 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In cow-calf beef production systems, the growth and development of replacement heifers deserve special attention because they affect the age at puberty and the onset of reproductive activity. Therefore, the objective of this study was to test if changes in average daily gain (ADG) have an effect on eye muscle area, rump fat depth and back-fat thickness in developing beef heifers. These traits of 42 beef heifers were measured over time. The results of the current study allow inferring that heifers older than 385 days and with high rates of ADG (above 0.950 kg per day) have the greatest impact on longissimus muscle area growth (above 57 cm2). On the other hand, it is observed that younger heifers (less than 230 days old) do not exceed 48 cm2 of the longissimus muscle area, even with ADGs around 1.25 kg. A positive relationship between the rate of ADG and rump fat depth was also evidenced, observing that rump fat depth deposition is influenced by the rate of ADG. The results of the current study show a linear trajectory of rump fat depth, indicating that for every 0.10 kg of ADG during a period exceeding 56 days, an increase of 1.09 mm of rump fat depth occurs. In conclusion, this study illustrates a simple way on how ADG and age affect the body composition traits in beef heifers and shows that changes in average daily gain affect muscle and fat tissue growth, as determined by longissimus muscle area and rump fat depth in growing beef heifers.
Introduction
Brazil is one of the main global producers of beef and in cow-calf beef production systems and replacement heifers are the genetic basis of the herd. The Brazilian Ministry of Agriculture (MAPA Citation2017) estimates that beef production will increase by 20.5%, consumption by 15.8% and exports by 3.5% per year until 2027.
However, to achieve these forecasts, the heifer systems need to be fully understood and optimised, as heifers improve the genetics of cow herds because they are the backbone of beef production systems (Hersom et al. Citation2013). Therefore, in the beef cattle industry, the growth and development of replacement heifers deserve special attention because they affect the age at puberty and the onset of reproductive activity (Randel and Welsh Citation2013; Gonzalez et al. Citation2016), as well as heifer retention rate and cow longevity (Endecott et al. Citation2013; Roberts et al. Citation2015).
In beef heifers, these factors are affected by the rate of average daily gain (ADG) post-weaning, among other factors (Greer et al. Citation1983; Bagley Citation1993; Barcellos et al. Citation2014). There are several reports in the international literature on the development of replacement beef heifers (Lancaster et al. Citation2009; Lardner et al. Citation2014; Moriel et al. Citation2014; Landarin et al. Citation2016). However, there are few studies evaluating the impact of ADG on body composition traits during heifer development. In this context, the objective of this study was to test if changes in ADG have an effect on eye muscle area, rump fat depth and back-fat thickness in developing beef heifers.
Materials and methods
The experiment was carried out at the Research Station of the Federal University of Rio Grande do Sul, Brazil (30°05’51”S, 51°40’42”W). The climate is subtropical humid (Cfa classification, Köppen), with annual precipitation of 1440 mm, well distributed throughout the year; June is the wettest month (168.2 mm) and December is the driest (97.7 mm).
Animals
The experimental protocol followed the guidelines and was approved by the Ethics Committee on Animal Use at the Federal University of Rio Grande do Sul (CEUA-UFRGS) under project number 27,478. In this study, 42 Brangus females, born in the spring (September–November) of 2012 were evaluated between 16 April 2013 (weaning) and 25 February 2014.
The health management of heifers was in accordance with the recommendations of the veterinarian of the experimental station of UFRGS and consisted of preventive treatments against infectious, viral, ectoparasite and endoparasite diseases. The bovine brucellosis (Brucella abortus) vaccine was applied at four months of age. At five months of age, the vaccine against anthrax (Bacillus anthracis) and clostridiosis (Clostridium spp.) was applied, the latter having been reinforced in 21 days. At seven months of age, rabies vaccine (rabies virus) was applied with reinforcement within 30 days after the first application. At 11 months of age, heifers were immunised against infectious bovine rhinotracheitis, bovine viral diarrhoea and leptospirosis (Leptospira spp.), with repetition of these vaccines after 21 days. For the strategic control of the ectoparasites, the active principles cypermethrin with chlorpyrifos and fipronil with fluazuron 2.5% were used, while for the control of endoparasites, the active principle ivermectin 1 and 3.5% was used, according to the recommendation of the veterinarian.
Feeding management systems
At the beginning of the study, heifers with average body weight (BW) of 167.8 ± 3.9 kg and 187 ± 8.0 days of age were randomly distributed into four feeding management systems (FS). The number of heifers per treatment corresponded to FS1 = 9, FS2 = 8, FS3 = 9 and FS4 = 16 heifers, setting an unbalanced experimental design (Quinn and Keough Citation2002) due to the carrying capacity of the paddocks available in this study.
