Abstract
This study aimed to investigate the effect of supplementing the sow diet with fish oil on litter characteristics, fatty acid composition and desaturase expression of sows and their progeny. Sixteen second-parity sows were allocated equally into two groups. Sows were fed either the soybean oil diet (SD) or soybean oil + fish oil diet (FD) during the late gestation and lactation periods. Fatty acid composition was evaluated in the red blood cell (RBC) of sows, full-term placenta, RBC of cord blood, milk and livers of piglets. The expression of fatty acid desaturases mRNA was measured in placenta and livers of piglets. The proportion of 20:5n-3 (p < .001) in placenta and RBC of cord blood increased in the FD group. The proportion of 22:5n-3 (p < .01) decreased in placenta and cord blood and the fatty acid desaturases gene 1 (FADS1) mRNA expression (p < .05) in placenta decreased in the FD group. The proportion of 22:6n-3 in the RBC of piglets reduced from birth to day 16 in both groups, while the proportion of 22:6n-3 in the livers of piglets continuously increased during the same time period in both groups. These results indicate that fish oil supplementation during late gestation and lactation alters the n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFA) composition in the RBC and livers of piglets. The decrease of the 22:5n-3 and the increase of the 22:6n-3 in placenta might be related to the regulation of n-3 LC-PUFA metabolism at the 22:5n-3 formation step.
Fish oil supplementation in maternal diet alters n-3 LC-PUFA composition in progeny.
Fish oil might regulate n-3 LC-PUFA metabolism in placenta.
Highlights
Introduction
Long-chain polyunsaturated fatty acids (LC-PUFA), such as 20:5n-3 (EPA), 22:6n-3 (DHA), 22: 5n-3 (DPA) and 20:4n-6 (ARA), play an important role in foetal growth and development (Amusquivar et al. Citation2010), and a deficit of n-3 LC-PUFA during the gestation period leads to impairment of cognitive and/or physiological functions in rats (Catalan et al. Citation2002), and the impairment is considered to be irreversible (Nesheim and Yaktine Citation2006). Fish oil has been recognised as a good source of n-3 LC-PUFA in diet (Bimbo and Crowther Citation1992). The previous reports about the effects of n-3PUFA in the sow diet on sow reproduction and piglet performance are very inconsistent, as reviewed by (Tanghe and De Smet Citation2013). Although it is unclear whether n-3 PUFA affect sow reproduction and piglet performance, many feed companies nowadays include small amounts of n-3 PUFA in sow diets. Therefore, a good knowledge of the n-3PUFA metabolism and transfer is needed.
First, the transfer of n-3 PUFA composition from sow to their offspring has been evidenced before by evaluating the change of n-3 PUFA in the red blood cell (RBC), liver, placenta, cord blood or milk from sow or their offspring after different n-3PUFA source supplement in maternal diet (Rooke et al. Citation2000; de Quelen et al. Citation2010; Sampels et al. Citation2011). However, there is no report on the effect of fish oil supplementation in sow diet on the fatty acid composition of placenta and cord blood. Therefore, the first objective of this study was to examine that the effect of fish oil supplement in sow diet on the fatty acid composition of the RBC of sows and their piglets, RBC of cord blood, placenta, milk and livers of piglets.
Second, the D-5 desaturase (D5D) and D-6 desaturase (D6D) are associated with the LC-PUFA level in different tissue (Nakamura and Nara Citation2004). It is inconsistent that the previous reports about the effect of supplementing n-3PUFA in sow diet on the hepatic D5D and D6D mRNA desaturase expression of piglets (McNeil et al. Citation2005; de Quelen et al. Citation2013). So far, there is no report about the expression of fatty acid desaturases genes (FADS) and the possible regulatory mechanism in the pig placenta. Considering all mentioned above, we hypothesised that the supplementation of fish oil in sow diet might regulate the metabolism of n-3 LC-PUFA in the placenta and livers of piglets. The second objective of this study was to examine that the effect of supplementation of fish oil in sow diet on the mRNA expression of fatty acid desaturase genes in the placenta and livers of piglets.
Materials and methods
The experiment was carried out according to Chinese guidelines for animal welfare and the National Institutes of Health guide for the care and use of Laboratory animals. All animal experimental protocols were approved by the Animal Care and Use Committee of the Shanghai Jiaotong University.
