Abstract
Foods of animal origin are controlled for antibiotic and coccidiostat residues. The rapid residue detection is possible using reliable broad-spectrum screening tests. This study’s objective using four microbial inhibition tests for the detection and identification of antibiotic and coccidiostat residues in different foods of animal origin: Premi®Test, EXP Ampulle test, Milchtest and Screening Test for Antibiotic Residues (STAR). Four hundred and thirty (430) food samples (165 animal tissues, 152 raw cow’s milk and 113 eggs) were randomly collected and screened. Using the Premi®Test, 18 samples were positive and 6 samples dubious. Using the EXP Ampulle test, 31 samples were positive and 2 samples dubious. Using the Milchtest, 15 samples were positive and 12 samples dubious. Using the STAR, 65 samples were positive with 62 samples positive on plates specific for beta-lactams and sulphonamides, 4 samples on plates specific for aminoglycosides, 8 samples on plates specific for macrolides and beta-lactams; and 7 samples on the plates specific for tetracyclines. Retesting using penicillinase and para-aminobenzoic acid (PABA) to confirm the presence of beta-lactams or sulphonamides all potentially positive tube test samples revealed 21 samples positive for beta-lactams and 27 samples positive for sulphonamides. Further testing of sulphonamide positive chicken samples revealed the positivity for coccidiostat salinomycin which was confirmed by testing with PABA, which counteracting salinomycin inhibition. Three hundred and sixty six (366) animal food samples were negative for antibiotic and coccidiostat residues. Microbial inhibition tests are preferred for initial antibiotic screening and have also proven useful for coccidiostat screening and post-screening.
Foodstuffs of animal origin are subject to controls on antibiotic and coccidiostat residues.
Microbial inhibition tests are still the preferred choice for the initial screening of antibiotic residues in food matrices and could become a useful tool for the screening of coccidiostat residues also.
More specific post-screening analysis with PABA proved unexpectedly to be a reliable tool in the preliminary detection of coccidiostat residues in poultry meat and eggs.
HIGHLIGHTS
Introduction
The use of antibiotics or coccidiostats for therapeutic, preventative and/or growth promotion purposes in food-producing animals is causing serious problems associated with the presence of residues in foods of animal origin. The presence of residues in food of animal origin above the maximum levels or residue limits is recognised worldwide as an important food safety and health hazards. The presence of residues in foods may lead to the development of resistant strains of microorganisms, hypersensitivity reactions in sensitive individuals, intestinal microflora disturbances and economic losses in the food industry especially by interfering with starter culture (Kožárová, Mačanga, et al. Citation2011; Ezenduka et al. Citation2014; Samandoulougou et al. Citation2015; Bacanli and Başaran Citation2019).
Screening of food-producing animals for the presence of antibiotic and coccidiostat residues is one of the main pillars of the European Union (EU) and national monitoring programmes. Commission Decision 2002/657/EC describes a screening method as a method used to detect the presence of a substance or class of substances at the level of interest. The method must be able to detect the marker residue (specific metabolite) or parent compound at/or below the appropriate regulatory limit (RL). In the EU, the RL for authorised veterinary medicinal products is referred to as the maximum residue limit (MRL). The MRLs serve as the points for the control of residues in foods of animal origin in Member States and at border inspection posts; and have to be properly controlled in accordance with the Council Directive 96/23/EC (Council of the European Union Citation1996; European Commission Citation2002; European Parliament and the Council of the European Union Citation2009; Wang et al. Citation2012; Gondová et al. Citation2014).
