Abstract
This study investigates the expression of the muscle growth factors Myostatin (MSTN) and Insulin-like growth hormone type I (IGF-I) and muscle marker genes MyoD and MyoG in relation to growth performance and meat characteristics in four different commercial broiler strains. Eight hundred, one-day-old chicks of Hubbard Classic (HC), Cobb500 (Cobb), Ross308 (Ross) and Indian River (IR) strains were randomly distributed in a completely randomised design into four groups for 28 days. At the end of the growth trial, 10 birds from each strain were weighed and slaughtered. A sample of Pectoral muscle was taken and kept in RNA solution for mRNA expression level measurements. Gene expression in the pectoral muscle at 20 days revealed that MSTN expression was higher for Ross than HC and IR. IGF-I expression was highest in IR and lowest in HC. MyoD expression was lowest in HC but higher in Ross and Cobb, while MyoG expression was similar. At the end of the experiment, IR gained the highest (p < .0001) body weight, while Ross the lowest, yet still with no significant differences in body weight gain among the four strains. HC scored the lowest (p = .01) efficiency in feed consumption (1.60 ± 0.03 kg/kg). Hot and cold carcase weights of IR were significantly heavier (p < .0001) than the other strains, with no significant difference in dressing percentages. Cooking loss was the highest (p = .058) for Cobb, IR, HC, and Ross, in decreasing order. The meat-tenderness value was highest for Ross, while differences in pH, colour and water holding capacities were insignificant among the strains.
IGF-I and MyoG genes can be used in Broiler artificial selection programmes for improving body weight and carcase cuts.
Indian River strain gained the highest in body, and hot and cold carcase weights.
HIGHLIGHTS
Introduction
Several commercial broiler chicken strains (Arbor Acres, Cobb, Hubbard, Indian River, Lohman, Ross) are reared in the Mediterranean environment. Although many studies attempted to investigate the effect of strain on growth performance, carcase characteristics, and meat quality in broiler chickens worldwide (Dransfield and Sosnicki Citation1999; Young et al. Citation2001; Santiago et al. Citation2005; Berri et al. Citation2006), only few of them focussed on the molecular investigation of gene expression of muscle marker genes (Weintraub Citation1993; Oustanina et al. Citation2004) and muscle growth factors (Adams and McCue Citation1998; Adams et al. Citation1999; Halevy et al. Citation2004). Chicken body weights have increased twice at about half the time compared to five decades ago (Barbut et al. Citation2008); improvements were mainly due to the high heritability of body composition and body weight during breeding (Le Bihan-Duval et al. Citation2003). Furthermore, genetic selection and enhancement of chickens produced heritable changes in their genetic capacity and muscle tissue characteristics by imposing stress on the growing birds (Barbut et al. Citation2008) and altering the majority of chicken growth processes and their relevant environmental requirements. The expression of the genes that control these quantitative traits in chicken is affected by both environmental and genetic factors (Falconer and Mackay Citation1996). Therefore, only an optimal environment would allow the expression of their genetic potential. Several genes have been identified with differing effects on growth performance in broiler chickens (Zhou et al. Citation2005; Bhattacharya et al. Citation2016); differences in gene expression, and body and breast muscle weights have been observed and correlated among birds within the same strain (Xiao et al. Citation2017). Another study showed how upregulation of muscle marker and growth factor genes induced the improvement of body weight of chickens near and at market age (Al-Zghoul et al. Citation2016). Candidate genes involved in growth and meat production traits include IGFs that regulate and control body and muscle growth in chickens (Duclos et al. Citation1999; Kadlec et al. Citation2011). Insulin-like Growth Factor I (Igf-I) is another factor that stimulates proliferation, differentiation and metabolism of myogenic cell lines in different species (Florini et al. Citation1996). Meanwhile, Myostatin (MSTN) has a negative regulatory effect on muscle growth determining both muscle fibre number and size (Carnac et al. Citation2007). Additionally, muscle regulatory factors (MRFs) such as Myogenic Differentiation Antigen (MyoD) and Myogenin (MyoG) play important roles in muscle growth and development (Te Pas and Soumillion Citation2001).
During the last few decades, the inflating global population and the consumer’s perception of the health benefits of chicken meat has led to an increasing demand for poultry meat (FAO Citation2008; López et al. Citation2011), in addition to a shift of consumer's preferences towards the consumption of cuts (particularly breast fillets) and processed products instead of whole chickens (McKee and Sams Citation1998; Mehaffey et al. Citation2006; Abdullah et al. Citation2010). This growing demand has resulted in pressure on nutritionists, breeders and livestock keepers to enhance growth performance and meat quality of birds (Petracci and Cavani Citation2012). Therefore, poultry breeding companies have focussed more on many commercial broiler breeds with superior traits and higher growth performance capabilities (Scheuermann et al. Citation2003; Havenstein et al. Citation2003).
The objectives of this study were to investigate the gene expression of muscle growth factors (MSTN and IGF-1) and muscle marker genes (MyoD and MyoG) in relation to the growth performance and meat characteristics of four different commercial broiler strains reared in the Mediterranean area. The results generated from this study will aid in marker-assisted selection for improved broiler production satisfying both consumers and producers, in addition, to increase knowledge of poultry breed performance in the Middle East.
Materials and methods
All procedures and experiments were approved by the Animal Care and Use Committee (ACUC) of Jordan University of Science and Technology.
A total number of 800, one-day-old chicks from a commercial hatchery were randomly distributed by a completely randomised design to four equal groups, with 10 replicates (pens) per treatment and 20 birds per pen, representing the four broiler strains; Ross 308 (Ross), Indian River (IR), Cobb500 (Cobb) and Hubbard Classic (HC) strains. The chicks were transferred to the animal house at Jordan University of Science and Technology, where the field experiment was conducted.