Animals and diets
The back fat thickness of a group sows was measured at day 84 of gestation, and 16 sows (hybrid Topigs 20 breed sows, Dutch Landrace × Great York) which had similar back fat thickness (soybean oil group: 15.14 ± 0.51 versus fish oil group: 15.43 ± 0.48 mm; p = .69) were selected for the experiment. The back fat thickness was measured at the level of the last rib on each side and 65 mm from the midline by using the digital back fat indicator (BQT-521, Renco Lean-meater, Renco Electronics Inc., Rockledge, FL).
Late gestation and lactation diets were formulated according to the National Research Council (NRC) requirements for sows in gestation and lactation, respectively (NRC Citation2012). The fatty acid composition of the soybean oil (Four Grade GB 1535-2003, Yihai Kerry Group) and fish oil (Product name: refined fish oil, Batch number: 811393, NovoSana (Taicang) Co. Ltd., Taicang City , Jiangsu Province, China) was analysed before use (Supplementary Table S1). Briefly, the oil were saponified with sodium methylate and then esterified with 6% H2SO4 in anhydrous methanol. After methylation, the fatty acid composition was determined by gas chromatography, following the method of Molina et al. (Citation1989).
On day 85 of gestation, sows were equally divided into two groups, with eight replicates per group and one sow per replicate. One group was fed soybean oil diet (SD) and the other group fed soybean oil + fish oil diet (FD) during the gestation and lactation periods. For gestation diet, the SD was composed of 3% of soybean oil, while the FD was composed of 0.5% of soybean oil +2.5% of fish oil; for lactation diet, the SD was composed of 3.5% of soybean oil, while the FD was composed of 0.7% of soybean oil +2.8% of fish oil (Table ). Feed samples of each diet were taken and analysed according to Association of Official Analytical Chemists (AOAC) methods (Thiex Citation2002). The fatty acid composition in the diets was determined as described by (Raes et al. Citation2001). Dietary fatty acid composition for the gestation and lactation diets is presented in Table . All diets were mash feed and were stored in 20 kg vacuum dark storage bags, and were kept in a warehouse with a constant temperature of 24–28 °C before using.
Table 1. Ingredient composition and nutrient levels of experimental diets (as fed basis, %).
Table 2. Fatty acid composition of experimental diets.
On day of farrowing and day 16 after birth, 12 piglets (3 males and 3 females in both the SD group and the FD group) were selected from 16 litters for RBC and liver collection, respectively. The average body weight of the selected new-born piglets (before suckling) was similar between the SD group and the FD group (SD: 1.54 ± 0.14 versus FD: 1.41 ± 0.05 kg; p = .42). The average body weight of the selected piglets on day 16 after birth (no access to suckle milk 1 hour before euthanasia) was similar between the SD group and the FD group (SD: 4.12 ± 0.05 versus FD: 4.11 ± 0.02 kg; p = .87).
Animal care and feeding
All pregnant sows were housed individually in gestation crates (2.1 × 0.65 m) and were transferred to a farrowing unit (2.23 × 2.2m) on day 110 of gestation. The room temperature of the gestation and farrowing units were approximately 26 ± 2 °C. At parturition, litter size was recorded and each new-born piglet was immediately weighed. Within 24 h post-farrowing, litter size was equalised by cross-fostering to achieve 12–14 piglets per sow within the same treatment group. The suckling piglets had no access to creep feed and free access to water. The piglets were weaned at day 24, and litter weight and litter size were recorded on weaning day.
The gestation diets were supplied twice a day (06:00 and 13:00) at 3.0 kg/d per sow until day 5 before farrowing, and then feed allowance was reduced by 0.5 kg/d until farrowing day when no diet was provided. The lactation diets were supplied three times a day (06:00, 13:00 and 18:00) beginning at 2.0 kg/d per sow. The transition from 2.0 kg/d to ad libitum was gradual, by adding 0.5 kg/d within the first week of lactation. Sows had free access to water during the entire experiment.
Blood and tissue sample collection
Full-term placental samples: Each piglet and the corresponding placenta were carefully marked. Sows were supervised throughout farrowing and each new-born piglet was matched to its placenta using the umbilical tagging procedure described by Wilson et al. (Citation1998). Placenta samples around the central cord region of the placenta (avoiding vessel) were obtained immediately after farrowing and collected into a 5 mL freezing tube, frozen in liquid N2, and then stored at −80 °C for further analysis.
