Abstract
Probiotics beneficially affect human health by improving the balance of intestinal microbiota and mucosal defence against pathogens. Many probiotic bacteria, mostly belong to the genera Lactobacillus, are used in commercial probiotic fermented milks throughout the world. The aim of this study was to investigate the probiotic potential of lactic acid bacteria (LAB) isolated from various Iranian traditional yogurts. During an 18-month study from October 2013 to May 2014, 96 samples were equally collected from traditional yogurts produced from goat, sheep and cow milk in Yazd Providence, Iran. Samples were transferred into MRS broth for enrichment and then subcultured on MRS agar. Isolates were studied for morphological and biochemical characteristics, using Gram staining and catalase test. The ability to tolerate acidic pH and resistance to bile salts were used as restrictive criteria for probiotic potential. Selected LAB were further identified using 16S rDNA sequence analysis. Of 96 yogurt samples, 47 LAB were isolated; from which 12 were candidates for probiotics. Six probiotic isolates belonged to Pediococcus acidilacticii and other six isolates to Lactobacillus plantarum, L. brevis, L. fermentum and L. kefiri. In summary, LAB strains isolated from Iranian traditional yogurts were considered as viable candidates of probiotics, based on their properties such as acid and bile tolerance.
Keywords:
Introduction
Probiotic isderived from two Greek words that mean ‘for life’. Probiotics are live microorganisms that make health benefits to their host when are adequately administrated (FAO Citation2001). They positively improve the balance of intestinal microbiota and strength mucosal defence mechanism against microbial pathogens. Probiotics include further health benefits such as better immune system responses, vitamin synthesis, reduced serum cholesterol levels, anticarcinogenic effects and antimicrobial activities (Medici et al. Citation2005; Gibson Citation2008; Urbanska et al. Citation2009; Soltan Dallal et al. Citation2012; Karimi et al. Citation2011). Lactic acid bacteria (LAB) are a broadly well-known group of probiotic bacteria (Naidu et al. Citation1999). Lactic acid bacteria, including Lactobacillus and Bifidobacterium species, are commonly used in various food products. Dairy products (such as yogurt and fermented milk) are the most common foods which contain probiotic microorganisms (Bergamini et al. Citation2005). Furthermore, LAB are used in kefir, sour dough and fermented meat (Caglar et al. Citation2008). Traditional fermented dairy products such as yogurt are the major sources for the isolation of potential probiotic microorganisms (Ambadoyiannis et al. Citation2005). The aim of this study was to investigate the probiotic potential of LAB isolated from traditional yogurts in Iran.
Materials and methods
Bacterial isolation
In total, 96 traditional yogurt samples (32 goat’s, 32 sheep’s and 32 cow’s yogurt) were collected in sterile tubes in Yazd Province, Iran. Fifteen grams of each sample were enriched in MRS broth (Scharlau, Spain) for 24–72 h at 37 °C with atmospheric CO2. Then, enrichments were cultured on MRS agar (Scharlau, Spain) and incubated as described. Colonies were selected based on morphology, catalase test, gas producing from glucose at 15 and 45 °C, arginine hydrolysis and fermentation of carbohydrates. Isolates were kept in 20% glycerol buffer at −20 °C until used.
Tolerance to acidic pH
One millilitre of MRS broth containing approximately 109 CFU/ml of LAB was transferred into 9 ml phosphate-buffered saline (PBS) (pH 2.0) and incubated at 37 °C for 3 h. Viable bacteria were counted by plating serial dilutions on MRS agar. Bacterial acid tolerance was assessed by calculating the ratio of viable LAB cultured on MRS agar to surviving cells after incubation at pH 2.0 for 3 h (Tsai et al. Citation2008).
Tolerance to bile salts
Lactic acid bacteria that survived in acid tolerance test were selected for bile salt tolerance test. These isolates were cultured in 9 ml of MRS broth with and without 0.3% (w/v) oxgall bile (Sigma) for 8 h. Bacterial growth rate was recorded after 8 h by measuring the light absorbance of cultures at 600 nm using spectrophotometer (Pharmacia Biotech Ultrospec 2000). Coefficient of inhibition (Cinh) was calculated using an original method by Gopal et al. (Citation2001) as follows:
Where, Δ represented the differences in absorbance between T0 (reading at 0 h) and T8 (reading at 8 h). Experiment was repeated to ensure the reproducibility of the result. Based on the calculated coefficient of inhibition (Cinh), isolates were classified into non-sensitive (resistant) to 0.3% bile salts (Cinh ≈ 0), with a retarded growth rate (0.2 < Cinh < 0.4) and poor tolerance (Cinh > 0.4).
