization cannot be the sole cause of reduced disease severity in this pathosystem. Additions of carbonized chaff were also seen to significantly and substantially reduce disease indices in non-AM inoculated plants (92.5% in the soil-only plot versus 48.9% in the carbonized chaff-amended plot) [Citation7]. What is more, it was reported that in field trials, while biochar-amended plots had greater asparagus yield in the first year, in the second year, biochar-amended plots produced smaller plants [Citation5]. This was attributed to higher soil moisture in the biochar-treated soil, which was conducive to the development of Fusarium root rot.
Zwart and Kim [Citation8] studied the influence of biochar amendment on severity of stem canker caused by Phytophthora spp. in two landscape tree species: red oak (Quercus rubra L.) and red maple (Acer rubrum L.). While Phytophthora are filamentous oomycetes that live in the soil, the stems were wound-inoculated. In this way, the study isolated and tested the impact of biochar on the systemic IR pathway. Horizontal expansion of lesions in both hosts was significantly reduced in potting media amended with 5% (v/v) pine wood biochar. In addition, there was significantly reduced vertical expansion of lesions and increased stem biomass in red maple compared with inoculated control plants. These results confirm that biochar can elicit a systemic resistance response to pathogens that can thrive in the soil as well as to pathogens whose life cycle is confined to above-ground parts of plants. A U-shaped dependency of disease severity on biochar dose was reported, whereby severity was least at 5% (v/v) biochar and greater at 0, 10, and 20% (v/v) biochar [Citation8].
The hypothesis that differences in biochar physical and chemical properties would lead to clearly differentiated behaviour in terms of disease development was tested on diseases caused by the soilborne fungal pathogen, R. solani, in cucumber (Cucumis sativus L.) [Citation6,Citation113] and common bean (Phaseolus vulgaris L.) [Citation113]. Distinctive biochars prepared from two feedstocks (eucalyptus wood chips (EUC) and greenhouse waste (GHW)), each produced at 350°C and 600°C [Citation30], were tested for their influence on damping-off at biochar concentrations of 0–3% (w/w). In general, relatively lower biochar concentrations suppressed damping-off, but at higher biochar concentrations, disease incidence and severity were the same as or greater than in the 0% biochar control ( & ). In other words, these systems also produced U-shaped dose–response curves. In contrast, plant growth parameters were generally improved by all levels of biochar addition. Biochars produced from the same feedstock but at different temperatures were equally effective against the tested disease and growth parameters, despite substantial differences in their chemical and physical characteristics. However, disease suppression was affected by biochar feedstock, and there was a significant interaction between feedstock and concentration for the incidence of final damping-off and other disease parameters. It was notable that the maximum protective effect of the two GHW biochars occurred at a lower biochar concentration (0.5% w/w) than that of EUC biochars (1% w/w), and that at 3% w/w, GHW biochars were no better than the control or were even conducive to the disease ( & ). The same patterns have been observed for both cucumber [Citation6,Citation113] and bean [Citation113].
Figure 2. Effect of biochar produced from eucalyptus wood chips and greenhouse waste, both at a highest treatment temperature of 600°C at different concentrations (0, 0.5, 1 and 3% w/w) on (A) final damping-off, (B) area under mortality progress curve (AUMPC), (C) epidemic period and (D) disease severity in cucumber caused by Rhizoctonia solani. Columns labelled by a common capital letter and small letter are not significantly different at p < 0.05 according to Tukey-Kramer HSD test within eucalyptus wood chips and greenhouse waste biochars, respectively. Asterisk denotes the significant difference at p ≤ 0.05 according to Tukey-Kramer HSD test between feedstock at the same concentration. Bars = standard error. Reprinted from [6] with permission.
![Figure 2. Effect of biochar produced from eucalyptus wood chips and greenhouse waste, both at a highest treatment temperature of 600°C at different concentrations (0, 0.5, 1 and 3% w/w) on (A) final damping-off, (B) area under mortality progress curve (AUMPC), (C) epidemic period and (D) disease severity in cucumber caused by Rhizoctonia solani. Columns labelled by a common capital letter and small letter are not significantly different at p < 0.05 according to Tukey-Kramer HSD test within eucalyptus wood chips and greenhouse waste biochars, respectively. Asterisk denotes the significant difference at p ≤ 0.05 according to Tukey-Kramer HSD test between feedstock at the same concentration. Bars = standard error. Reprinted from [6] with permission.](/cms/asset/b52984d6-7e9d-4aca-9964-e61ad4ce4a83/tcmt_a_913360_f0002_b.gif)
Figure 3. Damping-off of cucumber caused by Rhizoctonia solani as a function of biochar concentration in the potting media. (A) Above-ground plants in media amended with 0, 0.5, 1, or 3% w/w biochar made from eucalyptus wood chips at a highest treatment temperature of 600°C (EUC-600), day 15 after infection. (B) Above-ground plants in media amended with 0, 0.5, 1, or 3% w/w biochar made from greenhouse waste (mainly pepper plants) at a highest treatment temperature of 600°C (GHW-600), day 15 after infection. (C) Roots of cucumber plants shown in panel B, day 20 after infection. Slanted line differentiates between roots from non-infected plants and roots from infected plants. From [113].