Blood sample of sows and umbilical cord blood samples: Blood samples from sows were collected from the jugular vein on day 84 of gestation (G84d), farrowing day (Fd) and day 16 of lactation (L16d). Umbilical cord blood was collected by squeezing from the retracted side of the umbilical cord.
Blood and liver samples of piglets: On day of farrowing and day 16 after birth, 12 piglets (3 males and 3 females in both the SD group and the FD group) were randomly selected from 16 litters for blood and liver sample collection, respectively. The selected piglets were anaesthetized with an intramuscular neck injection of pentobarbital sodium (35 mg/kg body weight). The blood samples were collected from the vena jugulars and kept in evacuated heparinised tubes. The selected piglets were slaughtered after taking the blood samples. Liver samples were obtained immediately after slaughtering. The posterior half of liver samples was rinsed twice in physiological saline, a sample was collected into 5 mL freezing tube, frozen in liquid nitrogen, and then stored at −80 °C until further analysis.
Blood sample treatment: All of the blood samples were centrifuged at 2550 × g for 10 min at 4 °C. The upper plasma was divided and the white blood cells were removed by using pipette. The RBC were stored at −80 °C until further analysis.
Colostrum and milk sample collection
Colostrum samples (50 mL) were collected within 4 h after the birth of the first piglet as previously described by Li et al. (Citation2014). Milk samples (50 mL) were collected at day 15 of lactation after an intramuscular neck injection of oxytocin (20 U/sow). All milk samples were collected from the four thoracic pairs of functional teats. The colostrum and milk samples were frozen at −80 °C immediately pending subsequent analysis.
Analysis of fatty acids
The lipid content of placenta and liver was determined gravimetrically. The composition of colostrum and milk was analysed by using an automatic milk analyser (Milk-Yway-CP2, Beijing, China) (Supplementary Table S3). Lipids in placenta, liver and breast milk samples were extracted and purified in chloroform/methanol (2:1, by vol) according to the method given by Folch et al. (Citation1956). Lipids in RBC of blood were extracted and purified in methanol/toluene (4:1, by vol) according to the method of Olliver et al. (Citation2016). The extracted lipids were saponified with sodium methylate and then esterified with 6% H2SO4 in anhydrous methanol. After methylation, the fatty acid composition was determined by gas chromatography, following the method of Molina et al. (Citation1989).
Quantitative real-time PCR analysis of fatty acid desaturase (FADS1 and FADS2) expression
The total RNA was isolated from placenta and liver samples using total RNA Kit I (Cat no. R6834-01; OMEGA BIO-TEK, Norcross, GA). RNA quality was verified by both agarose gel (1%) electrophoresis and spectrophotometry (A260/A280 ratio, Beckman DU-800; Beckman Coulter Inc., Brea, CA). One μg of RNA was reverse transcribed using the Primescript™ RT Reagent Kit with gDNA Eraser (Cat#RR047A; Takara, Berkeley, CA). Primers for all target genes are shown in Supplementary Table S4. Quantitative real-time PCR (RT-qPCR) was used to determine the relative expression level of target gene according to the method of de Quelen et al. (Citation2013). β-Actin was used as the endogenous control gene to normalise the expression of target genes. The relative quantification of gene amplification by RT-qPCR was performed using the value of the threshold cycle (Ct). The comparative Ct value method using the formula 2-ΔΔCt was employed to quantify the expression levels of target genes relative to those of β-actin gene (Livak and Schmittgen Citation2001).
Statistical analyses
The data of average daily lactation sow intake, litter size at birth (total or alive) and weaning, litter weight at birth and weaning, expression of desaturase genes and fatty acid composition in placenta and RBC of cord blood were analysed using the one-way ANOVA. The χ2 test was used to test for stillborn rate and piglet mortality. The data of fatty acid composition in RBC of piglets and sows, milk and livers were assessed by using the multivariate GLM analysis. The model included the main effects of diet and time, as well as the interaction between these variables. The model was validated testing the goodness-of-fit to the data with R2 (Supplementary Tables S4–S7). All analyses were carried out with SPSS software (IBM SPSS Statistics 20, Chicago, IL).