All birds were fed a well-balanced commercial corn-soybean meal diet as recommended by the National Research Council (NRC Citation1994) shown in . Feed and water were present ad libitum for the duration of the experiment which lasted 28 days. Birds were kept in cages (1.00 × 1.00 m), with a stocking density of 20 birds/m2 (<36 kg/m2). Body Weight (BW) and feed consumption measurements were recorded on a weekly basis. Average daily gain, average daily feed intake (ADFI), and feed:gain ratio was calculated subsequently. At the end of the growth performance trial of 28 days, 10 birds from each strain were weighed and slaughtered following the procedure described by Merkley et al. (Citation1980) on post-hatch day 28. Fasting live weight was recorded, and after scalding (58 to 60 °C, 45 s), the carcases were de-feathered and manually eviscerated. Carcasses were then chilled for 6 h at 5 °C, and carcase cold weight, and cuts weight and percentages were measured and recorded. Meat quality measurements were performed on the major pectoral muscle of the broiler breast according to Hamm (Citation1981).
Table 1. Composition of the experimental diets.
The left side muscle was used for the evaluation of the ultimate pH and water holding capacity. While the muscles from the right side were weighed, placed in plastic bags and cooked at 85 °C for 25 min (Liu et al. Citation2004). Cooking loss was determined by weighing muscles before and after drying and allowed to cool at room temperature, after which tenderness was evaluated on a Salter model (Warner-Bratzler meat shear, G-R Electric Manufacturing Co., Kansas, USA) using the iodo-acetate method (Jeacocke Citation1977; Sams and Janky Citation1986). The pH values were determined in duplicate homogenated samples of 1.5 g each according to standard procedures (Ultra-Turrax T8, IKA Labortechnik, Janke & Kunkal GmbH & Co., Germany). Water holding capacity was measured using 5 g of raw meat (initial weight) samples as described by Grau and Hamm (Citation1953) and modified by Sañudo et al. (Citation1986); they were expressed as percentages of reported weight lost during sample pressing divided by initial sample weight.
Pectoral muscle mRNA expression levels of muscle growth factors (IGF-I and MSTN) and muscle marker genes (MyoD and MyoG) were evaluated using relative-quantitative RT-PCR using a CFX96 Touch TM Real-time PCR thermal cycler (BIO-RAD, California, USA). Sequences of broiler chicken MyoD (NM_001004384.2), MyoG (FJ882411.1), IGF-I (NM_001004384.2), MSTN (AF019621.1), and GAPDH (NM_204305.1) were obtained from the gene bank. All primers were designed using IDT Primer Quest software (http://eu.idtdna.com/PrimerQuest/Home/Index), as listed in .
Table 2. Oligonucleotides used for expression analysis of chicken genes and the annealing temperatures (AT).
Pectoral muscle samples (100 μg) were collected from 10 chicks from each treatment group per day, on days 1, 14, 21 and 28. Total RNA was extracted from homogenised muscle tissue samples using the DirectZol/chloroform/isopropanol method with DirectZol® (Zymo Research) reagent according to manufacturer’s instructions, and DNA was removed using DNase I kit (Ambion, Austin, TX). Furthermore, RNA samples were checked for concentration and purity (260:280 nm absorbency) using Elisa reader (BioTeK PowerWave HT microplate Spectrophotometer). Next, 2 μg of RNA was reverse transcribed to cDNA using iScript cDNA Synthesis Kit (BIORAD, California, USA), after which the cDNA was amplified using semi-quantitative RT-PCR with Advanced TM SYBR Green Supermix kit (BIO-RAD, California, USA). Briefly, 10 µL of master mix, 2 µL forward primer (10 pmol), 2 µL reverse primer (10 pmol), 2 µL sample cDNA and 4 µL of nuclease-free water were amplified using BIORAD CFX96 (BioRad, Hercules, CA, USA) with the thermal cycling conditions of 50 °C for 2 min, 95 °C for 15 min for denaturation, and 40 cycles of 95 °C for 10 s denaturation, followed by 30 s at 55 °C for annealing and 72 °C for 10 s for extension with final melting at 95 °C for 20 s. Duplicates from each cDNA were analysed, fluorescence emission was detected, and using the relative comparative threshold method, values were automatically calculated using CFX manager TM software V3.1 (BioRad, Hercules, CA, USA).
Data were analysed as a completely randomised design using General Linear Model procedure of SAS 9.1 (SAS Institute Inc Citation2004). Means were considered to be significantly different at p < .05. Body weight, relative expression of mRNA levels of MyoD, MyoG, IGF-I and MSTN were expressed as means ± standard error (SE). One-way analysis of variance, followed by all-pairs Bonferroni test, was used to compare different parameters among treatment groups. Simple correlations between body weight and gene expression data were calculated using Pearsons correlations (PROC CORR in SAS 9.1).
Results
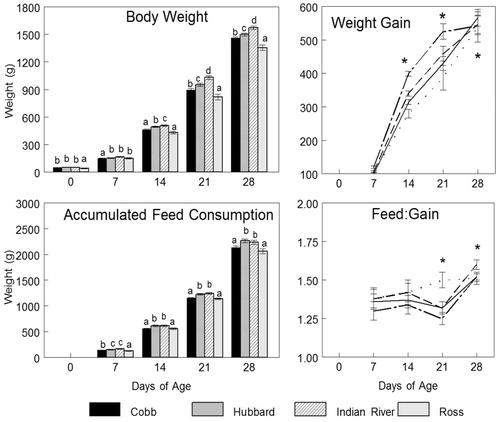
Body weights (g/chick/week) of the four commercial broiler strains are shown in . Day 7 onwards, IR had the heaviest body weight at both 7 d (166.34 g) and 21 d (1030.51 g), while Ross was the lightest (p ≤ .05) at 14 d (429.92 g) and 21 d (816.42 g). At the end of the experiment (28 d), IR gained the highest (p < .0001) body weight (1573.62 g) compared to HC and Cobb (1499.03 and 1459.06 g, respectively), while Ross remained the lowest (1355.16 g).