From April to July (92 days, autumn-winter), all heifers were managed as a single group. In April, they grazed on pearl millet (Pennisetum americanum) and received a supplement at 1.5% of average body weight three times a week. The supplement consisted of 87% corn, 10% soybeans and 3% mineral salt with 80 g phosphorus/kg and contained 17% crude protein and 79% total digestible nutrients.
In May, heifers grazed on a deferred Brachiaria decumbens pasture and received the above supplement three times a week. In the period from June 2 to July 19 (47 days, autumn-winter), heifers continued to graze a single group on a black oat pasture (Avena strigosa Schreb) and were supplemented as mentioned above.
From July 20 until November 9 (112 days, winter-spring), heifers in treatments FS2, FS3 and FS4 grazed on a ryegrass pasture (Lolium multiflorum Lam). During this period, heifers were supplemented daily with increasing levels of cracked corn, according to treatment. Control heifers (FS1) received only 40 g/head/d of mineral salt on the same pasture, corresponding to the control treatment, whereas FS2 heifers were offered 0.5% cracked corn, FS3 1% of cracked corn and FS4 1.5% cracked corn relative to body weight. From 10 November to 3 January (54 days, spring-summer), all heifers grazed on a natural pasture with moderate pasture allowance (12% DM relative to BW), and received mineral salt containing 80 g of phosphorus per kg ad libitum. During the last 54 days of the study, in January and February 2014 (summer), all heifers grazed on pearl millet (Pennisetum americanum) and were offered mineral salt with 80 g of phosphorus per kg. Further details regarding feeding management systems are illustrated in Table .
Table 1. Feeding management systems during the study period.
A chemical analysis of the pastures and supplements was carried out to know the nutrient density of the feed offered to the heifers throughout the evaluation period (Table ).
Table 2. Diet nutrient density during the evaluation period.
Measurements and equipment
Heifer individual BW was obtained using an electronic scale (True-test 3000® GR) after a 12 h feed and water fasting, and body composition was assessed by ultrasound.
The ultrasound images were obtained on the left side of the animals, according to the guidelines of the Ultrasound Council (UGC) , as described by Hays and Meadows (Citation2012). Ultrasound images were obtained using an echo-camera as a master unit (Aloka SSD 500V, Corometrics Medacals Systems, Wallingford, CT) coupled with a 17.2 cm long linear transducer UST 5049 at 3.5 MHz frequency, using vegetable oil as an acoustic couplant.
The images were used to estimate eye muscle area (longissimus muscle area; ULMA) and back-fat thickness (UBFT). ULMA was obtained from the anatomical site intercostal space between the 12th and 13th ribs, with the aid of acoustic probe, including the total muscle area, as was expressed in square centimetres (cm2). In order to prevent tissue distortions during ULMA measurement, the transducer was fitted with a Super flab waveguide standoff pad (Mick Radio-Nuclear Instruments, Inc., Mt Vernon, NY). UBFT was measured at the anatomical site located three-fourth distances from the medial side of the longissimus muscle to its lateral side and expressed in millimetres (mm). Rump fat depth (URFD) was measured, placing the transducer parallel between the ilium and ischium, precisely at the intersection between the muscles gluteus medius and biceps femoris in millimetres (mm). An image per animal was collected for ULMA, UBFT and URFD measurements, stored on the hard disk of a laptop and later interpreted using a specific software by a UGC certified technician. The analysis was conducted according to the protocols of the laboratory CUP lab-Brazil, UFRGS, RS, Brazil. The serial measurements in live animals started to be made in April 2013 (autumn), in total six measurements per treatment, corresponding to 0, 77, 140, 189, 244 and 315 days of the experimental period. The characteristics ADG, ULMA and UBFT were measured from 0 to 315 days of the experimental period, while URFD was measured from 77 to 315 days (Table ).
Table 3. Descriptive summary of body composition of beef heifers submitted to FS1, FS2, FS3, FS4 feeding systems with different periods at the time of ultrasound evaluation (0, 77, 140, 189, 244 and 315 days).
Statistical analysis
Repeated measure analysis was adjusted for the model:
where Yijk is the phenotype value for ULMA, URFD or UBFT evaluated on animal k in the feed system i and at evaluation day j; u is the constant average; Si(Dj) is the classification effect of system i at the evaluation day j; αijk (ADG) is the covariate effect of ADG of the animal k in the feed system i and at evaluation day j; βijk (AGE) is the covariate effect of animal’s age (days) k in the feed system i and at evaluation day j. Different covariance matrix structures between different evaluation days were tested using options in the PROC MIXED of Statistical Analysis System (SAS Citation2004; SAS Institute Inc., Cary, NC). Bayesian information criterion (BIC) was used to choose the better covariance matrix and the TOEPH structure was chosen for adjusting ULMA, UBFT and URFD.