Results
Feed intake, gestation outcomes and piglet growth performance
Average daily feed consumption of the lactation sows, gestation outcomes and piglet growth performance are summarised in Table . Average daily feed consumption of the lactation sows, litter size at birth and weaning, litter weight at birth and weaning, stillborn rate and piglet mortality did not differ between the SD and FD groups.
Table 3. Effect of fish oil supplement on reproductive performance of sow.
Fatty acid composition in RBC of sows
The proportions of 18:2n-6 (p < .01) and 20:4n-6 (p < .01) in RBC of sows was lower in the FD group compared to the SD group (Table ). The proportion of 20:4n-6 was affected by day in both groups (p < .001). The proportions of 20:5n-3 (p < .001), 22:5n-3 (p < .01), 22:6n-3 (p < .001) in RBC of sows were higher in the FD group than those in the SD group. Furthermore, the 20:5n-3 (p < .001), 22:5n-3 (p < .05) and 22:6n-3 (p < .01) were affected by time in the FD group. Diet × day interaction affected 20:5n-3 and 22:6n-3 (p < .001).
Table 4. Effect of fish oil supplement on the fatty acid composition in the RBC of sow.
Fatty acid composition in full-term placenta and RBC of cord blood
The proportion of 18:2n-6 in placenta did not differ between the two groups, but the 20:4n-6 in placenta was lower in the FD group than that in the SD group (p < .01; Table ). The proportions of 20:5n-3 (p < .001) and 22:6n-3 (p < .05) were higher, but the proportion of 22:5n-3 (p < .001) was lower in placenta in the FD group compared to the SD group. In RBC of cord blood, the proportion of 20:4n-6 was lower in the FD group than that in the SD group. Interestingly, the proportion of 20:5n-3 (p < .001) was higher, but the 22:5n-3 (p < .01) was lower in the FD group in comparison to the SD group.
Table 5. Effect of fish oil supplement on the fatty acid composition in placenta and RBC of cord blood.
Colostrum and milk fatty acid composition
The proportion of n-6PUFA in the colostrum and milk from the FD group was lower than that from the SD group (p < .001), while the proportion of n-3PUFA in the colostrum and milk from the FD group was higher than that from the SD group (p < .001) (Table ). The FD decreased the proportions of 18:2n-6 (p < .001) and 20:4n-6(p < .001) but increased the proportion of 20:5n-3 (p < .001), 22:5n-3 (p < .001) and 22:6n-3 (p < .001) in the colostrum and milk. The proportion of 18:2n-6, 20:4n-6, 18:3n-3, 20:5, 22:5 and 22:6 was affected by day in both groups (p < .001). Diet × day interaction affected 20:4n-6 (p < .01), 20:5n-3 (p < .001), 22:5n-3 (p < .001) and 22:6n-3 (p < .001).
Table 6. Effect of fish oil supplement on the fatty acid composition of colostrum and milk.
Fatty acid composition in RBC of piglets
The FD treatment had higher proportions of 20:5n-3 (p < .001) and 22:6n-3 (p < .001) in RBC of piglets compared with the SD treatment, and both of them were affected by day and diet × day effect (day effect p < .01 and diet × day effect p < .001 for 20:5n-3; day effect p < .001 and diet × day effect p < .05) (Table ). The proportion of 22:5n-3 in RBC of piglets was affected by day effect (p < .001) but not by diet effect. The proportion of 18:3n-3 in RBC of piglets was lower in the FD group than that in the SD group (p < .001).
Table 7. Effect of fish oil supplement on the fatty acid composition in the RBC of piglets.
Fatty acid composition in livers of piglets
The proportion of 18:2n-6 in livers of piglets was lower in the FD group than that in the SD group (p = .01), but it increased from birth to day 16 in both groups (p < .001) (Table ). The diet × day interaction effect affected the proportion of 18:2n-6 (p < .05). The proportion of 20:4n-6 was lower in the FD group than that in the SD group (p = .001). The proportion of 18:3n-3 in the livers of piglets increased with time in both groups (p = .002). The livers of the piglets contained greater proportions of 20:5n-3, 22:5n-3 and 22:6n-3 (p < .001) in the FD group than in the SD group during the entire experiment period. Furthermore, the proportions of 20:5n-3, 22:5n-3 and 22:6n-3 in piglet liver increased (p < .001) from birth to day 16 in both groups, and the diet × day interaction affected the proportions of 20:5n-3, 22:5n-3 and 22:6n-3 (p < .001 for 20:5n-3 and 22:6n-3, p < .05 for 22:5n-3).
