Abstract
The present study was undertaken to investigate the effects of dietary lycopene and tomato paste on plasma lipids, malondialdehyde (MDA) concentrations and fatty acid composition of fresh belly meats from finishing pigs. In addition, lycopene concentrations in fresh belly meat were measured. A total of 60, 18-week-old crossbred pigs (Landrace × Yorkshire × Duroc) were fed four dietary treatments for four weeks. The diets included a base diet of maize and soybean meal (control treatment) and the base diet mixed with lycopene (20 mg/kg of diet); or tomato paste (3.4 g/100 g diet); or both (10 mg/kg lycopene and 1.7 g tomato paste/100 g diet). Supplementing pigs with lycopene and/or tomato paste did not affect production traits, plasma lipids including total lipids, total cholesterol, high-density and low-density cholesterols, and triglycerides. The dietary lycopene or tomato paste resulted in significantly lower (p < .05) MDA concentrations in fresh pork bellies compared with the control group. Lycopene was not detected in fresh belly meat of pigs fed the control diet, but the lycopene concentration was significantly higher (p < .05) in lycopene- or tomato paste-fed pigs. The fatty acid composition of fresh belly meat was not affected by dietary treatment. We conclude that the dietary lycopene or tomato paste failed to affect growth performance and lipid parameters in late finishing pigs but resulted in lower MDA concentrations in fresh pork belly meats. It is likely that enhanced oxidative stability in pork belly meats is mediated via the incorporation of diet-origin lycopene in the analysed tissues.
Dietary lycopene improves oxidative stability of fresh belly meats from pigs.
Dietary lycopene is incorporated into fresh belly meats from pigs.
Highlights
Introduction
Lipid oxidation is a major deteriorative process that negatively affects the flavour, colour and nutritional value of meats (Rossi et al. Citation2013). Thus, many attempts have been made to reduce lipid peroxidation in meat with the use of antioxidants in animal feeds. Antioxidants are components that can donate hydrogen radicals for pairing with other available free radicals to prevent the propagation reaction during the oxidation process (Kumar et al. Citation2015). Currently, synthetic and natural antioxidants are available, but the former, including butylated hydroxytoluene (BHT), are raising concerns among consumers due to the fact that they have potential adverse effects that natural antioxidants do not (Lee et al. Citation2017; Nguyen et al. Citation2017).
Lycopene is a red coloured pigment that occurs naturally in fruits and vegetables. Tomatoes are known to be an important source of lycopene, and tomatoes and tomato products have a lycopene concentration of 3100–8600 µg per 100 g (Stahl and Sies Citation1996). The most well-known biological effect of lycopene is to act as a cholesterol-lowering or antioxidant compound (Di Mascio et al. Citation1989). Dietary lycopene and tomato products have been known to improve meat quality in poultry via inhibiting lipid peroxidation due to their antioxidative capacity (Leal et al. Citation1999; Botsoglou et al. Citation2004; Sahin et al. Citation2006; Bou et al. Citation2009; Sun et al. Citation2014a). Lycopene-rich eggs have been also reported in laying hens (Kim et al. Citation2008; Sahin et al. Citation2008; Rotolo et al. Citation2010; Akdemir et al. Citation2012; Sun et al. Citation2014a). To our surprise, the effects of dietary lycopene have not been previously tested in pigs, which prompted us to design the current experiment. Recently, Correia et al. (Citation2017) reported that tomato pomace-fed piglets had improved meat oxidative stability, although lycopene and beta-carotene were not detected in the meat. We hypothesised that the dietary lycopene could exhibit in vivo antioxidant characteristics in pigs. To verify the antioxidant characteristics of lycopene and/or tomato paste, concentrations of malondialdehyde (MDA), an indicator of lipid peroxidation, were measured in fresh pork bellies. To further assess the hypocholesterolemic effects of lycopene (Di Mascio et al. Citation1989), plasma lipid concentrations were also measured. In addition, we analysed the fatty acid composition of fresh pork bellies to evaluate if lycopene could alter fatty acid metabolism in pigs.