![Figure 3. Damping-off of cucumber caused by Rhizoctonia solani as a function of biochar concentration in the potting media. (A) Above-ground plants in media amended with 0, 0.5, 1, or 3% w/w biochar made from eucalyptus wood chips at a highest treatment temperature of 600°C (EUC-600), day 15 after infection. (B) Above-ground plants in media amended with 0, 0.5, 1, or 3% w/w biochar made from greenhouse waste (mainly pepper plants) at a highest treatment temperature of 600°C (GHW-600), day 15 after infection. (C) Roots of cucumber plants shown in panel B, day 20 after infection. Slanted line differentiates between roots from non-infected plants and roots from infected plants. From [113].](/cms/asset/359a5c76-0db3-4418-865d-9d27fefbafdb/tcmt_a_913360_f0003_c.jpg)
The question whether different biochars can have direct in vitro toxicity towards soil micro-organisms was tested by Jaiswal [Citation113] using the same four biochars as by Jaiswal et al. and Graber et al. [Citation6,Citation30] (i.e., EUC-350, EUC-600, GHW-350, GHW-600) for a variety of soil pathogens (Pythium aphanidermatum, Sclerotium rolfsii, Verticillium dahliae, Macrophomina phaseolina, F. oxysporum f. sp. melonis and Sclerotinia sclerotiorum) and one beneficial fungus (Trichoderma harzianum). Despite the physical and chemical differences among the four tested biochars [Citation6,Citation30], the overall pattern of pathogen response to the biochar presence in the growing media was strikingly similar between the biochars (). Mycelial radial growth of F. oxysporum f. sp. melonis was promoted in all circumstances, while that of V. dahliae and M. phaseolina was hardly affected. Mycelial radial growth of the remaining three pathogens was inhibited by all the biochars. Differences in the mycelial radial growth among the biochars were expressed in a secondary pattern superimposed over the primary pattern: the radial mycelial growth of all the organisms was, in general, least affected by the EUC-350 biochar and most affected by the GHW-600 biochar (). This secondary pattern is seen clearly in the behaviour of T. harzianum, a plant growth-promoting and biocontrol fungus [Citation116]. The fact that biochar may affect the growth of different micro-organisms, both pathogenic and plant growth promoting, may be in part responsible for the effect of biochar on progression of diseases caused by soilborne pathogens.
Figure 4. Per cent inhibition (–) or promotion (+) of radial mycelial growth of six soilborne pathogens (Pythium aphanidermatum, Sclerotium rolfsii, Verticillium dahliae, Macrophomina phaseolina, Fusarium oxysporum f. sp. melonis and Sclerotinia sclerotiorum) and one beneficial fungus (Trichoderma harzianum) as a function of biochar type and amount in the growing media as compared with biochar-free controls. Biochars were produced from two contrasting feedstocks (eucalyptus wood chips (EUC) and greenhouse waste (GHW)) at temperatures of 350°C and 600°C, respectively. Direct toxicity was studied using an in vitro contact assay to evaluate hyphal growth inhibition or promotion in growing media amended with varying concentrations of biochar (0, 0.5, 0.75, 1 and 3% w/v) and fortified with agar before autoclaving. Mycelial growth (in centimetres) was measured as the average of two perpendicular diameters of each thallus. The percentage growth inhibition (I, %) for treated (T) and control (C) was calculated as –[(C – T)/C] × 100. Standard deviation of three replicate plates is shown. Inverted red triangles designate treatments that were not significantly different from biochar-free controls at p ≤ 0.05 according to Tukey-Kramer (HSD) test. Detailed description of methodology, experimental design and statistical analysis is given in Supplemental data, which is available on the article's Taylor & Francis online page, at http://dx.doi.org/10.1080/17583004.2014.913360. Data from [113].