Table 3. Least squares mean for broiler initial body, final body, hot carcase and cold carcase weights and dressing percentages as affected by strain.
Body weight gain for IR was significantly greater (p = .0084) than the other strains (525.16 g/chick/week) at 14 d, while there were no significant differences among Cobb, HC and Ross at 21 d, neither (p > .1) in body weight gain among all strains at 28 d.
Initial and final body weights, dressing percentages, and hot and cold carcase weights are shown in . Final body weight was significantly heavier (p < .0001) for IR (1878.46 g), while final body weight between Ross (1688.51 g) and HC (1678.73 g) was not significantly different. Cobb final body weight (1588.15 g) was the significantly lowest, while IR hot carcase weight was the significantly highest (1460.66 g), among the four strains. Only IR (1489.25 g) had a significantly higher cold carcase weight with no significant differences among HC (1367.17 g), Cobb (1293.28 g) and Ross (1285.73 g). Dressing percentage had no significant difference (p > .1) among all strains with values ranging between 75–80% at 28 d.
shows the cumulative feed consumption (kg feed/kg gain), where no significant differences (p > .1) were observed among all strains at 7 d (p = .39) and 14 d (p = .37). Whereas at 21 d, Ross had the highest (1.50 ± 0.05 kg/kg) and at 28 d HC the lowest (p = .01) feed conversion ratio (1.60 ± 0.03 kg/kg). At 21 d; IR, HC and Cobb scored the lowest (p = .04) cumulative feed consumption and were more efficient in feed conversion than Ross (1.25 ± 0.04, 1.32 ± 0.04 and 1.38 ± 0.04 kg/kg vs. 1.50 ± 0.05 kg/kg, respectively). At 28 d, HC had the lowest (p = .01) efficiency in feed consumption (1.60 ± 0.03 kg/kg).
indicates that, at slaughter (28 d), Cobb had the significantly (p < .01) lowest value of breast percentage, while HC had the lowest (p = .04) value of leg percentage. Back percentages were significantly (p = .13) higher for HC (19.96%) and IR (19.13%) as opposed to Ross (17.91%) and Cobb (17.94%). Cobb had the highest (p < .0001) abdominal fat percentage (1.48%) among all strains with the lowest significant value for IR (0.57%), followed by Ross with a moderate percentage of 0.93%. Wing, neck and breast fillets were not affected by broiler strain since there were no significant differences among the strains.
Table 4. Least squares mean for carcase cuts and abdominal fat percentages as affected by strain (percentages expressed as a part of cold carcase weight).
shows the non-carcase components with a significant difference (p = .05) in Gizzard % among the different strains. Ross (1.73%) and HC (1.44%) had greater percentages. There were no significant differences in proventriculus, liver and heart percentages expressed as part of cold carcase weights among all strains. Gizzard percentage in HC tended to be significantly different (p ≤ .06) between Cobb and Ross.
Table 5. Least squares mean for non-carcase components as affected by strain (percentages expressed as a part of cold carcase weight).
illustrates meat quality parameters; there was a significant (p = .0468) difference in yellowness among the strains with the lowest value assigned to IR(1465.28). Cooking loss was significantly the highest (p = .0583) for Cobb (29.37). Shear force values indicated that meat from the Ross strain carcases were significantly (p = .0397) more tender (2.23 kg/cm2) than other strains. There were no significant differences in pH, Lightness (L*), Redness (a*) and water holding capacity (WHC) among all strains.
Table 6. Least squares means for meat quality parameters as affected by strain.
As shown in , feed consumption at 7 d was significantly the highest (p = .0004) for IR (165.209 ± 4.92 g/week), while Ross (127.74 ± 5.87 g/week) was the lowest (p = .0004). At 14 d (p = .0070) and 21 d (p = .0037), HC and IR had the highest feed consumption values (at 21 d: 1225.00 ± 13.10 and 1241.10 ± 13.68 g/week, respectively). At 28 d, Ross (2063.57 ± 46.20 g/week) and Cobb (2135.57 ± 29.34 g/week) had the lowest (p = .0112) feed consumption.
highlights a highly significant difference in IGF-I expression. IR had the highest (p < .05) expression of IGF-I gene that was about 35% higher than Ross, one fold higher than Cobb, and more than two folds higher (p < .05) than HC. Multiple comparisons show that IGF-I expression in Ross is higher (p < .05) by 40% to Cobb and 60% to HC. Cobb had an IGF-I expression level about 45% greater than HC. There was no significant difference in MYO-D gene expression between Ross and Cobb, although it was higher (p < .05) than IR by about 20%, while HC had the lowest value with nearly one fold lower than Cobb and Ross, whereas IR’s was about 30% folds higher (p < .05) than HC. There were significant differences (p < .05) in Myo-G expression among the four strains. IR and Ross had the greatest values of Myo-G among the four strains with about 20%, 35% and 110% higher (p < .05) expression in IR than in Ross, HC and Cobb, respectively. Meanwhile, Myo-G levels in Ross was about 30% higher (p < .05) than HC and 110% than Cobb with HC 35% higher (p < .05) than Cobb.Ross had the highest (p < .05) MSTN expression levels but it was not significantly different than Cobb. Whereas IR and HC had 20% lower (p < .05) expression levels than Ross. Notwithstanding, MSTN showed no significant (p > .05) differences among all breeds.
Figure 1. Growth and consumption parameters of tested broiler strains during four weeks of experimentation. Data presented as LSMeans ± SE. Means without a common superscript differ significantly (p ≤ .05); *means differ significantly (p < .05, see text).