16S rDNA gene sequencing
Selected LAB with acid and bile salt tolerance were confirmed using 16S rDNA sequence analysis. Genomic DNA was extracted according to a previously described method by Antonsson & Molin Gardo (Citation2003). PCR primer sequences were as follows: F: 5′-CTCGTTGCGGGACTTAA-3′ and R: 5′-GCAGCAGTAGGGAATCTTC-3′ (Davoodabadi et al. Citation2015). The reaction mixture consisted of 5 μl of 10 × PCR buffer, 1.5 mM of MgCl2, 0.2 mM of dNTPs, 3 pmol of each primer, 1.5 U of Taq DNA polymerase and 2 μl of genomic DNA (Sinaclon, Iran) in a final volume of 50 μl. Thermal cycling programme included an initial denaturation at 94 °C for 2 min followed by 30 cycles; each cycle consisted of denaturation at 94 °C for 30 s, annealing at 53 °C for 1 min and elongation at 72 °C for 1 min, final elongation was carried out at 72 °C for 7 min. PCR products were electrophoresed on 1.5% agarose gels and visualised under UV light. Furthermore, PCR products were sequenced using the Sanger method (Bioneer, Korea). 16S rDNA sequences were compared with known sequences in GenBank using BLAST online tool (www.ncbi.nlm.nih.gov/blast).
Antibiotic susceptibility
Lactic acid bacteria were cultured in 10 ml of a mixture of 90% (w/v) Mueller–Hinton broth (Merck, Germany) and 10% (w/v) MRS broth (Merck, Germany). When broth cultures reached 0.5 McFarland’s standard turbidity at 37 °C, cultures were streaked on agar plates containing a mixture of Mueller–Hinton agar added with 10% (w/v) MRS dehydrated broth. Antibiotic disks (Mast, UK) were placed on the agar and plates were incubated under anaerobic condition at 37 °C for 48 h. Inhibition zone diameters were calculated and results ≤15 mm reported as resistant, 16–20 mm as moderate susceptible and ≥21 mm as susceptible (Hashemi et al. Citation2014).
Results and discussion
Of the 96 samples, 47 LAB were isolated; from which, 12 isolates were identified as probiotics potential (Table ; Figure ). Six isolates belonged to Pediococci and six to Lactobacillus spp. subspecies Lb. plantarum (n = 2), Lb. brevis (n = 2), Lb. fermentum (n = 1) and Lb. kefiri (n = 1). Lb. fermentum 27 showed a higher and P. acidilactici 23 showed a lower acid tolerance than other isolates. Lb. brevis 25 had a higher and Lb. brevis 86 had a lower bile salt tolerance than other isolates. All LAB isolates were resistant to vancomycin and streptomycin. Nine isolates (75%) were resistant to chloramphenicol and seven isolates (58.3%) to tetracycline. All isolates were susceptible to clindamycin, erythromycin and gentamicin (Table ). Probiotic foods have become popular over the past decades. Nowadays, a wide variety of foods contain probiotics. In this study, LAB were isolated from Iranian traditional yogurt samples and assessed in vitro for their probiotics properties, including acid and bile acid tolerance. LAB, such as Lactobacillus spp., are naturally found in various raw foods. They are also present as microbiota of the human and mammal gastrointestinal tract. In the current study, LAB isolates were selected for their tolerance and survival in acidic environment as well as growth in the presence of 0.3% bile salts; a similar concentration to that of bile acids in small intestine (Davoodabadi et al., Citation2015). Similar results have previously been reported by other researchers analysing LAB strains from various environments (Saarela et al. Citation2000; Ramos et al. Citation2013).
Figure 1. 16S rDNA PCR products (757 bp): Lane M: 100 bp DNA marker; Lane P: positive control; Lane N: negative control; Lanes 1–12: LAB isolates.
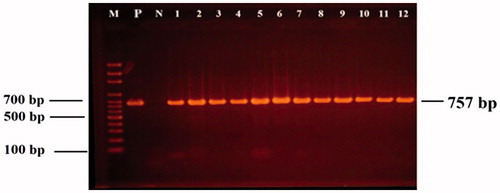
Table 1. Acid and bile acid tolerance of the LAB isolates.
Table 2. Susceptibility of the LAB isolates to antibiotics using disc diffusion method.
In the present study, 12 LAB with probiotic potential were isolated from traditional yogurts in Yazd Province, Iran. These bacteria belonged to two genera, Pediococcus (6 P. acidilacticii isolates) and Lactobacillus (2 Lb. plantarum, 2 Lb. brevis, 1 Lb. fermentum and 1 Lb. kefiri isolates). In another study in Tehran Province of Iran, Ebrahimi et al. (Citation2011) reported four different Lactobacillus spp., including Lb. casei (n = 6), Lb. acidophilus (n = 1), Lb. salivarius (n = 1) and Lb. alimentarium (n = 1) in traditional yogurts. Other research studies have independently analysed the microbial composition of probiotics in dairy products (Zago et al. Citation2011). In this study, most LAB were resistant to vancomycin and streptomycin. Various species of LAB (e.g. Lb. rhamnosus, Lb. casei, Lb. plantarum, Lb. fermentum, Lb. brevis and Lb. curvatus) are intrinsically resistant to vancomycin (Saarela et al. Citation2000; Kirtzalidou et al. Citation2011). This resistance is not inducible or transferable. High level of resistance to vancomycin and streptomycin has been observed in previous study on Lactobacillus spp. with probiotic potential isolated from stool microbiota of healthy infants (Davoodabadi et al. Citation2015). Furthermore, high level of resistance to streptomycin in LAB has been reported in other studies (Saarela et al. Citation2000; Katla et al. Citation2001). Since these resistances are chromosomally encoded, they do not usually cause safety concerns (Saarela et al. Citation2000; Ammor et al. Citation2007).