![Figure 4. Per cent inhibition (–) or promotion (+) of radial mycelial growth of six soilborne pathogens (Pythium aphanidermatum, Sclerotium rolfsii, Verticillium dahliae, Macrophomina phaseolina, Fusarium oxysporum f. sp. melonis and Sclerotinia sclerotiorum) and one beneficial fungus (Trichoderma harzianum) as a function of biochar type and amount in the growing media as compared with biochar-free controls. Biochars were produced from two contrasting feedstocks (eucalyptus wood chips (EUC) and greenhouse waste (GHW)) at temperatures of 350°C and 600°C, respectively. Direct toxicity was studied using an in vitro contact assay to evaluate hyphal growth inhibition or promotion in growing media amended with varying concentrations of biochar (0, 0.5, 0.75, 1 and 3% w/v) and fortified with agar before autoclaving. Mycelial growth (in centimetres) was measured as the average of two perpendicular diameters of each thallus. The percentage growth inhibition (I, %) for treated (T) and control (C) was calculated as –[(C – T)/C] × 100. Standard deviation of three replicate plates is shown. Inverted red triangles designate treatments that were not significantly different from biochar-free controls at p ≤ 0.05 according to Tukey-Kramer (HSD) test. Detailed description of methodology, experimental design and statistical analysis is given in Supplemental data, which is available on the article's Taylor & Francis online page, at http://dx.doi.org/10.1080/17583004.2014.913360. Data from [113].](/cms/asset/67670cd1-ddca-4422-8096-86276b100784/tcmt_a_913360_f0004_c.jpg)
Until now there is but one report regarding biochar impact on diseases caused by soilborne bacterial pathogens [Citation9]. The effect of soil treatment with charcoal made from municipal biowaste and wood on bacterial wilt of tomato caused by Ral. solanacearum was tested in growth chambers and the field [Citation9]. Disease incidence of tomato plants grown in soil infested with Ral. solanacearum was lower in soils amended with charcoal from municipal biowaste than in untreated soils or in soils amended with wood charcoal. An optimum effect on disease suppression and tomato growth was found at an intermediate amendment rate of 20% (v/v). Disease suppression was attributed to the presence of Ca containing compounds as well as to other unspecified physical, chemical and biological factors. Interestingly, it was recently found that biochar absorbs N-acylhomoserine lactones that are used as signalling molecules by many quorum-sensing proteobacteria such as Ral. solanacearum [Citation98] and are used by many gram-negative soil microbes to regulate gene expression, including virulence genes. Much more information regarding biochar impact on bacterial pathogens is needed, as the interactions in the rhizosphere between different bacterial populations and plant roots can be distinctive from those of the more commonly studied fungal and fungus-like pathogens.
Knowledge gaps and future research needs
The severity of diseases caused by plant pathogens under the introduction of biochar to the soil environment is related to a complex interplay of factors affecting the rhizosphere microbiome, host susceptibility, environmental conditions and pathogen virulence. Scant published data is suggestive that biochar additions to soil do impact the severity of diseases caused by soilborne pathogens. A U-shaped biochar dose–response relationship appears to be emerging as a general feature, whereby relatively lower doses of biochar reduce disease severity and relatively higher biochar doses promote disease severity. However, given the paucity of data and the plethora of possible mechanisms, more research is clearly warranted. To date, only a handful of biochars and pathosystems have been tested, and most of the tested disease organisms have been fungi or related organisms. It remains to be seen if biochar additions can impact diseases caused by bacteria, viruses, nematodes and other soil organisms. Moreover, it is not known whether the biochar effect will be protective in field situations over a number of seasons. This need is particularly acute considering that plant growth may be less sensitive to biochar dose in the absence of disease-causing pathogens than in its presence. There is no clarity regarding the role of other micro-organisms in biochar-elicited effects, versus the role of biochar-borne chemicals, and no information at all on longevity of the effects. These knowledge gaps exist for foliar pathogens as well as for soilborne pathogens. Given the complexity of the biochar–soil–rhizosphere–pathogen system, it is apparent that attempts should be made to design experimental setups that can isolate factors for testing and evaluating potential mechanisms. Future studies should address the utility of specific biochars for diseases of representative crops from different plant families, and for several diseases on a specific crop, in an attempt to identify a biochar that is effective in a number of pathosystems. The most useful biochar types and concentrations should be tested in scaled-up experiments on various soil types and in different climate zones. Furthermore, disease dynamics over the course of several years needs to be documented.
One of the major challenges will be optimizing the use of biochar by finding an effective biochar type and concentration that will have a positive effect on a broad range of pathosystems. It is reasonable to assume that for many markets, disease control will not be the main goal of biochar application, but rather, plant growth and performance, carbon sequestration and management, soil physical characteristics, or other goals. Whether disease control is or is not the main purpose for application, it is important to recall that soilborne pathogens survive in the soil for many years, and population build-up of the pathogen may take several years before significant damage occurs. Since biochar half-life in the soil is very long, it is possible that its misuse could lead to undesirable increases in epidemics and crop loss by soil diseases. This suggests that the influence of biochar on soilborne pathogens needs to be an important consideration in managing and optimizing biochar additions to soil or horticultural media. If adding biochar to soil ultimately suppresses diseases caused by soilborne pathogens, or, conversely, promotes diseases, then the potential utility of biochar addition to soil as a carbon management tool will be affected.