Furthermore, correlation analysis between body weight and the various muscle marker genes showed a negative correlation between BW and MSTN (r= −0.76), a positive correlation of 0.547 with both MyoD and MyoG, and 0.50 with IGF-I; indicating that MyoD, MyoG or IGF-I can be used as effective selection markers for weight variations.
Discussion
In this study, we measured growth performance, and carcase and meat quality, in relation to the expression levels of muscle marker genes of four commercial broiler strains reared in Jordan. Hristakieva et al. (Citation2014) showed that the weight of hatchlings differed significantly according to genotype between one-day-old Cobb 500 which were heavier than Ross 308 broilers; differences are possibly attributed to the variation in the weight of eggs at incubation. Further, López et al. (2011) found significant differences in average weight per bird at 3 week and 6 week of age between two commercial broiler strains.
Furthermore, variations in body weight gain in the present study are consistent with the findings of Sarker et al. (Citation2001) who reported that body weight gain was significantly higher in ISA Vedette than Arbor Acres and Hybro; the three different fast-growing broiler strains from day-old to 6 weeks. Amao et al. (Citation2011) confirmed that the significant differences in body weight, average daily gain, and average feed intake could be attributed to variations in genetic makeup, as in several other studies where genotype affected initially, weekly average and final body weights, feed consumption, and feed conversion ratio among different commercial strains at different ages (Yakubu et al. Citation2010; Siaga et al. Citation2017). Abdullah et al. (Citation2010) reported a significant strain effect on the weight gain of broilers at 7–21 and 28–42 days of age. Difference in feed efficiency among modern broiler strains is attributed to physical activity and genotype which can affect the growth performance of broiler chicken strains (Agaviezor Citation2005).
In addition, the present study shows differences in feed consumption among the studied strains that were consistent with Ravindran et al. (Citation1999), where nutrient utilisation between breeds could be attributed to differences in the structure of the digestive tract and absorptive capacity, changes in digestive enzyme output, and passage rate of digesta. Latshaw and Moritz (Citation2009) suggested that increasing feed efficiency for the modern strain could be related to lower maintenance requirements because maintenance energy requirements decreased with rapid growth. In fact, digestive function or nutrient uptake per unit of gut mass was not influenced by selection, but increased gut size was responsible for increased digestive and absorptive capacity in modern broilers (Jackson and Diamond Citation1996). Additionally, Gonzales et al. (Citation1998) reported that weight gain and FCR were affected by strain; Ross broilers achieved higher weight gain compared to other strains (Arbor Acres, Avian Farms, Cobb-500, Hubbard-Peterson, ISA and Naked Neck). Similarly, Smith et al. (Citation1998) reported that strain had an effect on feed intake and FCR when they compared strain crosses (Ross x Ross208) and fast-growing strains (Peterson x Arbor Acres). Rondelli et al. (Citation2003) concluded that the Ross line showed higher final weight and weight gain, and better intake and feed conversion rate than the AVIAN farm line through weeks 2–4. However, Farran et al. (Citation2000) reported that there were no significant differences in live body weight and feed to gain ratio among Ross, Arbor acres and Lohman broilers from day 0 to 21. Yet still, several reports (Taha et al. Citation2011; Udeh et al. Citation2015) showed that genotype does affect body weight, body weight gain, feed intake, and feed conversion ratio of broiler chickens.
Altogether, it would be reasonable to argue that the differences observed in growth performance traits among the studied strains may be attributed to differences in the genetic potential of each strain.
In this study, genotype showed a decisive influence on the relative fasted body weight and carcase weight (%) of broilers, with greater weights recorded for Arbor Acres. Genotype had a significant effect on the carcase yields and some cut parts (neck, wing, and back relative weights %) of the birds. These differences in breast percentage are consistent with the results presented by Reddish and Lilburn (Citation2004) who showed that these could be related to the differences in breast muscle dimensions and muscle fibre number and size. Similarly, several studies (Johnson and Asmundson Citation1957; Havenstein et al. Citation1994; Zuidhof et al. Citation2014) suggested a high positive correlation between pectoral weight and body weight due to emphasis on genetic improvement and body weight selection.
The significant differences in abdominal and carcase fat calculated in our study were in agreement with the findings reported by Chambers et al. (Citation1981). They compared commercial and experimental strains of broilers and reported that selection for heavier body weight increases abdominal fat in selected birds, and part of the increased fat deposition could be due to genetic differences in feed conversion. Furthermore, Abeni and Bergoglio (Citation2001) and Gonzales et al. (Citation1998) showed that the percentage of abdominal fat differed among broiler strains, while Nestor et al. (Citation1988) showed line differences in the relative weight of drumstick muscles; both findings are in accordance with our reported results. However, Siaga et al. (Citation2017) found no significant differences in carcase, breast, back, thigh, wing, drumstick, heart, gizzard, liver, and abdominal fat among different strains. Differences in gizzard weights (expressed as a percentage of cold carcase weight) are supported by the findings of Maisonnier et al. (Citation2001); they reported that absolute size of gizzard is susceptible to genetic change as a result of selection for higher digestive efficiency.
The results of the current study indicated significant differences in growth performance and meat quality. The heart percentages are consistent with the results of a previous work conducted by Schmidt et al. (Citation2009), who reported no change in liver allometry due to commercial selection pressure. Katanbaf et al. (Citation1988) concluded that there were no differences in heart relative weights between two lines selected for high or low juvenile body weight. O’Sullivan et al. (Citation1991) reported similar results for heart relative weight at 21 days in lines divergently selected for low and high body weights. Benyi et al. (Citation2015) found that genotype effect on the percentage gizzard weight when comparing Ross 308 with Cobb Avian 48 broiler strains. In the present study, liver relative weights also remained constant among the strains, in agreement with a previous study by Schmidt et al. (Citation2009).