Materials and methods
Pigs, diets, and experimental design
The experimental procedures were approved by the Institutional Animal Care and Use Committee at Konkuk University (Seoul, Korea). A total of 60 18-week-old crossbred pigs (Landrace × Yorkshire × Duroc) with an average body weight of 87.6 kg (±0.7 kg standard deviation) were allotted to four dietary treatments. Each treatment included 10 castrated boars and 5 gilts, housed in a pen (length 3.9 m × width 2.4 m) with a slatted floor. The initial body weights of pigs in the different groups were balanced in order to avoid masking effects caused by different initial body weights. To formulate the experimental diets, a maize and soybean meal base diet (Table ) was mixed with either lycopene (LP), tomato paste (TP) or both (LTP). The LP diet contained 20 ppm of lycopene, the TP diet contained 3.4% tomato paste, and the LTP diet contained 10 ppm of lycopene and 1.7% of tomato paste. The base diet was used as a control diet (CONT). Commercially available lycopene (DSM Nutritional Products Inc., Basel, Switzerland) and tomato paste (Heinz) were used. The tomato paste contained 300 mg of lycopene per kg, according to the manufacturer’s specification. Thus, the TP diet contained 10 ppm of lycopene and the LTP diet contained 15 ppm of lycopene. Feed and water were provided on an ad-libitum basis. All pigs were fed the CONT for 1 week before the initiation of the experiment. Experimental diets were fed to pigs for 4 weeks starting when the pigs were 18 weeks old.
Table 1. Ingredients and composition of the base diet (as-fed basis).
Sampling
Individual body weight was measured at the start and end of the experiment. At the end of the experiment, blood samples were collected from the jugular vein into heparinised tubes from six castrated boars per treatment. Plasma was obtained by gentle centrifugation and stored at −20 °C until use. After blood sampling, the pigs were fasted for one day, and then slaughtered in a slaughter house. The eviscerated carcases were cooled at 0 °C for 24 h in a chilling room, and portions of belly (5th through 7th ribs) on the left side of the carcases were sampled and stored at −20 °C until use.
Measurement of lycopene concentrations in belly meat
Lycopene concentrations in fresh belly meats were extracted using the method described by Boileau et al. (Citation2000) and analysed following Wei et al. (Citation2001), using high performance liquid chromatography (HPLC). In brief, a portion of belly meat was homogenised thoroughly, dissolved in a potassium hydroxide/ethanol (1:5) solution containing 1 g/L BHT, and saponified at 60 °C for 30 min. Lycopene was extracted twice under yellow light using hexane and distilled water. The extracts were dried and stored at −20 °C for no longer than two days before HPLC analysis. The HPLC system included Waters 510 pumps, a Waters 717 Plus Auto Sampler, a Waters 486 Tunable Absorbance Detector, and a Waters Nova-Pak (5 µm, 3.9 cm ×300 mm) C18 column. The mobile phase was methanol:acetonitrile:chloroform (47:47:6, v/v/v), and the flow rate was 1.0 mL/min. The HPLC was controlled by Waters Millennium chromatography software, and the lycopene peak was monitored at 472 nm. The lycopene concentration was calculated using a calibration curve prepared with a pure lycopene standard (L-9879, Sigma Co., St. Louis, MO).
MDA concentrations in belly meat
The MDA concentrations in minced belly meat were estimated according to the method described by Ohkawa et al. (Citation1979). The MDA concentration was determined using the specific absorbance coefficient (1.56 × 105 µmol cm−3). The produced MDA was expressed as nM per g of fresh belly meat.
Fatty acid composition of belly meat
Total lipids were extracted from 3 g samples of belly meat with chloroform/methanol (2:1, v/v), following Folch et al. (Citation1957), and methylated according to Sukhija and Palmquist (Citation1988). Fatty acid methyl esters (FAME) were analysed using gas chromatography (HP 6890 series GC System, Hewlett Packard, PA), equipped with a flame ionisation detector, an autoinjector, and a fused silica capillary column (30 m × 0.32 mm ×0.25 µm column, Supelco Inc., Ellefonte, PA). Fatty acids were identified using the standard mixture of FAME mix (C4-C24, Supelco, Bellefonte, PA).
Statistical analysis
Each pig was considered an experimental unit. Data obtained in this study were analysed by one-way analysis of variance (ANOVA) using the general linear model procedure of SAS (Citation1986). Body weight was analysed by analysis of covariance (ANCOVA), with final weight as the dependent variable and initial body weight as the covariable. If the F-test for treatment effect was significant, differences between treatment means were evaluated using Duncan’s multiple range test (Duncan Citation1955). A value of p < .05 was considered significant.