Future perspectives
Clearly, the understanding of biochar impacts and effects on the progress of soilborne diseases is in its infancy. Information about its impacts on foliar diseases is only a bit more advanced. We expect that over the course of the coming 5–10 years, progress will be made in elucidating some of the mechanisms that control biochar impacts on plant diseases caused by both soilborne and foliar pathogens. We suspect that the positive impact of biochar addition on soil microbial diversity will be found to be one of the keys to the biochar effect. Indeed, we anticipate that biochar impact on the rhizosphere microbiome will be found to be of critical importance for many different aspects of biochar effects in soil. In the coming years, we expect to see more interest in biochar from the plant's perspective, and better understanding of how biochar addition impacts plant physiological traits, adaptation responses, cause-and-effect signalling and organism interaction chains.
Supplemental data
Supplemental data for this article can be seen here.
Financial & competing interests disclosure
This work was funded by the Chief Scientist of the Ministry of Agriculture and Rural Development, Israel (project number 132-1653-13). This paper is contribution number 602/14 from the Agricultural Research Organization, P.O. Box 6, Bet Dagan 50250, Israel.
Executive summary
Introduction
Applying biochar to soil can alter the intensity of diseases caused by soilborne plant pathogens.
Plant Disease - Background
The severity of diseases caused by plant pathogens is a function of the interplay between host susceptibility, pathogen virulence, and environmental conditions, all of which are influenced by the rhizosphere microbiome.
Biochar influences on pathogen-caused diseases
Biochar can affect host susceptibility, pathogen virulence, environmental conditions, and the rhizosphere microbiome directly and indirectly via a number of physical and chemical properties such as nutrient content, water holding capacity, redox activity, adsorption, pH, and content of toxic and hormone-like compounds.
In turn, the direct and indirect impacts of biochar on these factors can have domino effects on plant development and disease progress.
Current Knowledge
The effect of biochar on progression of diseases caused by soilborne pathogens has been reported in 6 distinct pathosystems, nearly all of them fungal or fungus-like pathogens.
Biochar addition may suppress, promote or have no effect on plant disease severity, usually as a function of biochar dose.
Frequently, disease severity follows a U-shaped response curve, with a minimum at some intermediate biochar dose and greater severity at lower and higher doses.
Knowledge Gaps and Future Research Needs
It is unknown if biochar additions can impact diseases caused by bacteria, viruses, nematodes, or other soil organisms. The longevity of the biochar effect on disease severity is unknown.
The role of microorganisms in biochar-elicited effects, versus the role of biochar-borne chemicals or biochar structure, is unknown.
Future Perspectives
Positive impacts of biochar addition on soil microbial diversity in the rhizosphere microbiome will be found to be one of the keys to the ‘Biochar Effect’.
Supplementary material
Download MS Word (19.4 KB)Acknowledgements
We gratefully acknowledge the assistance of Dalia Rav-David, Ludmilla Tsechansky and Fauzi Abu-Moch.
References
▪▪ of considerable interest
- Retan GA. Charcoal as a means of solving some nursery problems. J. Forestry 13, 25–30 (1915).
- Elad Y, Rav David D, Meller Harel Y et al. Induction of systemic resistance in plants by biochar: a soil-applied carbon sequestering agent. Phytopathology 100(9), 913–921 (2010).
- Meller Harel Y, Elad Y, Rav-David D et al. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 357(1–2), 245–257 (2012).
▪▪ First study to show that the presence of biochar in the potting media can induce upregulation of plant defence-related genes and priming.
- Elad Y, Cytryn E, Harel YM, Lew B, Graber ER. The biochar effect: plant resistance to biotic stresses. Phytopath. Mediterr. 50(3), 335–349 (2011).
- Elmer WH, Pignatello JJ. Effect of biochar amendments on mycorrhizal associations and Fusarium crown and root rot of asparagus in replant soils. Plant Dis. 95(8), 960–966 (2011).
▪▪ Study exploring interactions between mycorrhizal fungi and disease development as affected by biochar, with an emphasis on mechanisms, particularly adsorption of allelopathic compounds by biochar.
- Jaiswal AK, Elad Y, Graber ER, Frenkel O. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 69, 110–118 (2014).
▪Explores the relationship between biochar physical and chemical characteristics and suppression or promotion of diseases caused by a soilborne pathogen.
- Matsubara Y, Hasegawa N, Fukui H. Incidence of Fusarium root rot in asparagus seedlings infected with arbuscular mycorrhizal fungus as affected by several soil amendments. J. Jpn. Soc. Hort. Sci. 71(3), 370–374 (2002).
- Zwart DC, Kim S-H. Biochar amendment increases resistance to stem lesions caused by Phytophthora spp. in tree seedlings. HortScience 47(12), 1736–1740 (2012).
- Nerome M, Toyota K, Islam TM et al. Suppression of bacterial wilt of tomato by incorporation of municipal biowaste charcoal into soil. Soil Microorgan. 59(1), 9–14 (2005) [ in Japanese].
- Bruehl GW. Soilborne Plant Pathogens. Macmillan, New York, USA (1987).
- Katan J. Soil disinfestation: environmental problems and solutions. In: Modern Agriculture and the Environment. Rosen D, Tel-Or E, Hadar Y, Chen Y (Eds). Rehovot, Israel Kluwer Academic Publishers, Dordrecht. 41–45 (1997).