Results and discussion
Here, we provide evidence that changes in ADG and age, affect longissimus muscle area in beef heifers. In addition, the results of this study suggest that ADG rates also determine rump fat depth. The ADG rate determined increases longissimus muscle area and rump fat depth (p < .05). Age also determined longissimus muscle area (p < .05), however, there was no effect of ADG and age on back-fat thickness (p > .05; Table ).
Table 4. Mixed model estimates for ADG, age and intercept associated with body composition of 42 beef heifers.
A model was obtained for the longissimus muscle area (Figure ; ULMA (cm2) = 25.7617 + 0.06919 Age (days) + 5.0737
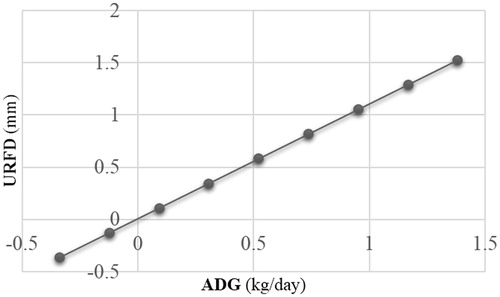
ADG) where for every 0.1 kg of ADG, an increase of 5 cm2 of longissimus muscle area occurs, and for each day of age an increase of 0.07 cm2 for the same trait occurs. In the model obtained for rump fat depth (Figure ; URFD (mm) = 0.005183 + 1.0996
ADG), there was no effect of age. (p = .11). The mixed model estimates for ADG, age and intercept associated with the body composition of 42 beef heifers are shown in Table .
Figure 1. Effect of average daily gain (ADG) and age on longissimus muscle area. ULMA: longissimus muscle area.
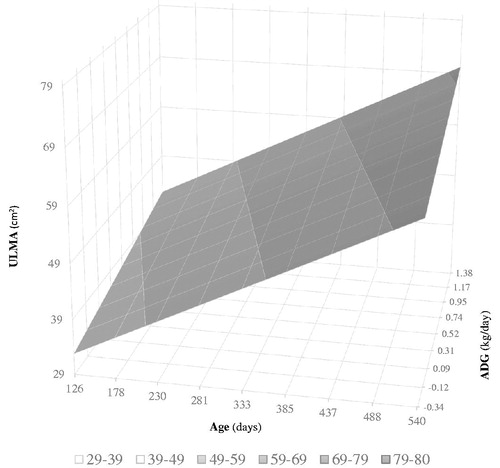
Figure 2. The relationship between average daily gain (ADG) and rump fat depth. URFD: rump fat depth.
Heifers with higher age and higher ADG rate were also the heifers that achieved higher longissimus muscle area. This means that heifers with phenotypic changes of the longissimus muscle area are largely associated with body weight differences, as reported in other studies (Berg and Butterfield Citation1976; Truscott et al. Citation1980).
Although there are studies associating BW with age (Bond et al. Citation2015; Titterington et al. Citation2015; Gonzalez et al. Citation2016) and longissimus muscle in heifers (Guggeri et al. Citation2014; Reis et al. Citation2015), few studies have evaluated the impact of ADG on body composition in beef heifers under grazing conditions.
Also, the present study had a different feeding system, this effect was added into the statistical model to reduce residual variance and this was the reason why the effect of feeding system was not discussed. Thus, the model which evaluated the effects of ADG and age on the characteristics like longissimus muscle area, rump fat depth and back-fat thickness was tested.
The results of the current study allow inferring that heifers older than 385 days and with high rates of ADG (above 0.950 kg per day) have the greatest impact on longissimus muscle area growth (above 57 cm2). On the other hand, it is observed that younger heifers (less than 230 days old) do not exceed 48 cm2 of the longissimus muscle area, even with ADGs around 1.25 kg. In a study by Moriel et al. (Citation2014), evaluating growth (Brahman × British crossbred heifers) under grazing conditions, heifers achieved a longissimus muscle area of 43.6 cm2 at 342 days of age, with an ADG of 0.69 kg from 252 to 462 days of age. These heifers achieved puberty at 397 days of age with a BW of 316 kg, which represented 58.7% of mature BW. In this age and ADG (342 days and 0.69 kg per day, respectively), a simulation was performed with the estimates of the ULMA mixed model of the current study (ULMA (cm2) = 25.7617 + 0.06919 × Age (days) + 5.0737 × ADG), obtaining a longissimus muscle area of 52.9 cm2, that is 21.3% higher. This difference may be probably associated with genetic or environmental aspects (Lancaster et al. Citation2009).