The current residue control strategy is based on two sequential steps: screening and confirmation. Screening techniques can generate qualitative (positive or negative), semiquantitative (high, medium/low, or negative) or quantitative results. The primary aim of a qualitative screening assay is to sift through large numbers of compliant samples. These methods are less expensive than immunochemical and chromatographic methods able to screen a large number of samples and most relatively simple to use. Because of these characteristics, microbial inhibition tests are preferred and routinely used as the methods of choice for the rapid detection and the large scale surveillance programmes used for testing antibiotic residues in food. These methods comprise of a medium inoculated with a susceptible bacterium and rely on agar diffusion of the antibiotic residues, which is expressed by the production of an inhibition zone (plate tests) or by the absence of colour change from purple to yellow (tube tests) (European Commission Citation2002; Stead et al. Citation2004; Gaudin et al. Citation2008; Pikkemaat et al. Citation2008; Pikkemaat Citation2009; Gaudin et al. Citation2010; Stead and Stark Citation2012; Serraino et al. Citation2013; Ezenduka et al. Citation2014; Gondová et al. Citation2014; Beltrán et al. Citation2015; Gaudin et al. Citation2017; Granados-Chinchilla and Rodríguez Citation2017; Sophila et al. Citation2018). For coccidiostats, confirmatory chromatographic methods still remain at the core of residue control (Olejnik et al Citation2009; Olejnik et al Citation2011; Clarke et al. Citation2014; Danaher et al. Citation2016; Barreto et al. Citation2017; Pietruk et al. Citation2018; Dasenaki and Thomaidis Citation2019; Radko and Cybulski Citation2019; Roila et al. Citation2019). However, also rapid and user-friendly antibody-based screening methods in new assay formats (Hagren Citation2009) or microbial inhibition tests (Kožárová and Máté Citation2000; Kožárová et al. Citation2002; Kožárová, Janošová, et al. Citation2009; Kožárová, Mačanga, et al. Citation2011, Kožárová, Šimková, et al. Citation2011) can find their way into routine coccidiostat residues testing.
The adequate detection of a broad-spectrum of antibiotics is only possible using multi-plate test systems based on a combination of different test organisms (Pikkemaat Citation2009). In 1999, the Community Reference Laboratory (CRL) in Fougeres (France) developed the Screening Test for Antibiotic Residues (STAR) for the detection of antibiotic residues in milk and meat. With respect to the requirements laid down by the Commission Decision 2002/657/EC, which concerns the performance of analytical methods and the interpretation of results, the STAR method has been validated for antibiotic residue detection in milk (Gaudin et al. Citation2004), and in meat (Gaudin et al. Citation2010). The STAR method comprises five test plates sensitive to group or groups of antibiotics as follows: Bacillus subtilis BGA at pH 8.0 for aminoglycosides, Kocuria rhizophila ATCC 9341 at pH 8.0 for macrolides and beta-lactams, Bacillus cereus ATCC 11788 at pH 6.0 for tetracyclines, Escherichia coli ATCC 11303 at pH 8.0 for quinolones, and Bacillus stearothermophilus var. calidolactis ATCC 10149 at pH 7.4 for beta-lactams and sulphonamides. The higher specificity and detection capability of this method predestined the STAR method to be a preferential method for the screening of antibiotic residues in many European countries (Pikkemaat et al. Citation2008; Kožárová, Mačanga, et al. Citation2009; Gaudin et al. Citation2010; Cháfer-Pericás et al. Citation2010; Kožárová, Mačanga, et al. Citation2011; Gondová and Kožárová Citation2012; Gondová et al. Citation2014).
From a practical perspective, commercial tube tests form an attractive alternative to multiplate methods (Pikkemaat Citation2009). From these tests, the Premi®Test manufactured and supplied by R-Biopharm AG (Darmstadt, Germany) is the most commonly used today. This test has been validated for the detection of ß-lactam residues, cephalosporin, macrolide, tetracycline, sulphonamide, aminoglycoside, quinolone, amphenicol, and polypeptide antibiotics in meat, fish, shrimp, eggs, liver, kidney, plasma/serum, urine and feed in line with EU MRLs (Stead et al. Citation2004; European Commission Citation2010). The Premi®Test contains viable spores of a strain of Bacillus stearothermophilus var. calidolactis. The use of spores instead of vegetative cells allows prolonged storage and enables commercial distribution. Because of its fast growing properties at elevated temperature, it is possible to obtain a result within a few hours (Pikkemaat et al. Citation2009).
An attractive alternative to the Premi®Test are two similar commercially available Bacillus stearothermophilus tube tests: EXP Ampulle and Milchtest (Packhaus Rockmann GmbH, Sendenhorst, Germany). They have a high degree of sensitivity, thus enabling the detection of a many antibiotics and sulphonamides in line with EU MRLs. The EXP Ampulle is used for the detection of antibiotic residues in meat, liver, kidney, eggs and feed and the Milchtest for the detection of antibiotic residues in cow, sheep and goat’s milk, including raw and heat-treated milk as well as powdered milk.