- Katan J. Role of cultural practices for the management of soilborne pathogens in intensive horticultural systems. XXVI International Horticultural Congress: Managing Soil-Borne Pathogens: A Sound Rhizosphere to Improve Productivity. Toronto, Canada, 11–18 (2004).
- Katan J, Waisel Y, Eshel A, Kafkafi U. Interactions of soilborne pathogens with roots and aboveground plant organs. In: Plant Roots: The Hidden Half. Kafkafi U, Waisel Y, Eshel A (Eds). CRC Press, Boca Raton, USA, 949–959 (2002).
- Koike S, Subbarao K, Davis RM, Turini T. Vegetable Diseases Caused by Soilborne Pathogens. UCANR Publications University of California, Oakland CA. (2003).
- Pimentel D, McLaughlin L, Zepp A et al. Environmental and economic effects of reducing pesticide use. BioScience 41(6), 402–409 (1991).
- Lumsden RD, Lewis JA, Fravel DR. Formulation and delivery of biocontrol agents for use against soilborne plant-pathogens. In: Biorational pest control agents: Formulation and delivery. American Chemical Society, Washington, USA, 166–182 (1995).
- Mazzola M, Reynolds MP. Management of resident soil microbial community structure and function to suppress soilborne disease development. Climate Change Crop Prod. 1, 200–218 (2010).
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant Bio. 57, 233–266 (2006).
- Agrios GN. Plant Pathology. Elvesier/Academic Press, London, UK (2005).
- Aoki H, Sassa T, Tamura T. Phytotoxic metabolites of Rhizoctonia solani. Nature 200, 575 (1963).
- Bartz FE, Glassbrook NJ, Danehower DA, Cubeta MA. Elucidating the role of the phenylacetic acid metabolic complex in the pathogenic activity of Rhizoctonia solani anastomosis group 3. Mycologia 104(4), 793–803 (2012).
- Orellana RG, Mandava NB. m-Hydroxyphenylacetic and m-methoxyphenylacetic acids of Rhizoctonia solani: their effect on specific root-nodule activity and histopathology in soybean. J. Phytopath. 107(2), 159–167 (1983).
- Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbio. Rev. 37(5), 634–663 (2013).
▪▪ Comprehensive review of the importance of the rhizosphere microbiome.
- Junker RR, Tholl D. Volatile organic compound mediated interactions at the plant-microbe interface. J. Chem. Ecol. 39(7), 810–825 (2013).
- Farag MA, Zhang HM, Ryu CM. Dynamic chemical communication between plants and bacteria through airborne signals: Induced resistance by bacterial volatiles. J. Chem. Ecol. 39(7), 1007–1018 (2013).
- Bitas V, Kim HS, Bennett JW, Kang S. Sniffing on microbes: diverse roles of microbial volatile organic compounds in plant health. Mol. Plant Microbe Interact. 26(8), 835–843 (2013).
- Bakker P, Doornbos RF, Zamioudis C, Berendsen RL, Pieterse CMJ. Induced systemic resistance and the rhizosphere microbiome. Plant Pathol. J. 29(2), 136–143 (2013).
▪▪ Detailed review regarding the role of rhizosphere microbiome in plant resistance to disease.
- Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nature Rev. Microbiol. 11, 789–799. (2013).
- Silber A, Levkovitch I, Graber ER. pH-Dependent mineral release and surface properties of cornstraw biochar: agronomic implications. Environ. Sci. Technol. 44(24), 9318–9323 (2010).
- Graber ER, Tsechansky L, Lew B, Cohen E. Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe. Eur. J. Soil Sci. 65, 162–172 (2014).
- Datnoff LE, Elmer WH, Huber DM. Mineral Nutrition and Plant Disease. American Phytopathological Society Press, St. Paul, USA (2007).
- Bateman DF, Basham HG. Degradation of plant cell walls and membranes by microbial enzymes. In: Physiological Plant Pathology, Heitefuß R, Williams P (Eds). Springer-Verlag, Berlin, 316–355 (1976).
- Kelman A, McGuire RG, Tzeng KC. Reducing the severity of bacterial soft rot by increasing the concentration of calcium in potato tubers. In: Soilborne Plant Pathogens: Management of Diseases with Macro- and Microelements. Engelhard, AW (Ed.): American Phytopathological Society, St Paul, USA, 102–123 (1989).
- Hafez AAR, Stout PR, DeVay JE. Potassium uptake by cotton in relation to Verticillium wilt. Agron. J. 67, 359–361 (1975).
- Prendergast-Miller MT, Duvall M, Sohi SP. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 65, 173–185 (2014).
- Newsham KK, Fitter AH, Watkinson AR. Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 10, 407–411 (2005).