Results
Supplementing finishing pigs with dietary lycopene, tomato paste or a combination of lycopene and tomato paste did not affect production traits (final body weight and body weight gain, see Supplementary material). None of the dietary treatments affected (p > .05) plasma lipid concentrations, such as total lipids, total cholesterol, high-density lipoprotein and low-density lipoprotein (LDL) cholesterols, or triglycerides (Table ). The dietary lycopene and dietary tomato paste resulted in significantly lower (p < .05) MDA concentrations in fresh belly meats compared with the control group (Table ). The MDA concentrations in fresh belly meat were significantly lower (p < .05), by on average 16.8%, 23.0%, and 24.9% in pigs fed the diets containing lycopene, tomato paste, or a combination of lycopene and tomato paste, respectively, compared with the CONT-fed pigs. Lycopene was not detected in belly meats of pigs fed a CONT, but lycopene concentrations were substantially higher it in all treated groups (Figure ). In general, the highest concentration of lycopene was found in belly meat from pigs in the LP group. Finally, none of the dietary treatments affected (p > .05) the fatty acid composition of fresh belly meat (Table ).
Figure 1. Lycopene concentrations in fresh belly meat of finishing pigs fed diets containing lycopene or tomato paste, or both lycopene and tomato paste. Values are shown as means (±SE) of six castrated pigs (n = 6 pigs/treatment). a,bMeans with different superscript letters above the bars differ significantly (p < .05). LP: synthetic lycopene at 20 mg/kg of diet; TP: tomato paste at 3.4%; LTP: synthetic lycopene at 10 mg/kg of diet and 1.7% tomato paste; ND: lycopene not detectable with a detection limit of 0.01 μg/g.
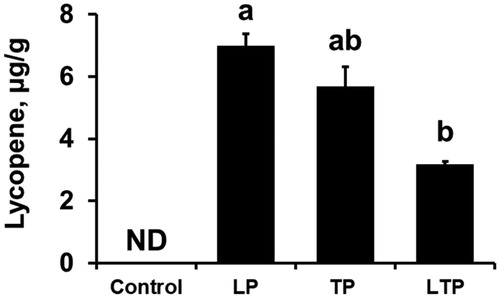
Table 2. Effect of lycopene, tomato paste, or both lycopene and tomato paste, on serum lipid profiles in finishing pigs.
Table 3. Effect of lycopene or tomato paste, or both lycopene and tomato paste, on MDA concentrations in fresh pork belly meats.
Table 4. Effect of lycopene or tomato paste, or both lycopene and tomato paste, on fatty acid profiles (g/100 g total fatty acid methyl esters) of fresh pork belly meats.
Discussion
It is clear from this study that lycopene or tomato paste, when added into the diet fed to pigs, can be incorporated into the tissues (i.e. belly meat). It has been known that lycopene is transported mainly by LDL, and the absorbed lycopene is known to primarily accumulate in the liver, seminal vesicles and prostate tissue in rodents (Palozza et al. Citation2012). Similarly, beta-carotene was predominantly detected in the liver, adrenal gland or intestinal tissues, but trace amounts were detected in muscle tissues and fat depots in ferrets (Ribaya-Mercado et al. Citation1992). In studies using poultry, the incorporation of diet-origin lycopene in serum, liver or eggs has been reported (Sahin et al. Citation2006; Rotolo et al. Citation2010; Englmaierova et al. Citation2011; Sun et al. Citation2014a, Citation2015). It is well documented that lycopene is the predominant carotenoid in plasma, and lycopene concentrations range from 0.15 to 21.66 nmol/g tissue in humans (Kong et al., Citation2010). Collectively, our finding that diet-origin lycopene accumulated in fat and muscle tissues (i.e. belly meat) in pigs agrees with earlier findings. In contrast to our finding, Correia et al. (Citation2017) failed to detect lycopene or beta-carotene in various tissues in piglets fed a diet containing tomato pomace. The major difference between our study and the study by Correia et al. (Citation2017) is the age of the pigs used: we used late finishing pigs, while Correia et al. (Citation2017) used piglets. Thus, differences in voluntary feed intakes could be a plausible explanation, but this needs to be clarified further.