Table 8. Effect of fish oil supplement on the fatty acid composition in the livers of piglets†.
Expression of fatty acid desaturase mRNA in placenta and livers of piglets
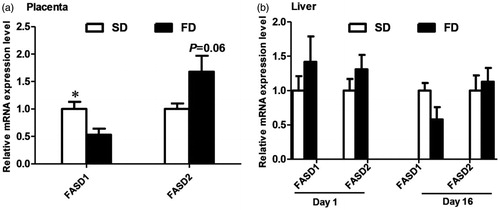
The expression levels of fatty acid desaturase genes in the placenta and livers of piglets are shown in Figure . The expression of FADS1 mRNA in the placenta significantly decreased in the FD group (p < .05), but the expression of FADS2 mRNA tended to increase in the placenta of piglets in the FD group (p = .06).
Figure 1. RT-qPCR analysis of FADS1 and FADS2 expression in the (a) placenta and (b) livers (1 d and 16 d) of piglets born to sows fed either the diet with soybean oil (SD) or the diet with fish oil (FD). Values are mean ± SEM (n = 6). *Mean values were significantly different between the two diet groups (p < .05).
Discussion
Many feed companies nowadays include small amounts of n-3 PUFA in sow diets, but the previous reports about the effects of n-3PUFA in the sow diet on sow reproduction and piglet performance are very inconsistent, as reviewed by Tanghe and De Smet (Citation2013). Therefore, a good knowledge of the n-3PUFA metabolism and transfer between sows and piglets is needed.
Our study showed that supplementing the diet of sow with fish oil did not affect reproductive performance, which is in agreement with other studies on sows fed n-3 PUFA (Boudry et al. Citation2009).The lack of dietary effect on gestation outcomes could be due to the comparable nutrient level in sow diets but also to the similar feed intake for sows at gestation and lactation period. Moreover, the lack of dietary effect on the growth performance of weaning piglets could be associated with no diet effect on the composition of colostrum and milk.
The FD increased the proportions of 20:5n-3, 22:5n-3 and 22:6n-3 in RBC of sow, which is consistent with other studies (Rooke et al. Citation2000), but the FD decreased the proportion of 22:5n-3 in the placenta. The possible reasons for the different change tendency in the placenta might be attributed not only to the incorporation of these fatty acids from the diet but also to the regulation of long-chain polyunsaturated fatty acids (LC-PUFA) metabolism in placenta. It is unclear how the metabolism of LC-PUFA was regulated in different tissue (especially in placenta), but the metabolism of LC-PUFA is thought to be linked with the catalysis of elongation and desaturation enzymes, the competition for the same set of enzyme between n-6 and n-3 LC-PUFA and the nutrients in diet (Sul and Wang Citation1998; Sprecher Citation2000).
The previous studies have confirmed that hepatic and cardiac cells have the capacity to synthesise n-3 LC-PUFA from 18:3n-3 (Barcelo-Coblijn and Murphy Citation2009), but there is no report about the synthesise LC-PUFA from 18:3n-3 in placenta. The delta-5 desaturase (D5D) and the delta-6 desaturase (D6D), encoded by fatty acid desaturase 1 (FADS1) and fatty acid desaturase 2 (FADS2), respectively, are the rate-limiting enzymes in synthesis of the long-chain PUFA 20:4 (n-6) and 20:5 (n-3) and 22:6 (n-3) from their dietary precursors linoleic acid 18:2(n-6) and 18:3(n-3), respectively (Schaeffer et al. Citation2006; Molto-Puigmarti et al. Citation2010). For n-3 PUFA, the delta-5 desaturase (D5D) and elongase enzymes participate in the conversion of 18:3n-3 to 20:5n-3, and the delta-6 desaturase (D6D) and elongase enzymes participate in the conversion of 22:5n-3 to 22:6n-3 (de Quelen et al. Citation2010). According to the n-3 PUFAs biosynthesis pathway, the production of 22:6n-3 from 18:3n-3 is metabolically limited at a step beyond 22:5n-3 formation in comparison with the 20:5n-3 and 22:5n-3 formation steps (Barcelo-Coblijn and Murphy Citation2009). These desaturase and elongase genes are tissue specific which enhances the efficiency of long-chain fatty acid synthesis and enhance survival by diminishing the attendant inflammatory response (Cheng et al. Citation2015). In our study, the decrease of 22:5n-3 but the increase of 22:6n-3 in placenta of the FD group implies that n-3 LC-PUFA metabolism in the placenta might be regulated at the step of 22:5n-3 formation. In addition, the FD also reduced the proportion of 22:5n-3 in RBC of cord blood, and down-regulated the expression of FADS1 but tended to up-regulate the expression of FADS2 in the placenta from the FD group. These results indicate that the decrease of 22:5n-3 and the increase of 22:6n-3 in placenta might be related to either the inhibition of the 22:5n-3 synthesis from beyond step or the improvement of the conversion of 22:5n-3 to 22:6n-3.