- Baker R, Martinson CA. Epidemiology of diseases caused by Rhizoctonia solani. In: Rhizoctonia solani, Biology and Pathology. Parmeter JR (Ed.): University of California Press, Berkeley, USA, 125–148 (1970).
- Wall PC, Neate SM, Graham RD, Reuter DJ, Rovira AD. The effect of rhizoctonia root disease and applied nitrogen on growth, nitrogen uptake and nutrient concentrations in spring wheat. Plant Soil 163(1), 111–120 (1994).
- Clough TJ, Condron LM, Kammann C, Moeller C. A review of biochar and soil nitrogen dynamics. Agronomy 3(2), 275–293 (2013).
- Joseph SD, Camps-Arbestain M, Lin Y et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 48(6–7), 501–515 (2010).
- Husson O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362(1–2), 389–417 (2012).
- Yuan J-H, Xu R-K. Effects of biochars generated from crop residues on chemical properties of acid soils from tropical and subtropical china. Soil Res. 50(7), 570–578 (2012).
- Lovley DR. Electromicrobiology. Ann. Rev. Microbiol. 66, 391–409 (2012).
- Cayuela ML, Sanchez-Monedero MA, Roig A, Hanley K, Enders A, Lehmann J. Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions? Sci. Rep. 3, DOI: http://dx.doi.org/10.1038/srep01732 (2013).
- Bornemann LC, Kookana RS, Welp G. Differential sorption behaviour of aromatic hydrocarbons on charcoals prepared at different temperatures from grass and wood. Chemosphere 67(5), 1033–1042 (2007).
- Chen BL, Chen ZM. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 76(1), 127–133 (2009).
- Graber ER, Tsechansky L, Gerstl Z, Lew B. High surface area biochar negatively impacts herbicide efficacy. Plant Soil 353(1–2), 95–106 (2011).
- Graber ER, Tsechansky L, Khanukov J, Oka Y. Sorption, volatilization and efficacy of the fumigant 1,3-dichloropropene in a biochar-amended soil. Soil Sci. Soc. Am. J. 75(4), 1365–1373 (2011).
- Han YX, Boateng AA, Qi PX, Lima IM, Chang JM. Heavy metal and phenol adsorptive properties of biochars from pyrolyzed switchgrass and woody biomass in correlation with surface properties. J. Environ. Manag. 118, 196–204 (2013).
- Uchimiya M, Bannon DI, Wartelle LH. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agric. Food Chem. 60(7), 1798–1809 (2012).
- Yu JQ, Lee KS, Matsui Y. Effect of the addition of activated charcoal to the nutrient solution on the growth of tomato in hydroponic culture. Soil Sci. Plant Nutr. 39(1), 13–22 (1993).
- Wardle DA, Zackrisson O, Nilsson MC. The charcoal effect in boreal forests: mechanisms and ecological consequences. Oecologia 115(3), 419–426 (1998).
- Daoud FB-O, Kaddour S, Sadoun T. Adsorption of cellulase Aspergillus niger on a commercial activated carbon: kinetics and equilibrium studies. Colloids Surf. B Biointerfaces 75, 93–99 (2010).
- Lammirato C, A. Miltner, and M. Kaestner. Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol. Biochem. 43, 1936–1942 (2011).
- Lee C-YC, Pedram EO, Hines AL. Adsorption of oxalic, malonic, and succinic acids on activated carbon. J. Chem. Eng. Data 31(2), 133–136 (1986).
- Das KC, Garcia-Perez M, Bibens B, Melear N. Slow pyrolysis of poultry litter and pine woody biomass: impact of chars and bio-oils on microbial growth. J. Environ. Sci. Health Part A 43(7), 714–724 (2008).
- Graber ER, Meller-Harel Y, Kolton M et al. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 337(1-2), 481–496 (2010).
▪▪ Conjectured two alternatives for improved plant performance under biochar treatment: influence on microbial populations or hormesis due to low doses of biochar chemicals.
- Spokas KA, Novak JM, Stewart CE et al. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 85(5), 869–882 (2011).
- Orihashi K, Kojima Y, Terazawa M. Deterrent effect of rosin and wood tar against barking by the gray-sided vole (Clethrionomys rufocanus bedfordiae). J. Forest Res. 6(3), 191–196 (2001).
- Yatagai M, Nishimoto M, Hori K, Ohira T, Shibata A. Termiticidal activity of wood vinegar, its components and their homologues. J. Wood Sci. 48(4), 338–342 (2002).
- Faix O, Fortmann I, Bremer J, Meier D. Thermal degradation products of wood: Gas chromatographic separation and mass spectrometric characterization of polysaccharide derived products. Holz als Roh-und Werkstoff 49(5), 213–219 (1991).
- Lingens A, Windeisen E, Wegener G. Investigating the combustion behaviour of various wood species via their fire gases. Wood Sci. Technol. 39(1), 49–60 (2005).
- McDonald JD, Zielinska B, Fujita EM, Sagebiel JC, Chow JC, Watson JG. Fine particle and gaseous emission rates from residential wood combustion. Environ. Sci. Technol. 34(11), 2080–2091 (2000).