Our results of proventriculus relative weight agree with the results of Mussini (Citation2012) who reported that proventriculus relative weights for all strains were similar until 28 days. In a previous study conducted by Berri et al. (Citation2001) who investigated the effect of selection for growth, breast muscle development, and decreased rates of post-mortem pH. This change could be a result of the noticeable decrease in the potential of glycolysis of the selected birds. However, our results are in agreement with those results obtained by López et al. (2011) who observed that there were no significant differences in meat pH, lightness and redness between two commercial broiler strains. Our present finding is consistent with previous results on broilers (Le Bihan-Duval et al. Citation1999), turkeys (Sante et al. Citation1991), and ducks (Baéza et al. Citation1997) showing a significant decrease in colour intensity or an increase in lightness in rapidly growing or high-yield strains compared to those less selected. Meat colour differed in redness due to a difference in the amount of myoglobin in meat. They also reported that selection had an influence on breast meat colour development during storage.
Water holding capacity values in the present study are similar to the findings of Abdullah and Matarneh (Citation2010). They observed that water-holding capacity percentage was not significantly affected by differences in carcase weight. This result could be explained by the lack of difference in muscle pH values among carcase weights. Our results are also supported by the findings of Hamm (Citation1986) who explained that post-mortem drop in pH resulted in changes in muscle protein electronic charge to reach isoelectric point that weakens the bond between actin and myosin resulting in expelling water outside, therefore reducing water holding capacity.
Mehaffey et al. (Citation2006) stated that variation among strains, particularly in tenderness, could be attributed to slower rates of rigour mortis development in some broiler lines than others, resulting in increased breast meat toughness. Shear force values are consistent with the results of Abdullah and Matarneh (Citation2010) that suggest that differences in tenderness could be associated with differences in bird age, size, strain and fat content in the breast muscle. Meat tenderness is influenced by the quantity and quality of connective tissue and by the contractile state of muscle fibres and bundles (Koohmaraie et al. Citation2002). Results of cooking loss percentage reported by López et al. (Citation2011), consistent with our results, indicated no significant differences in cooking loss percentage between two commercial broiler strains.
The levels of IGF-I expression for IR were consistent with the findings of Lalani et al. (Citation2000) who reported that IGF-I had a positive regulatory effect on muscle differentiation and growth. In addition, IR had the highest final body weight compared to the other studied strain. MyoD and MyoG levels obtained from the current study are partly (slightly) consistent with the findings of previous researches that found MSTN functions as an inhibitory factor for myogenic differentiation by downregulating the expression of myogenic regulators such as MyoD, MyoG, and Myf-5 (Langley et al. Citation2002; Ríos et al. 2002; Joulia et al. Citation2003). Furthermore, differences in MyoD and MyoG expression among strains could be attributed to long-term genetic selection or type of production (Zhang et al. Citation2018). Other factors such as physiological and environmental conditions (Yin et al. Citation2014), different genetic origins, skeletal muscle contents (Li et al. Citation2014), and polymorphisms (Zhu et al. Citation2010) could also be involved in gene expression differences among strains. Results of MSTN expression in the present study are in agreement with the findings of several previous studies that showed a negative association between MSTN levels and increase in size and number of skeletal muscle fibres or muscle mass (Grobet et al. Citation1997; Kambadur et al. Citation1997; McPherron et al. Citation1997; McPherron and Lee Citation1997; Lee Citation2004), and the results of final body weights of the four strains in the current study support these findings. Similarly, Kocamis and Killefer (Citation2002) reported that high expression levels of MSTN may be to prevent excessive muscle growth. Differences in MSTN expression among strains could be attributed to the inhibitory effect of propeptide on the biological activity of the MSTN (Lee and McPherron Citation2001; Thies et al. Citation2001).
From the expression levels () of the studied genes, it can be concluded that body weight () of IND and HUB were the highest compared to Ross and Cobb, in fact, associated with elevated IGF-I and MYO-G genes expression, with similar expression in the Myostatin gene for all studied strains.
Figure 2. Normalized fold expression of IGF-I, MYO-D, MYO-G and MSTN genes in four broiler strains (IND: Indian River, HUB: Hubbard, Cobb 500, Ross 308). Different letters (a, b, c, d) indicate significant difference among strains.
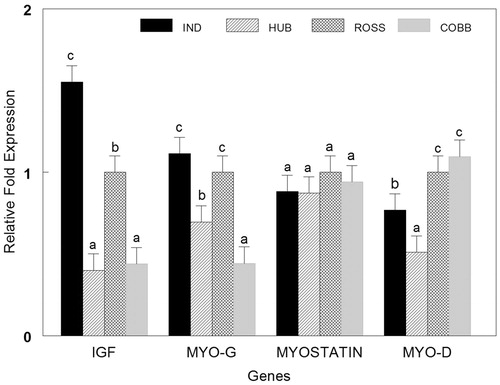
Our reported results and the findings of previous studies that investigated IGF-I expression levels may explain the superiority of IR to the other strains in growth performance and breast muscle percentages. Gene expression levels of IR and Ross could explain the relationship between MSTN and the other MRFs. As MSTN expression decreased, the expression of the other genes (MyoD, MyoG and IGF-I) increased and vice versa; that proves the theory proposed about MSTN acting as a regulatory factor for MRFs (Xiao et al. Citation2017). It is important to note that although the surrounding environmental conditions were the same for all strains, they may have been more suitable for the IR and Ross strains, possibly negatively impacting the mRNA expression of the IGF-I gene.
Conclusions
This study shows a physiological variation among several commercial broiler strains reared in Jordan. It also shows the superiority of the Indian River to the other strains regarding growth performance and carcase characteristics. Additionally, a small variation in carcase cuts was observed among the different strains. Further support for the Indian River strain’s superiority is demonstrated by its highest expression levels of IGF-I and MyoG, while IGF-I and MyoD were the lowest in the Hubbard strain. Moreover, IGF-I and MYO-G genes may play an important role in enhancing the growth of the IND and HUB strains. Hence, the IGF-1 gene can be used as a promising marker for selecting Broiler types with a higher growth rate.