Another important advantage of these microbial inhibition tests the post-screening confirmation of beta-lactams and sulphonamides in screen-positive samples using a suitable neutralisation solution, which selectively reverses the inhibitory activity of either beta-lactams or sulphonamides. Today, p-aminobenzoic acid (PABA) and penicillinase are used in direct combination with Bacillus stearothermophilus tube tests for confirming the presence of beta-lactams or sulphonamides in the screen-positive samples (Ferrini et al. Citation2006; Pikkemaat et al. Citation2009; Gondová et al. Citation2014; Sanz et al. Citation2015).
Because antibiotics and coccidiostats are still widely used for the treatment of infectious diseases in food-producing animals and because coccidiostats are also authorised as feed additives for target animal species and residues of all these pharmacologically active substances that are still detectable in foods of animal origin, this means the screening of residues in animal products still plays a significant role in controlling and ensuring food safety. The aim of our study was to assess the applicability of the microbial inhibition tests mentioned above for the effective detection and identification of antibiotic and coccidiostat residues in different foods of animal origin.
Materials and methods
Sample material
A total of 165 chicken, bovine, porcine and ovine samples were randomly collected from slaughterhouses, dairy and eggs farms, grocery stores and supermarkets located in various European countries between January 2018 and August 2019. The distribution of samples was as follows: [chickens: meat (37), liver (28), heart (27), gizzard (2), kidney (3), fat and skin (3), spleen (2); bovine animals: meat (12), liver (12), kidney (12); porcine animals: meat (7), liver (7), kidney (7); ovine animals: meat (2), kidney (2), spleen (2)]. Additionally, 152 samples of raw cow’s milk and 113 fresh table eggs (organic (12), free-range (18), barn laid (24), cage (59)] were also included in the study. The samples were stored at 4 °C (milk, eggs) and −20 °C (meat) until further processing. All samples were taken applying the simple random sampling method. The meat samples were harvested from randomly selected animals slaughtered in a slaughterhouse or purchased at a wholesale market. The chicken kidneys were manually removed from the back of the whole chicken carcases. The raw cow’s milk samples were taken from on-farm bulk tanks. The egg samples were purchased at the production site or a wholesale market. Several meat samples have been provided to us by the national authority within the monitoring and control of residues.
Screening Test for Antibiotic Residues (STAR)
Preparation of test plates
Test plates were prepared and used according to the STAR protocol Version 3 developed by the Community Reference Laboratory (AFSSA-Fougères, France) adopted by the competent national authority as the officially approved protocol for the screening of products of food-producing animals for antibiotics residues in Slovakia (R-25 2013). Antibiotic medium 11 (Difco 259310; Difco, Detroit, USA) adjusted to pH 8.0 was seeded with ready-to-use commercial spore suspension Bacillus subtilis BGA (5 × 104 spores/mL) (Merck 10649, Merck, Darmstadt, Germany). Test agar pH 8.0 (Merck 10664) was seeded with bacterial suspension Kocuria rhizophila ATCC 9341 (Czech Collection of Microorganisms, Brno, Czech Republic) to give a final concentration of 5 × 104 germs/mL in agar medium. Test agar pH 6.0 (Merck 10663) was seeded with bacterial suspension Bacillus cereus ATCC 11778 (Czech Collection of Microorganisms) to give a final concentration of 3 × 104 germs/mL in the agar medium. Test agar pH 8.0 (Merck 10664) was seeded with bacterial suspension Escherichia coli ATCC 11303 (Czech Collection of Microorganisms) to give a final concentration of 105 germs/mL in the agar medium. Diagnostic Sensitive Test (DST) agar (Oxoid CM 261; Oxoid, Basingstoke, UK) adjusted to pH 7.4 was seeded with ready-to-use commercial spore suspension Bacillus stearothermophilus var. calidolactis ATCC 10149 (5 × 105 spores/mL) (Merck 1.11499) and supplemented with trimethoprim (Fluka 92131; Fluka, Buchs, Switzerland) to obtain a final concentration of 0.005 µg/mL in the agar medium. Finally, 5 ml of the seeded media were poured into Petri dishes of 90 mm in diameter. Commercial agar media were prepared according to the manufacturer’s instructions. Quality control for each test plate was performed using paper discs 9 mm in diameter (Whatman Grade No. 1, Whatman International Ltd, Maidstone, UK) soaked with 30 µL of control standard solutions of reference antibiotics prepared and stored according to the procedures set by the method.