A positive relationship between the rate of GMD and rump fat depth was also evidenced, observing that rump fat depth deposition is influenced by the rate of ADG. The results of the current study show a linear trajectory of rump fat depth, indicating that for every 0.10 kg of ADG during a period exceeding 56 days, an increase of 1.09 mm of rump fat depth occurs. A similar effect was obtained in a study by Lardner et al. (Citation2014), where differences were detected for ADG, prebreeding BW, final rump fat and pregnancy diagnosis BW between development system. High ADG in beef heifers (0.7 kg per day) had greater final BW (396 vs. 353 kg) and rump fat depth (2.6 vs. 1.4 mm) compared with moderate ADG (0.5 kg per day).
The ADG had no effect on back-fat thickness. This can be explained by the rump fat depth that is deposited earlier than back-fat thickness. Similar results were found by Tait et al. (Citation2005) and Yokoo et al. (Citation2014).
Although this study did not evaluate reproductive parameters, it brings relevant information about the effect of ADG on the deposition of rump fat depth and longissimus muscle area growth, and it is known that the relation between these characteristics is associated with puberty in heifers. The deposition of rump fat depth before breeding season translates into body reserves and may be related to the anticipation of puberty (Randel and Welsh Citation2013). Heifers who have a higher ADG and higher BW before the breeding season, are heifers that can conceive at 14–15 months of age, as reported by Landarin et al. (Citation2016). Therefore, as the rate of ADG can be controlled through selective management practices, age at puberty can be predicted (Arije and Wiltbank Citation1974).
The limitation of the current study is the number of animals used in the experiment and the fact that there is no year repetition. However, the statistical models that were used provide adequate estimates and the 95% confidence level, accepted by the international scientific community. In addition, it was possible to verify that the ULMA model provides adequate estimates for what could be expected to occur on an average and this was observed when a simulation was carried out with the data generated by the study of Moriel et al. (Citation2014). It was also possible to verify that the mathematical solution of our URFD model is in agreement with the biological relationship between ADG and rump fat depth in heifers found by Lardner et al. (Citation2014). Despite the limitations mentioned above, the authors believe the results are reflective of what may be expected to occur in biological systems.
Conclusions
This study illustrates a simple way on how ADG and age affect the body composition traits in beef heifers and shows that changes in average daily gain affect muscle and fat tissue growth, as determined by longissimus muscle area and rump fat depth in growing beef heifers. Thus, the knowledge of the influence of animal growth rate on body composition allows planning feeding strategies aimed at improving the efficiency of beef cattle production systems.
We suggest that routine monitoring of heifer ADG and body composition will allow producers to better evaluate the success of their heifer management.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arije GF, Wiltbank JN. 1974. Prediction of age and weight at puberty in Beef Heifers. J Anim Sci. 38:803–810.
- Bagley CP. 1993. Nutritional management of replacement beef heifers: a review. J Anim Sci. 71:3155–3163.
- Barcellos JOJ, Pereira GR, Dias EA, McManus C, Canellas L, Bernardi ML, Tarouco A, Prates ÊR. 2014. Higher feeding diets effects on age and liveweight gain at puberty in crossbred Nelore × Hereford heifers. Trop Anim Health Prod. 46:953–960.
- Berg RT, Butterfield RM. 1976. New concepts of cattle growth. New South Wales, Australia: Sydney University Press, University of Sydney.
- Bond GB, von Keyserlingk MAG, Chapinal N, Pajor EA, Weary DM. 2015. Among farm variation in heifer BW gains. Animal. 9:1884–1887.
- Endecott RL, Funston RN, Mulliniks JT, Roberts AJ. 2013. Implications of beef heifer development systems and lifetime productivity. Paper presented at: The Joint Alpharma-beef Species Symposium of Journal of Animal Science and the American Society of Animal Science; 2012 July 15–19; Phoenix, AZ, USA. Vol. 91; p. 1329–1335.
- Gonzalez FAL, Patino HO, Swanson KC, Pereira CH, Tarouco JU, Diaz AMP, Silveira SRL. 2016. Performance of heifers supplemented with different levels of corn on pasture. Bol Ind Anim. 73:260–266.
- Greer RC, Whitman RW, Staigmiller RB, Anderson DC. 1983. Estimating the impact of management decisions on the occurrence of puberty in Beef Heifers. J Anim Sci. 56:30–39.
