Abstract
The aim of this study was to evaluate the effect of dietary Zingiber officinale Roscoe (ginger) powder on rabbit productive performances, meat quality and shelf-life of raw and cooked meat. Ninety hybrid rabbits of 60 days old were fed three different diets: basal diet (control, C), basal diet supplemented by 4 g of ginger powder on 100 g of feed (G4) and basal diet supplemented by 8 g of ginger powder on 100 g of feed (G8) (3.6 and 7.2 g/100 g of dry matter for G4 and G8, respectively). Live weight, average daily gain and feed intake were recorded. Ten rabbits of each group were slaughtered at 90 days of age and meat quality was assessed during seven days of storage at 4 °C. Live performance and slaughter traits did not show any significant differences. Dietary ginger powder induced modification in pH of raw samples and in colour indexes of both raw and cooked meat. Lipid oxidation of raw samples was delayed in time by ginger feed addition even if no modification was highlighted in antioxidant capacity. Ginger powder could be a potential supplementation in diet of rabbits for increasing meat shelf-life.
Keywords:
Introduction
In the last decades, much attention has been given in rabbit farming in order to increase productions without negatively affecting meat quality and animal wellness. In recent years, studies have been carried out on the addition of natural antioxidants as nutritional supplements in animal feeding to improve performance, health, meat quality and shelf-life of raw or cooked meat products (Jiang and Xiong Citation2016).
Dietary supplementation with natural antioxidant can improve the oxidative stability of rabbit meat which is susceptible to the deterioration because of its nutritional composition (Dalle Zotte Citation2002). To improve the performance, healthy conditions and meat quality, different aromatic herbs and essential oils have been used in rabbit feed (Dal Bosco et al. Citation2012; Peiretti et al. Citation2013; Gerencsér et al. Citation2014).
Ginger powder and extracts have been studied for their antioxidant and antimicrobial properties both in dietary supplementation and in food preservation (Zomrawi et al. Citation2012; Mancini, Paci, Fratini, et al. Citation2017; Mancini, Preziuso, et al. Citation2017). Hence, ginger could play an important role in rabbit feeding strategy. The aim of this study was to assess in rabbit the effects of two different concentrations of ginger powder as supplement in feed on the productive performances and meat quality.
Materials and methods
Ninety hybrid rabbits of 60 days old were randomly allotted into three groups and housed in cages. Groups were fed ad libitum, one with a commercial pellet (control diet, C; proximate composition as g/100 g of dry matter: crude protein 18.5, ether extract 3.0, crude fibre 17.8, ash 9.0) and the other two with the same pellet supplemented by ginger powder at the concentration of 4% (G4) or 8% (G8) of the feed (3.6 g/100 g and 7.2 g/100 g of dry matter, respectively). Water was available ad libitum from nipple drinkers. Body weights and feed intake were registered weekly, anyhow only initial and final weighs and total feed intake were considered for statistical analyses. The experimental protocol was designed according to the guidelines of the current European and Italian laws on the care and use of experimental animals (European directive 2010/63/UE, put into law in Italy with D.Lgs. 26/2014).
Ten rabbits from each experimental group were slaughtered at 90 days of age. Rabbits were electro-stunned and slaughtered by cutting carotid and jugular veins. The dissection procedures of warm and chilled carcases followed the WRSA recommendations (Blasco and Ouhayoun Citation1996). The pH after 45 minutes and 24 h of chilling was recorded on the Longissimus thoracis et lumborum (measured between sixth and seventh lumbar vertebrae) and in Biceps femoris muscle (pHmeter pH80 equipped with a S7 2 PORE SLIM electrode; XS instruments, Carpi, MO, Italy). At 1 day post mortem, left and right Longissimus thoracis et lumborum muscles were dissected and used for meat quality assessments.
Longissimus thoracis et lumborum muscle of each animal was individually packaged in Styrofoam trays overwrapped with polyethylene film and stored at 4 °C up to seven days (namely eight post mortem days) after been divided in sub-samples. Meat was analysed at days 2, 5 and 8 (T2, T5 and T8) post mortem days for water holding capacity, pH, colour, lipid oxidation and antioxidant capacity. Moreover, pH, colour, lipid oxidation and antioxidant capacity were also analysed on meat cooked at T2, T5 and T8. Right and left muscles of each animal were analysed as raw and cooked meat respectively. Water holding capacity was quantified as drip loss between T2 and T5 or T2 and T8 (Lundström and Malmfors Citation1985) and as cooking loss after cooking in a preheated oven at 163 °C to an internal temperature of 71 °C (AMSA Citation1995).
pH was recorded via electrode injection in three different points for each sample; colour (L* = lightness, a* = redness, b* = yellowness; CIE Citation1976) was measured using a Chroma metre Minolta CR300 (Minolta, Osaka, Japan) with an aperture size of 8 mm (illuminant D65, incidence angle of 0°). Hue (H*) and chroma (C*) parameters were calculated as reported by CIE (Citation1976) and the numerical total colour difference (ΔE) was calculated as proposed by Sharma and Bala (Citation2002) among samples of the same diet at different storage times (effect of storage time), among samples of two different diets at a fixed storage time (effect of diet) and among raw and cooked samples of the same diet at the same storage time (effect of cooking).