Screening of the samples
Meat: A cylindrical core 8 mm in diameter and approximately 2 cm long was removed from each frozen sample using a sterile cork borer (Ø 9 mm) and cut into slices of 2 mm in thickness with a sterile lancet. Cut slices were placed opposite each other on each of the five test plates. Milk: A total of 30 µL of raw homogenised cow’s milk was transferred to a filter paper discs 9 mm in diameter (A2668090, Hahnemühle FineArt GmbH, Dassel, Germany), which were placed opposite each other on each of the five test plates. Eggs: The egg shell was cracked manually and the content was thoroughly homogenised. Before analysis, all egg samples were pre-incubated in a thermoblock (Acublock Digital Dry Bath D 1200, Labnet, USA) at 80 °C for 10 min (according to the Premi®Test manufacturer’s instructions.). Eggs were further examined in the same way as milk samples. Test plates were incubated as follows: Bacillus subtilis BGA and Bacillus cereus ATCC 11778 test plates at 30 °C for at least 18 h, Kocuria rhizophila ATCC 9341 and Escherichia coli ATCC 11303 test plates at 37 °C for at least 24 h, and Bacillus stearothermophilus var. calidolactis ATCC 10149 test plates at 55 °C for 12 h.
Reading the test results
Samples were considered positive if they gave the inhibition zone (IZ) on at least one of the five test platesequal or superior to 2 mm wide on plates seeded with Bacillus subtilis BGA, Kocuria rhizophila ATCC 9341, Bacillus cereus ATCC 11778 and Escherichia coli ATCC 11303, and equal or superior to 4 mm (meat) or 2 mm (milk, eggs) on plates seeded with Bacillus stearothermophilus var. calidolactis ATCC 10149. The width of the IZ was measured as the distance between the edge of the slice of the tissue or the disc and the outer limit of the IZ in mm using a digital calliper (Mitutoyo, Kawasaki, Japan) with a precision of 0.01 mm. The diameter of the IZ were expressed as the mean ± standard deviation (SD) of six measures.
Premi®test
Screening of the samples
Premi®Test was performed according to the officially approved protocol for the for the determination of residues of inhibitory substances in meat by the Premi®Test (R-26 2013) in Slovakia which is in compliance with the manufacturer’s instructions for use. Meat: A total of 100 µL of the juice obtained by thawing the sample in a microwave oven set to defrost were pipetted onto the agar in the ampoule and allowed to stand at room temperature for 20 min for pre-diffusion. After the pre-diffusion, the juice was flushed out of the ampoule by washing twice with demineralised water. For the used kidney and liver samples, ampoules containing 100 µL of kidney and liver juice were pre-incubated at 80 °C for 10 min. After the heat pre-treatment, all ampoules were further tested uniformly by incubation in a digital dry bath (Labnet Accublock Digital Dry Bath D 1200, Labnet, Edison, USA) at 64 °C ± 0.5 °C for approximately 3 to 3.5 h until the negative control turned from purple to yellow. During the incubation period, ampoules were sealed with a plastic foil to avoid evaporation. Eggs: A total of 100 µL of the homogenised egg content were pipetted onto the agar in the ampoule. Ampoules were placed into a digital dry bath and pre-incubated at 80 °C for 10 min. After this heat pre-treatment, ampoules were further incubated in the same way as the meat and milk samples as aforementioned. During the incubation period, ampoules were covered with a plastic foil to avoid evaporation.