- Klinke HB, Thomsen AB, Ahring BK. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotech. 66(1), 10–26 (2004).
- Mu J, Yu Z-m, Wu W-q, Wu Q-l. Preliminary study of application effect of bamboo vinegar on vegetable growth. Forestry Stud. China 8(3), 43–47 (2006).
- Mun SP, Ku CS. Pyrolysis GC-MS analysis of tars formed during the aging of wood and bamboo crude vinegars. J. Wood Sci. 56(1), 47–52 (2010).
- Patrick Z, Toussoun T. Plant residues and organic amendments in relation to biological control. In: Ecology of Soil-Borne Plant Pathogens: Prelude to Biological Control. Baker, KF, Snyder, WC (Eds). University of California Press, Berkeley, USA, 440–459 (1965).
- Ye SF, Yu JQ, Peng YH, Zheng JH, Zou LY. Incidence of Fusarium wilt in Cucumis sativus l. is promoted by cinnamic acid, an autotoxin in root exudates. Plant Soil 263(1–2), 143–150 (2004).
- Dugardeyn J, Van Der Straeten D. Ethylene: fine-tuning plant growth and development by stimulation and inhibition of elongation. Plant Sci. 175(1–2), 59–70 (2008).
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci. 11(4), 176–183 (2006).
- Rigler E, Zechmeister-Boltenstern S. Oxidation of ethylene and methane in forest soils – effect of CO2 and mineral nitrogen. Geoderma 90(1-2), 147–159 (1999).
- Zhang J, You C. Water holding capacity and absorption properties of wood chars. Energy Fuels 27(5), 2643–2648 (2013).
- Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 45(8), 629–634 (2007).
- Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 46(5), 437–444 (2008).
- Busscher WJ, Novak JM, Evans DE, Watts DW, Niandou MAS, Ahmedna M. Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 175(1), 10–14 (2010).
- Zhang, Q, Wang, Y, Wu, Y, Wang, X, Du, Z, Liu, X, Song, J. Effects of Biochar amendment on soil thermal conductivity, reflectance, and temperature. Soil Sci. Soc. Am. J. 77(5), 1478–1487 (2013).
- Case SDC, McNamara NP, Reay DS, Whitaker J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil – the role of soil aeration. Soil Biol. Biochem. 51, 125–134 (2012).
- Kammann C, Ratering S, Eckhard C, Müller C. Biochar and hydrochar effects on greenhouse gas (CO2, N2O, CH4) fluxes from soils. J. Environ. Qual. 41(4), 1052–1066 (2012).
- Abel S, Peters A, Trinks S, Schonsky H, Facklam M, Wessolek G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 202, 183–191 (2013).
- Cornelissen G, Martinsen V, Shitumbanuma V et al. Biochar effect on maize yield and soil characteristics in five conservation farming sites in Zambia. Agronomy 3(2), 256–274 (2013).
- Liu J, Schulz H, Brandl S, Miehtke H, Huwe B, Glaser B. Short-term effect of biochar and compost on soil fertility and water status of a dystric cambisol in NE Germany under field conditions. J. Plant Nutr. Soil Sci. 175(5), 698–707 (2012).
- Buss W, Kammann C, Koyro H-W. Biochar reduced copper toxicity in Chenopodium quinoa Willd. in a sandy soil. J. Environ. Qual. 41(4), 1157–1165 (2012).
- Oguntunde PG, Abiodun BJ, Ajayi AE, van de Giesen N. Effects of charcoal production on soil physical properties in Ghana. J. Plant Nutr. Soil Sci. 171(4), 591–596 (2008).
- Uzoma KC, Inoue M, Andry H, Zahoor A, Nishihara E. Influence of biochar application on sandy soil hydraulic properties and nutrient retention. J. Food Agri. Environ. 9(3–4), 1137–1143 (2011).
- Fry WE, Grünwald NJ. Introduction to oomycetes. In: The Plant Health Instructor, American Phytopathological Society (APS), St. Paul USA. (2010)
- Verheijen, FGA, Jeffery, S, van der Velde, M, et al. Reductions in soil surface albedo as a function of biochar application rate: implications for global radiative forcing. Environ. Res. Lett. 8(4) 044008.
- Kolton M, Meller Harel Y, Pasternak Z, Graber ER, Elad Y, Cytryn E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 77(14), 4924–4930 (2011).
- Khodadad CLM, Zimmerman AR, Green SJ, Uthandi S, Foster JS. Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil. Biol. Biochem. 43(2), 385–392 (2011).
- Chen J, Liu X, Zheng J et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from southwest China. Appl. Soil Ecol. 71(0), 33–44 (2013).
- Anderson CR, Condron LM, Clough TJ et al. Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54(5–6), 309–320 (2011).
- Gomez JD, Denef K, Stewart CE, Zheng J, Cotrufo MF. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 65(1), 28–39 (2014).