Conclusions
In conclusion, this study has shown that LAB such as Pediococcus and Lactobacillus spp. with good probiotic potential can be isolated from traditional yogurt. Results suggest that these LAB isolates with probiotic potential can be used for the production of probiotic dairies. However, further in vitro and in vivo studies on these probiotic LAB isolates are required.
Acknowledgments
This paper was a part of a research project (Research Contract No. 20397) approved by the Food Microbiology Research Center, Tehran University of Medical Sciences, Tehran, Iran.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ambadoyiannis G, Hatzikamari M, Litopoulou-Tzanetaki ET, Zanetakis N. 2005. Probiotic and technological properties of enterococci isolates from infants and cheese. Food Biotech. 18:307–325.
- Ammor MS, Florez AB, Mayo B. 2007. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol. 24:559–570.
- Antonsson M, Molin Gardo Y. 2003. Lactobacillus strains isolated from Danbo cheese as adjunct cultures in a cheese model system. Int J Food Microbiol. 85:159–169.
- Bergamini CV, Hynes ER, Quiberoni A, Suarez VB, Zalazar CA. 2005. Probiotic bacteria as adjunct starters: influence of the addition methodology on their survival in a semi-hard Argentinean cheese. Food Res. Int. 38:597–604.
- Caglar E, Onder Kuscu O, Selvi Kuvvetli S, Kavaloglu Cildir S, Sandalli NT, Wetman S. 2008. Short-term effect of ice-cream containing Bifidobacterium lactis Bb-12 on the number of salivary mutans streptococci and lactobacilli. Acta Odontol. Scand. 66:154–158.
- Davoodabadi A, Soltan Dallal MM, Foroushani AR, Douraghi M, Amin Harati F. 2015. Antibacterial activity of Lactobacillus spp. isolated from the faeces of healthy infants against enteropathogenic bacteria. Anaerobe. 34:53–58.
- Ebrahimi MT, Ouwehand AC, Hejazi MA, Jafari P. 2011. Traditional Iranian dairy products: a source of potential probiotic lactobacilli. African J. Microbiol. Res. 5:20–27.
- FAO. 2001. Evaluation of health and nutritional properties of probiotics in food, including powder milk with live lactic acid bacteria. Food and Agricultural Organisation of United Nations and World Health Organisation Expert Consultation Report.
- Gibson GR. 2008. Prebiotics as gut microflora management tools. J. Clin. Gastroenterol. 42:S75–S79.
- Gopal PK, Prasad J, Smart J, Gill HS. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Inter. J. Food Microb. 67:207–216.
- Hashemi SM, Shahidi F, Mortazavi SA, Milani E, Eshaghi Z. 2014. Potentially probiotic Lactobacillus strains from traditional Kurdish cheese. Probiotics Antimicrob Proteins. 6:22–31.
- Karimi R, Mortazavian AMD, Cruz AG. 2011. Viability of probiotic microorganisms in cheese during production and storage: a review. Dairy Sci Technol. 91:283–308.
- Katla AK, Kruse H, Johnsen GHerikstad H. 2001. Antimicrobial susceptibility of starter culture bacteria used in Norwegian dairy products. Int. J. Food Microbiol. 67:147–152.
- Kirtzalidou E, Pramateftaki P, Kotsou MK, Yriacou A. 2011. Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe. 17:440–443.
- Medici M, Vinderola CG, Weill R, Perdigon G. 2005. Effect of fermented milk containing probiotic bacteria in the prevention of an enteroinvasive Escherichia coli infection in mice. J. Dairy Res. 72:243–249.
- Naidu AS, Bidlack WR, Clemens RA. 1999. Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr. 39:13–126.
- Ramos CL, Thorsen L, Schwan RF, Jespersen L. 2013. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 36:22–29.
- Saarela M, Mogensen G, Fondén R, Matto J, Mattila-Sandholm T. 2000. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol 84:197–215.
- Soltan Dallal MM, Mojarrad M, Salehipour Z, Raoofian R, Rajabi Z. 2012. Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on the behaviour of colorectal tumour cells. Tehran Univ Med J. 70:220–227.
- Tsai CC, Lin PP, Hsieh YM. 2008. Three Lactobacillus strains from healthy infant stool inhibit enterotoxigenic Escherichia coli grown in vitro. Anaerobe. 14:61–67.
- Urbanska AM, Bhathena J, Martoni C, Prakash S. 2009. Estimation of the potential antitumor activity of microencapsulated Lactobacillus acidophilus yogurt formulation in the attenuation of tumorigenesis in Apc (Min/+) mice. Dig Dis Sci. 54:264–273.
- Zago M, Fornasari ME, Carminati D, Burns P, Suarez V, Vinderola G, et al. 2011. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 28:1033–1040.