- Guggeri D, Meikle A, Carriquiry M, Montossi F, De Barbieri I, Viñoles C. 2014. Effect of different management systems on growth, endocrine parameters and puberty in Hereford female calves grazing Campos grassland. Livest Sci. 167:455–462.
- Hays C, Meadows A. 2012. Field technician study guide. Miles, MT: Ultrasound Guidelines Council. Chapter V, Ultrasound scanning technique.
- Hersom MJ, Bodine TN, Herring A. 2013. Redefining the replacement heifer paradigm. Paper presented at: The Joint Alpharma-Beef Species Symposium of Journal of Animal Science and the American Society Of Animal Science; 2012 July 15–19; Phoenix, AZ, USA. Vol 91. p. 1321–1322.
- Lancaster PA, Carstens GE, Crews DH, Welsh TH, Forbes TDA, Forrest DW, Tedeschi LO, Randel RD, Rouquette FM. 2009. Phenotypic and genetic relationships of residual feed intake with performance and ultrasound carcass traits in Brangus heifers. J Anim Sci. 87:3887–3896.
- Landarin CM, Lobato JFP, Tarouco AK, Tarouco JU, Eloy LR, Pötter L, Rosa AAG. 2016. Growth and reproductive performance of 14- to 15-month-old Hereford heifers. R Bras Zootec. 45:667–676.
- Lardner HA, Damiran D, Hendrick S, Larson K, Funston R. 2014. Effect of development system on growth and reproductive performance of beef heifers. J Anim Sci. 92:3116–3126.
- MAPA. 2017. Ministério da Agricultura, Pecuária e Abastecimento (MAPA): Projeções do agronegócio, Brasil 2016/2017 a 2026/2027. 8th edition. Brasilia, DF, Brasil.
- Moriel P, Johnson SE, Vendramini JMB, Mercadante VRG, Hersom MJ, Arthington JD. 2014. Effects of calf weaning age and subsequent management system on growth and reproductive performance of beef heifers. J Anim Sci. 92:3096–3107.
- Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge, United Kingdom: Cambridge University Press, University of Cambridge.
- Randel RD, Welsh TH. 2013. Interactions of feed efficiency with beef heifer reproductive development. Paper presented at: The Joint Alpharma Beef Cattle Nutrition and Beef Species Symposium of Journal of Animal Science and the American Society of Animal Science: 2012 July 15–19; Phoenix, AZ, USA. Vol 91; p.1323–1328.
- Reis MM, Cooke RF, Cappellozza BI, Marques RS, Guarnieri Filho TA, Rodrigues MC, Bradley JS, Mueller CJ, Keisler DH, Johnson SE, et al. 2015. Creep-feeding to stimulate metabolic imprinting in nursing beef heifers: impacts on heifer growth, reproductive and physiological variables. Animal. 9:1500–1508.
- Roberts AJ, Petersen MK, Funston RN. 2015. Can we build the cowherd by increasing longevity of females? Paper presented at: The Beef Species Symposium of Journal of Animal Science and the American Society of Animal Science; 2014 July 20–24; Kansas, MO, USA. Journal of animal science. Vol 93; p. 4235–4243.
- SAS. 2004. SAS 9.0. SAS user’s guide. Cary (NC): SAS Institute Inc. https://support.sas.com/documentation/onlinedoc/91pdf/sasdoc_913/whatsnew_10878.pdf
- Tait RG, Wilson DE, Rouse GH. 2005. Prediction of retail product and trimmable fat yields from the four primal cuts in beef cattle using ultrasound or carcass data. J Anim Sci. 83:1353–1360.
- Titterington FM, Morrison SJ, Lively FO, Wylie ARG, Gordon AW, Browne MR. 2015. An analysis of Northern Ireland farmers' experiences of using a target-driven beef heifer growth management plan and development of an empirical model leading to the launch of a decision support tool to promote first calving of beef heifers at 24 months. Agricult Sys. 132:107–120.
- Truscott T, Tulloh N, Whitfield D. 1980. A seriatim study using ultrasonic measurements of fat depth and M. longissimus area in Hereford bulls, steers and heifers under grazing conditions. Animal Sci. J. 30:199–209.
- Yokoo MJ-I, Ortelan AA, Sarmento JLR, de Magalhães Rosa GJ, Cardoso FF, de Albuquerque LG. 2014. Medidas repetidas no estudo de características de crescimento e carcaça avaliadas por ultrassom em novilhas de corte cruzadas. Bol Ind Anim. 71:200–210.