Lipid oxidation and antioxidant capacity were evaluated via thiobarbituric acid reactive substances (TBARS), ABTS (2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) reducing activity, DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity and FRAP ferric reducing ability on both raw and cooked samples. TBARS were evaluated spectrophotometrically according to Ke et al. (Citation1977) method, as modified by Dal Bosco et al. (Citation2009). The absorbance of TBARS was measured at 532 nm with a V-530 spectrophotometer (Jasco International, Milan, Italy). Results were expressed in mg of malondialdehyde on 100 g of sample via a calibration curve plotted with 1,1,3,3-tetraethoxypropane (0–15 μM).
Antioxidant capacity was measured on meat ethanol extracts as reported by Mancini et al. (Citation2015) using the radical probes of ABTS and DPPH, as reported respectively by Re et al. (Citation1999) and Blois (Citation1958), and the FRAP method as reported by Descalzo et al. (Citation2007) for meat samples. The effect of the diet on the rabbit performances (initial and final weights, feed intake) and the carcases characteristics were statistically analysed via one-way ANOVA. A two-way ANOVA repeated measured was applied on the raw and cooked meat quality parameters in order to evaluate the effects of the diet (D), of the storage time (T) and of their interaction (D × T). Tukey’s test was used to determine the differences when the p value was under .05. When the interaction D × T was not significant, the results were reported as the mean of the fixed effects D and T; the variability was expressed as root mean square error (RMSE). R software (R Core Team Citation2015) was used.
Results and discussion
Live performances and carcase characteristics did not show any significant differences between the three experimental groups (Tables and ). In literature studies on natural antioxidant supplementation in rabbit diet reported a wide range of effect (Abdel-Khalek Citation2013). Celia et al. (Citation2016) and Abd-El-Hady (Citation2014) reported that live performances of rabbits fed an herbal mixture (Digestarom®) were variable as affected also by other not-diet related parameters because animal age or rearing technique.
Table 1. Productive performance of rabbits.
Table 2. Effect of diets on slaughter traits.
Few differences were found in meat quality of both raw and cooked samples (Table ). In raw samples, diet affected pH, lightness, yellowness and chroma (p < .05 for pH and L*; p < .001 for b* and C*). G8 samples showed lower value of pH and higher values of L*, b* and C* than C and G4 samples. Modifications in pH values could affect lightness of meat (Swatland Citation2000), and G8 raw samples showed, as consequence of dietary regimen, a higher value than C samples as at a more acid matrix a lighter meat corresponds. This correlation was found in several studies in which rabbit meat physical characteristics were evaluated (Dal Bosco et al. Citation2014; Mancini et al. Citation2015).
Table 3. Effects of diets and storage times on meat quality.
Interestingly b* and C* indexes showed statistical differences due to the diet also on cooked samples, with higher values of G8 than other diet groups (p < .01) as influenced by the natural colour of ginger. Storage time affected pH, L*, b*, H* and C* in raw samples (p < .01 for pH, b*, H* and C*; p < .01 for L*) and pH and L* in the cooked samples (p < .001 and p < .01 for pH and L*, respectively). pH of raw samples decreased between T5 and T8, while pH of cooked samples reported a constant decrease from T2 to T8. Lightness of raw samples increased progressively during the storage time, whilst an opposite trend was shown by cooked samples. Raw samples showed to increase yellowness index between T5 and T8, and this modification in colour affected the values of H* and C* that consequently increased their value between T5 and T8.
Numerical colour differences (ΔEs) are reported in Table . ΔEs between raw samples of the same diet at different storage times reported that G8 samples appeared different between T5 and T8 and in the overall evaluation (T2–T8), whereas C samples modified their colour in a perceptible way only if compared T2–T8. No colour discernible differences were shown by cooked samples during storage time. Moreover, cooked samples of C and ginger diet groups (G4 and G8) showed to be not recognisable for difference in colour as the ΔEs calculated between the two diets in function of the storage time did not show values over 2.30 points (threshold value for a noticeable difference). Instead, raw C and G8 samples at T2 and T8 were slightly over the threshold leading to a partial difference in colour. As expected, cooking strongly modified the colours of samples, as showed by ΔEs calculated between raw and cooked samples of each diet’s samples as function of the storage time.
Table 4. Total colour difference (ΔE) between diets, storage times and between raw and cooked samples.