- Matos A, Kerkhof L, Garland JL. Effects of microbial community diversity on the survival of Pseudomonas aeruginosa in the wheat rhizosphere. Microb. Ecol. 49(2), 257–264 (2005).
- Irikiin Y, Nishiyama M, Otsuka S, Senoo K. Rhizobacterial community-level, sole carbon source utilization pattern affects the delay in the bacterial wilt of tomato grown in rhizobacterial community model system. Appl. Soil Ecol. 34(1), 27–32 (2006).
- Alexander BJR, Stewart A. Glasshouse screening for biological control agents of Phytophthora cactorum on apple (Malus domestica). N Z J. Crop Hort. Sci 29(3), 159–169 (2001).
- Gunasinghe W, Karunaratne AM. Interactions of Colletotrichum musae and Lasiodiplodia theobromae and their biocontrol by Pantoea agglomerans and Flavobacterium sp in expression of crown rot of “Embul” banana. Biocontrol 54(4), 587–596 (2009).
- Hebbar P, Berge O, Heulin T, Singh SP. Bacterial antagonists of sunflower (Helianthus annuus l.) fungal pathogens. Plant Soil 133(1), 131–140 (1991).
- Garbeva P, Hordijk C, Gerards S, Boer W. Volatiles produced by the mycophagous soil bacterium collimonas. FEMS Microbiol. Ecol. doi: 10.1111/1574-6941.12252 (2013) (Epub ahead of print).
- Masiello CA, Chen Y, Gao X et al. Biochar and microbial signaling: production conditions determine effects on microbial communication. Environ. Sci. Technol. 47(20), 11496–11503 (2013).
▪▪ Shows that adsorption of signalling molecules by biochar may influence microbial communications, providing a mechanism by which biochar may impact microbial communities.
- Vallad GE, Goodman RM. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 44(6), 1920–1934 (2004).
- Harman GE, Howell CR, Vitebro RA, Chet I, Lorito M. Trichoderma species – opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2(1), 43–56 (2004).
- Iriti M, Rossoni M, Borgo M, Faoro F. Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistance to botrytis cinerea. J. Agric. Food Chem. 52(14), 4406–4413 (2004).
- Perazzolli M, Dagostin S, Ferrari A, Elad Y, Pertot I. Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianumT39 and benzothiadiazole. Biol. Cont. 47(2), 228–234 (2008).
- Belhadj A, Saigne C, Telef N et al. Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator. J. Agric. Food Chem. 54(24), 9119–9125 (2006).
- Rajkumar M, Lee KJ, Freitas H. Effects of chitin and salicylic acid on biological control activity of Pseudomonas spp. against damping off of pepper. S. Afr. J. Bot. 74(2), 268–273 (2008).
- Aziz A, Trotel-Aziz P, Dhuicq L, Jeandet P, Couderchet M, Vernet G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 96(11), 1188–1194 (2006).
- Trouvelot S, Varnier AL, Allègre M et al. A β-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol. Plant Microbe Interact. 21(2), 232–243 (2008).
- Hamiduzzaman MM, Jakab G, Barnavon L, Neuhaus JM, Mauch-Mani B. β-aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Mol. Plant Microbe Interact. 18(8), 819–829 (2005).
- Reuveni M, Agapov V, Reuveni R. Induced systemic protection to powdery mildew in cucumber by phosphate and potassium fertilizers: effects of inoculum concentration and post-inoculation treatment. Can J. Plant Pathol. 17(3), 247–251 (1995).
- Walters D, Walsh D, Newton A, Lyon G. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 95(12), 1368–1373 (2005).
- Wiese J, Wiese H, Schwartz J, Schubert S. Osmotic stress and silicon act additively in enhancing pathogen resistance in barley against barley powdery mildew. J. Plant Nutr. Soil Sci. 168(2), 269–274 (2005).
- Ayres PG. The interaction between environmental-stress injury and biotic disease physiology. Ann. Rev. Phytopathol. 22, 53–75 (1984).
- Wiese J, Kranz T, Schubert S. Induction of pathogen resistance in barley by abiotic stress. Plant Biol. 6(5), 529–536 (2004).
- Jaiswal AK. Impact of biochar amendment to a potting medium on damping-off caused by Rhizoctonia solani. Hebrew University of Jerusalem, M.Sc. Thesis, (Elad, Y, Frenkel, O, Graber, ER), February 2013.
- Warnock DD, Lehmann J, Kuyper TW, Rillig MC. Mycorrhizal responses to biochar in soil – concepts and mechanisms. Plant Soil 300(1–2), 9–20 (2007).
▪ Classic review paper examining influence of biochar on mycorrhize.
- Solaiman ZM, Blackwell P, Abbott LK, Storer P. Direct and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. Aust. J. Soil Res. 48(7), 546–554 (2010).
- Elad Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 19(8–10), 709–714 (2000).