In order to gain more profitability, Indian River should be used in farmer’s fields because of its high growth performance. Marker-assisted selections, such as IGF-1, should be considered for choosing Broilers with the best performance. Nonetheless, more studies need to be completed on gene expressions and their role in affecting growth performance, and meat quality and characteristics of these strains and other strains in order to build a more comprehensive selection or screening process.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdullah AY, Matarneh SK. 2010. Broiler performance and the effects of carcass weight, broiler sex, and postchill carcass aging duration on breast fillet quality characteristics. J Appl Poult Res. 19:46–58.
- Abdullah Y, Al-Beitawi N, Rjoup M, Qudsieh R, Ishmais M. 2010. Growth performance, carcass and meat quality characteristics of different commercial crosses of broiler strains of chicken. J Poult Sci. 47:13–21.
- Abeni F, Bergoglio G. 2001. Characterization of different strains of broiler chicken by carcass measurements, chemical and physical parameters and NIRS on breast muscle. Meat Sci. 57:133–137.
- Adams GR, Haddad F, Baldwin KM. 1999. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol. 87:1705–1712.
- Adams GR, McCue SA. 1998. Localized infusion of IGF-I results in skeletal muscle hypertrophyin rats. J Appl Physiol. 84:1716–1722.
- Agaviezor BO. 2005. Genetic evaluation of laying performance of pure exotic and indigenous crossbred pullets’. M. Agric. Dissertation. Dept. of Animal Breeding and Genetics.Federal University of Agriculture, Abeokuta, Nigeria, 76.
- Al-Zghoul MB, Al-Natour MQ, Dalab AS, Alturki OI, Althnaian T, Al-ramadan SY, Hannon KM, El-Bahr SM. 2016. Thermal manipulation mid-term broiler chicken embryogenesis: effect on muscle growth factors and muscle marker genes. Rev Bras Cienc Avic. 18:607–618.
- Amao SR, Ojedapo LO, Sosina OA. 2011. Evaluation of growth performance traits in three strains of broiler chickens reared in derived savanna environment of Nigeria. World J Young Res. 1:28–31.
- Baéza E, De Carville H, Salichon MR, Marche G, Leclercq B. 1997. Effects of selection, over three and four generations, on meat yield and fatness in Muscovy ducks. Br Poult Sci. 38:359–365.
- Barbut S, Sósnicki AA, Lonergan SM, Knapp T, Ciobanu DC, Gatcliffe LJ, Huff-Lonergan E, Wilson EW. 2008. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 79:46–63.
- Benyi K, Netshipale AJ, Mahlako KT, Gwata ET. 2015. Effect of genotype and stocking density on broiler performance during two subtropical seasons. Trop Anim Health and Prod. 47:969–974.
- Berri C, Godet E, Hattab NH, Duclos MJ. 2006. Growth and differentiation of the chicken Pectoralis major muscle: effect of genotype and early nutrition. Arch Tierz. 49:31–32.
- Berri C, Wacrenier N, Millet N, Le Bihan-Duval E. 2001. Effect of selection for improved body composition on muscle and meat characteristics of broiler from experimental and commercial lines. Poult Sci. 80:833–838.
- Bhattacharya TK, Chatterjee RN, Dushyanth K, Paswan C, Guru Vishnu P. 2016. Activin receptor 2A and activin receptor 2B genes in chicken: effect on carcass traits. J Appl Anim Res. 44:480–486.
- Carnac G, Vernus B, Bonnieu A. 2007. Myostatin in the pathophysiology of skeletal muscle. Curr Genom. 8:415–422.
- Chambers JR, Gavora JS, Fortin A. 1981. Genetic changes in meat-type chickens in the last twenty years. Can J Anim Sci. 61:555–563.
- Dransfield E, Sosnicki AA. 1999. Relationship between muscle growth and poultry meat quality. Poult Sci. 78:743–746.
- Duclos M, Beccavin C, Simon J. 1999. Genetic models for the study of insulin-like growth factors (IGF) and muscle development in birds compared to mammals. Domest Anim Endocrinol. 17:231–243.
- Falconer DS, Mackay TFC. 1996. Introduction to Quantitative Genetics. 4th Edition. Longman Scientific Technical, Malaysia.
- FAO. 2008. Food outlook: global market analysis. Meat and meat products: poultry meat. [accessed 2010 July 29]. http://www.fao.org/docrep/010/ai466e/ai466e08.htm.
- Farran MT, Khalil RF, Uwayjan MG, Ashkarian VM, Hajj RN. 2000. Performance and Carcass Quality of Commercial Broiler Strains. J Appl Poult Res. 9:252–257.
- Florini JR, Ewton DZ, Coolican SA. 1996. Growth Hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 17:481–517.
- Gonzales E, Buyse J, Takita TS, Sartori JR, Decuypere E. 1998. Metabolic disturbances in male broilers of different strains.1.Performance, mortality, and right ventricular hypertrophy. Poult Sci. 77:1646–1653.
- Grau R, Hamm G. 1953. Eineenfache Methodezur Bestimmung der Wasserbindungim Muskel. Die Naturwissenschaften. 40:29–30.
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, et al. 1997. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle . Nat Gen. 17:71–74.
- Hamm D. 1981. Unconventional meat harvesting. Poult Sci. 60(Suppl. 1):166.
- Hamm R. 1986. Functional properties of the myofibrillar system and their measurements. In P.J. Bechtel, editor. Muscle as a Food. Orlando: Academic Press; p. 135.
- Halevy O, Piestun Y, Allouh MZ, Rosser BWC, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. 2004. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 231:489–502.