Lipid oxidation (TBARS) of raw samples showed a significant interaction D × T (p < .01, Figure ). Samples showed lowest values of oxidation at T2 with a constant increase during storage time. Anyhow, at T8, G4 and G8 samples showed to maintain their level of lipid oxidation at least comparable to T5 of the C samples, proving an enhancement of meat shelf life due to ginger addition in the diets. At T5, G8 samples showed lower value than C and G4 samples, delaying lipid oxidation in time. A different resistance to lipid oxidation in samples from groups differently fed was not shown in cooked samples as only storage time significantly affected TBARS values (p < .001, Table ), that increased with storage time with statistical differences among all the tested times. Similarly, Lo Fiego et al. (Citation2004) reported that dietary vitamin E (300 ppm) and vitamin C (500 ppm) negatively affected the lipid oxidation in rabbit meat stored at 2.0 °C for eight days, as well as Eid et al. (Citation2011) reported that also dietary green tea significantly decreased TBARS of thigh and loin meat stored for two months. Even if TBARS were affected by dietary antioxidant supplementation no modifications were highlighted in antioxidant capacity of raw and cooked samples (Table ). This lack of correlation between antioxidant characteristics and lipid oxidation in rabbit meat was reported by other Authors (Dal Bosco et al. Citation2014; Dalle Zotte et al. Citation2014) and in other meats from different animal species (Hernández-López et al. Citation2016; Mancini, Paci, Pisseri, et al. Citation2017) fed antioxidant products. On the other hand, few research articles on dietary supplementation report a decrease in lipid oxidation as consequence of a modification in antioxidant characteristics (Jung et al. Citation2010; Qwele et al. Citation2013; Mancini et al. Citation2016). These lack of coherence between studies lead to hypothesise that different mechanisms of action could be related to different antioxidants and no consistent results could be expected.
Figure 1. Effect of the interaction of diet (D) and storage time (T) on lipid oxidation (TBARS) of raw rabbit meat. C: control diet; G4: control diet +4% ginger powder; G8: control diet +8% ginger powder.a,b,c,dDifferent letters indicate significant differences for D × T at p < .05.
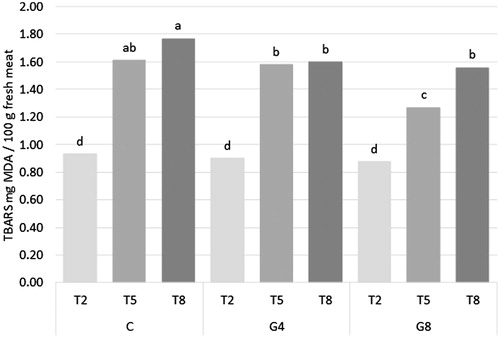
Table 5. Effects of diet and storage time on lipid oxidation and antioxidant capacity of rabbit meat.
Conclusions
Dietary supplementation with ginger powder represents a good opportunity to ameliorate meat quality of rabbits, resulting in a lowering of lipid oxidation susceptibility without any interference with productive performances of animals. Modifications were also highlighted in pH values and colour parameters of both raw and cooked samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abd-El-Hady AM. 2014. Performance, physiological parameters and slaughter characteristics in growing rabbits as affected by a herbal feed additives (Digestarom®). Agric Food. 2:353–365.
- Abdel-Khalek AM. 2013. Supplemental antioxidants in rabbit nutrition: a review. Livest Sci. 158:95–105.
- AMSA. 1995. Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat. Chicago (IL): National Live Stock and Meat Board.
- Blasco A, Ouhayoun J. 1996. Harmonization of criteria and terminology in rabbit meat research. Revised proposal. World Rabbit Sci. 4:93–99.
- Blois MS. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 181:1199–1200.
- Celia C, Cullere M, Gerencsér Z, Matics Z, Giaccone V, Kovàcs M, Bònai A, Szendrö Z, Dalle Zotte A. 2016. Dietary supplementation of Digestarom® herbal formulation: effect on apparent digestibility, faecal and caecal microbial counts and live performance of growing rabbits. World Rabbit Sci. 24:95–105.
- CIE. 1976. Official recommendations on uniform colour spaces, colour differences equations and metric colour terms. Paris (France): Commission Internationale de l’Eclairage.
- Dal Bosco A, Gerencsér Z, Szendrö Z, Mugnai C, Cullere M, Kovàcs M, Ruggeri S, Mattioli S, Castellini C, Dalle Zotte A. 2014. Effect of dietary supplementation of Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) on rabbit meat appearance, oxidative stability and fatty acid profile during retail display. Meat Sci. 96:114–119.
- Dal Bosco A, Mourvaki E, Cardinali R, Servili M, Sebastiani B, Ruggeri S, Mattioli S, Taticchi A, Esposto S, Castellini C. 2012. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 92:783–788.