It is clear from this study that MDA concentrations in fresh pork belly were significantly lower in all treated versus control groups. Similarly, an inverse relationship between egg yolk lycopene and MDA concentrations in serum and yolk has been reported in quail (Sahin et al. Citation2008). In addition, the antioxidant characteristics of dietary lycopene have been well documented (Kelkel et al. Citation2011; Joseph et al. Citation2014). Our finding suggests that enhanced oxidative stability of pork belly meat is due to the incorporation of lycopene, rather than lycopene-induced alteration of fatty acid composition or lipid metabolism. In this study, serum lipids and fatty acid composition of belly meat were not affected by the dietary lycopene or tomato paste. In contrast to our findings, a lycopene-mediated reduction in blood lipids via inhibition of de novo cholesterol synthesis and lipogenesis (Palozza et al. Citation2012; Chung et al. Citation2014) has been reported in feedlot lambs (Jiang et al. Citation2015), breeding hens (Sun et al. Citation2014b) and rodents (Palozza et al. Citation2012). On the other hand, it has been reported that the dietary lycopene or tomato products did not affect the fatty acid composition of meat in pigs (Chung et al. Citation2014) and broilers (Rozbicka-Wieczorek et al. Citation2014).
Conclusions
We conclude that the incorporation of diet-origin lycopene did not affect the fatty acid composition of pig belly meat but improved the oxidative stability of fresh belly meats in finishing pigs, as manifested by lower MDA concentrations.
Disclosure statement
No potential conflict of interest was reported by authors.
Additional information
Funding
References
- Akdemir F, Orhan C, Sahin N, Sahin K, Hayirli A. 2012. Tomato powder in laying hen diets: effects on concentrations of yolk carotenoids and lipid peroxidation. Br Poult Sci. 53:675–680.
- Boileau TWM, Clinton SK, Erdman JW. 2000. Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male F344 rats. J Nutr. 130:1613–1618.
- Botsoglou N, Papageorgiou G, Nikolakakis I, Florou-Paneri P, Giannenas I, Dotas V, Sinapis E. 2004. Effect of dietary dried tomato pulp on oxidative stability of Japanese quail meat. J Agric Food Chem. 52:2982–2988.
- Bou R, Codony R, Tres A, Decker EA, Guardiola F. 2009. Dietary strategies to improve nutritional value, oxidative stability, and sensory properties of poultry products. Crit Rev Food Sci Nutr. 49:800–822.
- Chung SH, Son AR, Lee SA, Kim BG. 2014. Effects of dietary tomato byproducts on pork nutrient composition and loin quality of pigs. Asian J Anim Vet Adv. 9:775–781.
- Correia CS, Alfaia CM, Madeira MS, Lopes PA, Matos TJS, Cunha LF, Prates JAM, Freire JPB. 2017. Dietary inclusion of tomato pomace improves meat oxidative stability of young pigs. J Anim Physiol Anim Nutr. 101:1215–1226.
- Di Mascio P, Kaiser S, Sies H. 1989. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 274:532–538.
- Lee MT, Lin WC, Yu B, Lee TT. 2017. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals - a review. Asian-Australas J Anim Sci. 30:299–308.
- Duncan DB. 1955. Multiple range and multiple F tests. Biom. 11:1–41.
- Englmaierova M, Bubancova I, Vit T, Skrivan M. 2011. The effect of lycopene and vitamin E on growth performance, quality and oxidative stability of chicken leg meat. Czech J Anim Sci. 56:536–543.
- Folch J, Lees M, Sloane-Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226:497–509.
- Jiang H, Wang Z, Ma Y, Qu Y, Lu X, Luo H. 2015. Effects of dietary lycopene supplementation on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot Bamei lamb. Asian-Australas J Anim Sci. 28:958–965.
- Joseph S, Chatli MK, Biswas AK, Sahoo J. 2014. Oxidative stability of pork emulsion containing tomato products and pink guava pulp during refrigerated aerobic storage. J Food Sci Technol. 51:3208–3216.
- Kelkel M, Schumacher M, Dicato M, Diederich M. 2011. Antioxidant and anti-proliferative properties of lycopene. Free Radic Res. 45:925–940.