The n-3 LC-PUFA in livers of piglets could be altered by incorporation of these fatty acids from the intake or by the biosynthesis of LC-PUFA. Indeed, hepatic expression of D5D and D6D in pig foetus increased as gestation progressed (McNeil et al. Citation2005), and their activities appear to be much higher in the postnatal period than before birth. In our study, the FD increased the proportion of 22:5n-3 and 22:6n-3 in livers of piglets at birth, and the proportion of 22:6n-3 was higher in the livers of 16d old piglets as shown also in another study (Lauridsen and Jensen Citation2007), although 22:6n-3 was reduced in milk and RBC of piglets. Our results suggest that the n-3 LC-PUFA composition in livers of piglets might be affected by the biosynthesis of LC-PUFA in liver.
Tu et al. (2010) reported that the n-3 LC-PUFA synthesis was regulated more by substrate competition for existing enzymes than by the increase of these enzymes mRNA expression. In our study, the FD did not modify the hepatic FADS1 and FADS2 mRNA expression in piglets, but the 22:6n-3 increase from birth to day 16 in the livers of piglets. These results implied that the increase of 22:6n-3 might be linked to the improvement of substrate competition for existing desaturase but not to the up-regulation of desaturase enzyme expression in the livers of piglets. Further research about enzyme kinetics of the hepatic desaturation enzymes in the livers of piglets will be needed to provide conclusive evidence.
Conclusions
Supplementing fish oil in the sow diet during late gestation and lactation period alters the fatty acid composition of the RBC of sows and their piglets, RBC of cord blood, placenta, milk and livers of piglets. The decrease of the 22:5n-3 and the increase of the 22:6n-3 in placenta of the FD group might be related to the regulation of n-3 LC-PUFA metabolism at the 22:5n-3 formation step. The increase of 22:5n-3 and 22:6n-3 in livers of piglets at birth and the increase of 22:6n-3 in the livers of 16d old piglets might probably be linked to the biosynthesis of LC-PUFA in the livers of piglets, and further research about the enzyme kinetics of the hepatic desaturation enzymes needs to be investigate.
Ethical approval
The protocol used in this experiment approved by Shanghai Jiao Tong University Institutional Animal Care and Use Committee.
Acknowledgements
The authors thank Xinnong Feed Co. Ltd for supplying pig experiment farm, feed process and people to care of the pigs. The authors also thank Dr M. Y. Y. for technical help.
Data availability statement
The data used to support the findings of this study are included within the article and the supplementary information file.
Disclosure statement
The authors disclose that there is no conflict of interest.
Additional information
Funding
References
- Amusquivar E, Laws J, Clarke L, Herrera E. 2010. Fatty acid composition of the maternal diet during the first or the second half of gestation influences the fatty acid composition of sows’ milk and plasma, and plasma of their piglets. Lipids. 45:409–418.
- Barcelo-Coblijn G, Murphy EJ. 2009. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res. 48:355–374.
- Bimbo AP, Crowther JB. 1992. Fish meal and oil: current uses. J Am Oil Chem Soc. 69:221–227.
- Boudry G, Douard V, Mourot J, Lallès JP, Le Huërou-Luron I. 2009. Linseed oil in the maternal diet during gestation and lactation modifies fatty acid composition, mucosal architecture, and mast cell regulation of the ileal barrier in piglets. J Nutr. 139:1110–1117.
- Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, Salem N. 2002. Cognitive deficits in docosahexaenoic acid-deficient rats. Behav Neurosci. 116:1022–1031.
- Cheng CL, Huang SJ, Wu CL, Gong HY, Ken CF, Hu SY, Wu JL. 2015. Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. J Biomed Sci. 22:103.
- de Quelen F, Boudry G, Mourot J. 2010. Linseed oil in the maternal diet increases long chain-PUFA status of the foetus and the newborn during the suckling period in pigs. Br J Nutr. 104:533–543.
- de Quelen F, Boudry G, Mourot J. 2013. Effect of different contents of extruded linseed in the sow diet on piglet fatty acid composition and hepatic desaturase expression during the post-natal period. Animal. 7:1671–1680.
- Folch J, Lees M, Sloane Stanley GH. 1956. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 226:497–509.
- Lauridsen C, Jensen SK. 2007. Lipid composition of lactational diets influences the fatty acid profile of the progeny before and after suckling. Animal. 1:952–962.
- Li Hao, Wan Haifeng, Mercier Yves, Zhang Xiaoling, Wu Caimei, Wu Xiuqun, Tang Li, Che Lianqiang, Lin Yan, Xu Shengyu, et al. 2014. Changes in plasma amino acid profiles, growth performance and intestinal antioxidant capacity of piglets following increased consumption of methionine as its hydroxy analogue. Br J Nutr. 112:855–867.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
- McNeil CJ, Finch AM, Page KR, Clarke SD, Ashworth CJ, McArdle HJ. 2005. The effect of fetal pig size and stage of gestation on tissue fatty acid metabolism and profile. Reproduction. 129:757–763.
- Molina MT, Vázquez CM, Ruiz-Gutierrez V. 1989. Changes in both acyl-CoA:cholesterol acyltransferase activity and microsomal lipid composition in rat liver induced by distal-small-bowel resection. Biochem J. 260:115–119.
- Molto-Puigmarti C, Plat J, Mensink RP, Muller A, Jansen E, Zeegers MP, Thijs C. 2010. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am J Clin Nutr. 91:1368–1376.
- Nakamura MT, Nara TY. 2004. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 24:345–376.
- Nesheim MC, Yaktine AL. 2006. Seafood choices: balancing benefits and risks. Report Brief by Institute of Medicine of the National Academies. Washington (DC): The National Academies Press.
- NRC. 2012. Nutrient requirements of swine. 11th revised ed. Washington (DC): National Academies Press (National Research Council).
- Olliver M, Veysey M, Lucock M, Niblett S, King K, MacDonald-Wicks L, Garg ML. 2016. Erythrocyte omega-3 polyunsaturated fatty acid levels are associated with biomarkers of inflammation in older Australians. J Nutr Intermediary Metab. 5:61–69.
- Raes K, Smet S. de, Demeyer D. 2001. Effect of double-muscling in Belgian Blue young bulls on the intramuscular fatty acid composition with emphasis on conjugated linoleic acid and polyunsaturated fatty acids. Anim Sci. 73:253–260.
- Rooke JA, Shanks M, Edwards SA. 2000. Effect of offering maize, linseed or tuna oils throughout pregnancy and lactation on sow and piglet tissue composition and piglet performance. Anim Sci. 71:289–299.
- Sampels S, Pickova J, Högberg A, Neil M. 2011. Fatty acid transfer from sow to piglet differs for different polyunsaturated fatty acids (PUFA). Physiol Res. 60:113–124.
- Schaeffer L, Gohlke H, Muller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. 2006. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 15:1745–1756.
- Sprecher H. 2000. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 1486:219–231.
- Sul HS, Wang D. 1998. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu Rev Nutr. 18:331–351.
- Tanghe S, De Smet S. 2013. Does sow reproduction and piglet performance benefit from the addition of n-3 polyunsaturated fatty acids to the maternal diet? Vet J. 197:560–569.
- Thiex N; AOAC International. 2002. Committee on Feeds, Fertilizers, and Related Agricultural Topics. Feeds. J AOAC Int. 85:270–273.
- Wilson ME, Biensen NJ, Youngs CR, Ford SP. 1998. Development of Meishan and Yorkshire littermate conceptuses in either a Meishan or Yorkshire uterine environment to day 90 of gestation and to term. Biol Reprod. 58:905–910.