- Dal Bosco A, Mugnai C, Mourvaki E, Cardinali R, Moscati L, Paci G, Castellini C. 2009. Effect of genotype and rearing system on the native immunity and oxidative status of growing rabbits. Ital J Anim Sci. 8:781–783.
- Dalle Zotte A, Cullere M, Sartori A, Szendrö Z, Kovàcs M, Giaccone V, Dal Bosco A. 2014. Dietary Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) supplementation to growing rabbits: Effects on raw and cooked meat quality, nutrient true retention and oxidative stability. Meat Sci. 98:94–103.
- Dalle Zotte A. 2002. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest Prod Sci. 75:11–32.
- Descalzo AM, Rossetti L, Grigioni G, Irurueta M, Sancho AM, Carrete J, Pensel NA. 2007. Antioxidant status and odour profile in fresh beef from pasture or grain-fed cattle. Meat Sci. 75:309–317.
- Eid Y, Zeweil H, Ahmed MH, Basyony M, Farok M. 2011. Effect of plant source of omega-3 fatty acids and green tea powder on the performance and meat quality of growing rabbits. Egypt J Rabbit Sci. 21:115–134.
- Gerencsér Z, Szendrö Z, Matics Z, Radnai I, Kovács M, Nagy I, Cullere M, Dal Bosco A, Dalle Zotte A. 2014. Effect of Dietary supplementation of Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) on apparent digestibility and productive performance of growing rabbits. World Rabbit Sci. 22:1–9.
- Hernández-López SH, Rodríguez-Carpena JG, Lemus-Flores C, Galindo-García J, Estévez M. 2016. Antioxidant protection of proteins and lipids in processed pork loin chops through feed supplementation with avocado. J Food Sci Tech. 53:2788–2796.
- Jiang J, Xiong YL. 2016. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: a review. Meat Sci. 120:107–117.
- Jung S, Choe JH, Kim B, Yun H, Kruk ZA, Jo C. 2010. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 86:520–526.
- Ke PJ, Ackman RG, Linke BA, Nash DM. 1977. Differential lipid oxidation in various parts of frozen mackerel. Int J Food Sci Tech. 12:37–47.
- Lo Fiego D, Santoro P, Macchioni P, Mazzoni D, Piattoni F, Tassone F, De Leonibus E. 2004. The effect of dietary supplementation of vitamins C and E on the α-tocopherol content of muscles, liver and kidney, on the stability of lipids, and on certain meat quality parameters of the longissimus dorsi of rabbits. Meat Sci. 67:319–327.
- Lundström K, Malmfors G. 1985. Variation in light scattering and water-holding capacity along the porcine Longissimus dorsi muscle. Meat Sci. 15:203–214.
- Mancini S, Paci G, Fratini F, Torracca B, Nuvoloni R, Dal Bosco A, Roscini V, Preziuso G. 2017. Improving pork burgers quality using Zingiber officinale Roscoe powder (ginger). Meat Sci. 129:161–168.
- Mancini S, Preziuso G, Dal Bosco A, Roscini V, Parisi G, Paci G. 2017. Modifications of fatty acids profile, lipid peroxidation and antioxidant capacity in raw and cooked rabbit burgers added with ginger. Meat Sci. 133:151–158.
- Mancini S, Paci G, Pisseri F, Preziuso G. 2017. Effect of turmeric (Curcuma longa L.) powder as dietary antioxidant supplementation on pig meat quality. J Food Process Pres. 41:e12878.
- Mancini S, Preziuso G, Dal Bosco A, Roscini V, Szendrő Z, Fratini F, Paci G. 2015. Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on physical characteristics and oxidative status of fresh and stored rabbit burgers. Meat Sci. 110:93–100.
- Mancini S, Preziuso G, Paci G. 2016. Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on antioxidant capacity and oxidative status in rabbit burgers after cooking. World Rabbit Sci. 24:121–127.
- Peiretti PG, Gai F, Rotolo L, Brugiapaglia A, Gasco L. 2013. Effects of tomato pomace supplementation on carcass characteristics and meat quality of fattening rabbits. Meat Sci. 95:345–351.
- Qwele K, Hugo A, Oyedemi SO, Moyo B, Masika PJ, Muchenje V. 2013. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 93: 455–462.
- R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med. 26:1231–1237.
- Sharma G, Bala R. 2002. Digital color imaging handbook. London: CRC Press.
- Swatland H. 2000. Meat science: an introductory text. P.D. Warriss. Wallingford: CABI Publishing.
- Zomrawi W, Abdel Atti K, Dousa B, Mahala A. 2012. The effect of ginger root powder (Zingiber officinale) supplementation on broiler chicks performance, blood and serum constituents. Online J Anim Feed Res. 2:457–460.