- Kim KS, Hong JA, Kim JW. 2008. Effects of lycopene as a feed additive on the quality of chicken eggs. Korean J Poult Sci. 35:275–281.
- Kong KW, Khoo HE, Nagendra Prasad K, Ismail A, Tan CP, Rajab NF. 2010. Revealing the power of the natural red pigment lycopene. Molecules. 15:959–987.
- Kumar Y, Yadav DN, Ahmad T, Narsaiah K. 2015. Recent trends in the use of natural antioxidants for meat and meat products. Compr Rev Food Sci Food Saf. 14:796–812.
- Leal M, Shimada A, Ruı́z F, González de Mejı́a E, 1999. Effect of lycopene on lipid peroxidation and glutathione-dependent enzymes induced by T-2 toxin in vivo. Toxicol Lett. 109:1–10.
- Nguyen TTK, Laosinwattana C, Teerarak M, Pilasombut K. 2017. Potential antioxidant and lipid peroxidation inhibition of Phyllanthus acidus leaf extract in minced pork. Asian-Australas J Anim Sci. 30:1223–1331.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Chem. 95:351–358.
- Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A. 2012. Effect of lycopene and tomato products on cholesterol metabolism. Ann Nutr Metab. 61:126–134.
- Ribaya-Mercado JD, Fox JG, Rosenblad WD, Blanco MC, Russell RM. 1992. Βcarotene Beta-carotene, retinol and retinyl ester concentrations in serum and selected tissues of ferrets fed beta-carotene. J Nutr. 122:1898–1903.
- Rossi R, Pastorelli G, Cannata S, Tavaniello S, Maiorano G, Corino C. 2013. Effect of long term dietary supplementation with plant extract on carcass characteristics meat quality and oxidative stability in pork. Meat Sci. 95:542–548.
- Rotolo L, Strazzullo G, Pagella M, Brugiapaglia A, Pozzo L, Schiavone A. 2010. Effect of a tomato extract-supplemented diet on egg yolk pigmentation and lycopene transfer efficiency. Ital J Food Sci. 22:180–185.
- Rozbicka-Wieczorek AJ, Więsyk E, Brzóska F, Śliwiński B, Kowalczyk J, Czauderna M. 2014. Fatty acid profile and oxidative stress on thigh muscles in chickens fed the ration enriched in lycopene, selenium compounds or fish oil. Ann Anim Sci. 14:595–609.
- Sahin N, Orhan C, Tuzcu M, Sahin K, Kucuk O. 2008. The effects of tomato powder supplementation on performance and lipid peroxidation in quail. Poult Sci. 87:276–283.
- Sahin N, Sahin K, Onderci M, Karatepe M, Smith MO, Kucuk O. 2006. Effects of dietary lycopene and vitamin E on egg production, antioxidant status and cholesterol levels in Japanese quails. Asian Australas J Anim Sci. 19:224–230.
- SAS User’s Guide. 1986. Statistical Analysis System. Cary (NC): SAS Institute Inc.
- Stahl W, Sies H. 1996. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 336:1–9.
- Sukhija PS, Palmquist DL. 1988. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J Agric Food Chem. 36:1202–1206.
- Sun B, Chen C, Wang W, Ma J, Xie Q, Gao Y, Chen F, Zhang X, Bi Y. 2015. Effects of lycopene supplementation in both maternal and offspring diets on growth performance, antioxidant capacity and biochemical parameters in chicks. J Anim Physiol Anim Nutr. 99:42–49.
- Sun B, Ma J, Zhang J, Su L, Xie Q, Bi Y. 2014. Lycopene regulates production performance, antioxidant capacity, and biochemical parameters in breeding hens. Czech J Anim Sci. 59:471–473.
- Sun B, Ma J, Zhang J, Su L, Xie Q, Gao Y, Zhu J, Shu D, Bi Y. 2014. Lycopene reduces the negative effects induced by lipopolysaccharide in breeding hens. Br Poult Sci. 55:628–634.
- Wei Y, Zhang T, Xu G, Ito Y. 2001. Application of analytical and preparative high-speed counter-current chromatography for separation of lycopene from crude extract of tomato paste. J Chromatogr A. 929:169–173.