- Havenstein GB, Ferke PR, Scheideler SE, Rives DV. 1994. Carcass composition and yield of 1991 vs 1957 broilers when fed 1957 and 1991 broiler diets. Poult Sci. 73:1795–1804.
- Havenstein GB, Ferket PR, Qureshi MA. 2003. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult Sci. 82:1509–1518.
- Hristakieva P, Mincheva N, Oblakova M, Lalev M, Ivanova I. 2014. Effect of genotype on production traits in broiler chickens. Slovac J Anim Sci. 47:19–24.
- Honikel KO. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49:447–457.
- Jackson S, Diamond J. 1996. Metabolic and digestive responses to artificial selection in chickens. Evolution. 50:1638–1650.
- Jeacocke RE. 1977. Continuous measurements of the pH of beef muscle in intact beef carcases. Int J Food Sci Technol. 12:375–386.
- Johnson AS, Asmundson VS. 1957. Genetic and environmental factors affecting size of body weight and body parts of turkeys. 2. The relation of body weight and certain body measurements to pectoral and tibial muscle weights. Poult Sci. 36:959–966.
- Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. 2003. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by Myostatin. Exper Cell Res. 286:263–275.
- Kadlec J, Hosnedlová B, Řehout V, Čítek J, Večerek L, Hanusová L. 2011. Insulin-like growth factor-I gene polymorphism and its association with growth and slaughter characteristics in broiler chickens. J Agrobiol. 28:157–163.
- Kambadur R, Sharma M, Smith TP, Bass JJ. 1997. Mutations in myostatin (GDF8) in Double-Muscled Belgian Blue and Piedmontese cattle. Gen Res. 7:910–915.
- Katanbaf MN, Siegel PB, Dunnington EA. 1988. Organ growth of selected lines of chicken and their F1 to a common body weight or age. Theor Appl Genet. 76:540–544.
- Kocamis H, Killefer J. 2002. Myostatin expression and possible functions in animal muscle growth. Domest Anim Endocrinol. 23:447–454.
- Koohmaraie M, Kent MP, Shackelford SD, Veiseth E, Wheeler TL. 2002. Meat tenderness and muscle growth: is there any relationship? Meat Sci. 62:345–352.
- Lalani R, Bhasin S, Byhower F, Tarnuzzer R, Grant M, Shen R, Asa S, Ezza S, Gonzalez-Cadavid NF. 2000. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J Endocrinol. 167:417–428.
- Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. 2002. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 277:49831–49840.
- Latshaw JD, Moritz JS. 2009. The partitioning of metabolizable energy by broiler chickens. Poult Sci. 88:98–105.
- Le Bihan-Duval E, Berri C, Baeza E, Sante V, Astruc T, Rémignon H, Le Pottier G, Bentley J, Beaumont C, Fernandez X. 2003. Genetic parameters of meat technological quality traits in a grand-parental commercial line of turkey. Gen Select Evol. 35:623–635.
- Le Bihan-Duval E, Millet N, Remignon H. 1999. Broiler Meat quality: effect of selection for increased carcass quality and estimates of genetic parameters. Poult Sci. 78:822–826.
- Lee SJ. 2004. Regulation of muscle mass by myostatin. Ann Rev Cell Develop Biol. 20:61–86.
- Lee SJ, McPherron AC. 2001. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 98:9306–9311.
- Li H, Zh C, Tao Z, Xu W, Song W, Hu Y, Zhu W, Song C. 2014. MyoD and Myf6 gene expression patterns in skeletal muscle during embryonic and posthatch development in the domestic duck (Anas platyrhynchos domestica). J Anim Breed Gen. 131:194–201.
- Liu Y, Lyon BG, Windham WR, Lyon CE, Savage EM. 2004. Principal component analysis of physical, color, and sensory characteristics of chicken breasts deboned at two, four, six, and twenty-four hours postmortem. Poult Sci. 83:101–108.
- López KP, Schilling MW, Corzo A. 2011. Broiler genetic strain and sex effects on meat characteristics. Poult Sci. 90:1105–1111.
- Maisonnier S, Gomez J, Chagneau A, Carré B. 2001. Analysis of variability in nutrient digestibilities in broiler chickens. Br Poult Sci. 42:70–76.
- McKee SR, Sams AR. 1998. Rigor mortis development at elevated temperatures induces pale exudative turkey meat characteristics. Poult Sci. 77:169–174.
- McPherron AC, Lawler AM, Lee SJ. 1997. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 387:83–90.
- McPherron AC, Lee SJ. 1997. Double muscling in cattle due to mutations in the myostatin gene. Proc Nat Acad Sci USA (PNAS). 94:12457–12461.
- Mehaffey JM, Pradhan SP, Meullenet JF, Emmert JL, Mckee SR, Owens CM. 2006. Meat quality evaluation of minimally aged broiler breast fillets from five commercial genetic strains. Poult Sci. 85:902–908.
- Merkley JW, Weinland BT, Malone GW, Chaloupka GW. 1980. Evaluation of five commercial broiler crosses. 2. Eviscerated yield and component parts. Poult Sci. 59:1755–1760.
- Mussini FJ. 2012. Comparative response of different broiler genotypes to dietary levels. Ph.D. Fayetteville, North Carolina: University of Arkansas.
- Nestor KE, Bacon WL, Havenstein GB, Saif YM, Renner PA. 1988. Carcass traits of turkeys from lines selected for increased growth rate or increased shank width. Poultr Sci. 67:1660–1667.
- NRC. 1994. Nutrient requirements of poultry. 9th Rev ed. Washington D.C.: National Academy Press.
- O’Sullivan NP, Dunnnington EA, Larsen AS, Siegel PB. 1991. Correlated responses in lines of chickens divergently selected for fifty-six-day body weight. 2. Organ growth, deoxyribonucleic acid, ribonucleic acid, and protein content. Poult Sci. 71:598–609.
- Oustanina S, Hause G, Braun T. 2004. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 23:3430–3439.
- Petracci M, Cavani C. 2012. Muscle growth and poultry meat quality issues. Nutrients. 4:1–12.
- Ravindran V, Hew L, Ravindran G, Gill R, Pittolo P, Bryden W. 1999. Influence of xylanase supplementation on the apparent metabolisable energy and ileal amino acid digestibility in a diet containing wheat and oats, and on the performance of three strains of broiler chickens. Aust J Agric Res. 50:1159–1163.
- Reddish JM, Lilburn MS. 2004. A comparison of growth and development patterns in diverse genotypes of broilers. 1. Male broiler growth. Poult Sci. 83:1067–1071.
- Ríos R, Carneiro I, Arce VM, Devesa J. 2002. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol- Cell Physiol. 282:C993–C999.
- Rondelli S, Martinez O, García PT. 2003. Sex effect on productive parameters, carcass and body fat composition of two commercial broilers lines. Rev Bras Cienc Avic. 5:169–173.
- Sams AR, Janky DM. 1986. The influence of brine chilling on tenderness of hot-boned, chill-boned, and age-boned broiler breast fillets. Poult Sci. 65:1316–1321.
- Sante V, Bielicki G, Renerre M, Lacourt A. 1991. Proceedings of the 37th International Congress of Meat Science and Technology. Kulmbach, Germany. Post mortem evolution in the pectoralis superficialis muscle from two turkey breeds: Relationship between pH and colour changes; p. 465–468.
- Santiago HL, Denbow DM, Emmerson DA, Denbow D, Graham P, Hohenboken W. 2005. Effects of strain, plane of nutrition, and age at slaughter on performance and meat quality traits of broilers. Poultry Science. 84(Suppl. 1):128.
- Sañudo C, Sierra I, Lopez M, Forcada F. 1986. La qualité de la viandeovion. Etude Des Differentsfacteurs Qui la Conditionnent.Commision C. E. Rapport EUR 11479. 67–81.
- Sarker MSK, Ahmed SU, Chowdhury SD, Hamid MA, Rahman MM. 2001. Performance of different fast growing broiler strains in winter. Pak J Biol Sci. 4:251–254.
- SAS Institute Inc. 2004. SAS/STAT 9.1 user’s guide. Cary, North Carolina, USA: SAS Institute Inc.
- Scheuermann GN, Bilgili SF, Hess JB, Mulvaney DR. 2003. Breast muscle development in commercial broiler chickens. Poult Sci. 82:1648–1658.
- Schmidt CJ, Persia ME, Feierstein E, Kingham B, Saylor WW. 2009. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult Sci. 88:2610–2619.
- Siaga R, Baloyi JJ, Rambau MD, Benyi K. 2017. Effects of stocking density and genotype on the growth performance of male and female broiler chickens. Asian J Poult Sci. 11:96–104.
- Smith ER, Pesti GM, Bakalli RI, Ware GO, Menten JF. 1998. Further studies on the influence of genotype and dietary protein on the performance of broilers. Poult Sci. 77:1678–1687.
- Taha AE, Abd El-Ghany FA, Sharaf MM. 2011. Strain and sex effects on productive and slaughter performance of local Egyptian and Canadian chicken strains. J World's Poult Res. 1:11–17.
- Te Pas MFW, Soumillion A. 2001. Improvement of livestock breeding strategies using physiologic and functional genomics information of the muscle regulatory factors gene family for skeletal muscle development. Curr Gen. 2:285–304.
- Thies RS, Chen T, Davies MV, Tomkinson KN, Pearson AA, Shakey QA, Wolfman NM. 2001. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors. 18:251–259.
- Udeh I, Ezebor PN, Akporahuarbo PO. 2015. Growth performance and carcass yield of three commercial strains of broiler chickens raised in a tropical environment. J Biol Agri Health. 5:62–67.
- Weintraub H. 1993. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 75:1241–1244.
- Xiao Y, Wu C, LiK Gui G, Zhang G, Yang H. 2017. Association of growth rate with hormone levels and myogenic gene expression profile in broilers. J Anim Sci Biotechnol. 8:43.
- Yakubu A, Ayoade JA, Dahiru YM. 2010. Effects of genotype and population density on growth performance, carcass characteristics, and cost-benefits of broiler chickens in north central Nigeria. Trop Anim Health Product. 42:719–727.
- Yin HD, Li DY, Zhang L, Yang MY, Zhao XL, Wang Y, Liu YP, Zhu Q. 2014. Housing system influences abundance of Pax3 and Pax7 in postnatal chicken skeletal muscles. Poult Sci. 93:1337–1343.
- Young LL, Northcutt JK, Buhr RJ, Lyon CE, Ware GO. 2001. Effects of age, sex, and duration of postmortem aging on percentage yield of parts from broiler chicken carcasses. Poult Sci. 80:376–379.
- Zhang R, Li R, Zhi L, Xu Y, Lin Y, Chen L. 2018. Expression profiles and associations of muscle regulatory factor (MRF) genes with growth traits in Tibetan chickens. Br Poult Sci. 59:63–67.
- Zhou H, Mitchell AD, McMurtry JP, Ashwell CM, Lamont SJ. 2005. Insulin-like growth factor-I gene polymorphism associations with growth, body composition, skeleton integrity, and metabolic traits in chickens. Poult Sci. 84:212–219.
- Zhu L, Li XW, Shuai SR, Chen Li Gu YR, Zhang K. 2010. The phylogeny analysis of MyoG gene in different pig breeds. Interdiscip Sci. 2:175–179.
- Zuidhof MJ, Schneider BL, Carney VL, Korver DR, Robinson FE. 2014. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult Sci. 93:2970–2982.