EXP Ampulle test
Screening of the samples
EXP Ampulle test was performed according to the manufacturer’s instructions for use supplied in the test kit (Packhaus Rockmann GmbH, Sendenhorst, Germany). Meat: A total of 100 µL of the extracted meat juice obtained by thawing the sample in a microwave oven set to defrost until the sample was fully cooked, were added to the ampoule. Ampoules were incubated at room temperature for 30 min, washed with demineralised water, and excess water was removed by turning the tubes upside down onto the absorbent paper. Ampoules were carefully sealed with an adhesive foil and placed in a digital dry bath incubator at 65 °C ± 1 °C for approximately 3 h and 15 min until the negative control turned from purple to yellow. Eggs: 10 mL of sterile demineralised water were added to 10 mL of the homogenised egg content. After thorough mixing, the diluted sample was warmed up for 3 min in a water bath at 100 °C with occasional mixing with a glass rod to prevent coagulation. A 100 µL of the diluted homogenised sample were transferred to the ampoule. The ampoule was covered with an adhesive foil and incubated in the same way as the meat samples.
Milchtest
Screening of the samples
Milchtest was performed according to the manufacturer’s instructions for use supplied in the test kit (Packhaus Rockmann GmbH, Sendenhorst, Germany). A total of 50 µL of the milk sample were transferred to the ampoule. The ampoule was sealed with a foil supplied with the kit and transferred to a digital dry bath incubator where the sample was incubated for about 3 h at a temperature of 65 ± 0.5 °C. The test was terminated when the colourof the agar medium of the negative control changed from violet to yellow.
Reading the results of the Premi®test, EXP Ampulle test and Milchtest
The test was terminated when the negative control sample turned yellow. A clear colour change from purple to yellow indicated that the sample contained no antibiotic residues or that the concentration of residues was below the detection limit of the respective test. When the colour remained purple or the colour of the sample was clearly different to that of the negative control, the sample contained antibiotic residues at a concentration above the detection limit of the respective test. The samples with light shades of purple colour indicated the presence of antibiotic residues around (near) the detection limit of the respective test and were considered dubious. The test results were evaluated using a colour card or a colour scale supplied by the manufacturer with the kit.
Post-screening confirmation of beta-lactams and sulphonamides
Identification of beta-lactam antibiotics
A total of 100 µL of penicillinase (Penase, SR0129, Oxoid, Basingstoke, UK, 50 IU/mL) was pipetted onto agar in test tubes and allowed to stand at room temperature for 30 min for pre-diffusion. After pre-diffusion, penicillinase was flushed away from the ampoules and the sample was further tested using the Premi®Test, EXP Ampulle test and Milchtest screening procedures mentioned above. If a previously positive sample on screening gave a negative result by a colour change of the medium from purple to yellow, it confirmed an indication of the presence of beta-lactam antibiotics in the sample.
Identification of sulphonamides
PABA stock solution (A 9878, Sigma-Aldrich, St. Louis, USA; 1000 µg/mL) was prepared by dissolving 10 mg PABA powder in 10 mL sterile demineralised water. 100 µL of working PABA solution at the concentration of 100 µg/mL was placed onto the agar in respective ampoules and allowed to stand at room temperature for 30 min for pre-diffusion. After pre-diffusion, PABA was flushed away from the ampoules and the sample was further tested using the Premi®Test, EXP Ampulle test and Milchtest as mentioned above. When a previously positive screened sample gave a negative result presented by a colour change of the medium from purple to yellow, it confirmed the presence of sulphonamides in the sample.
Statistical analysis
The Chi-square test was performed to compare the data of the Premi®Test and the EXP Ampulle test used for the screening of the same animal food samples with a degree of freedom 4. The significance level was 0.05. The statistical analysis was performed using the online tool http://www.quantpsy.org/chisq/chisq.htm.
Results
Screening results for the presence of antibiotic and coccidiostat residues using four microbial inhibition tests are presented in Table .
Table 1. Overview of the positive results obtained from the screening of animal food samples for the presence of antibiotic and coccidiostat residues using the STAR, Premi®Test, EXP Ampulle and Milchtest.
A total of 430 samples subjected to residues analysis by inhibition of growth of sensitive bacterial strains, 65 samples (15.12%) yielded a positive or dubious result on one or more screening tests.
Residue screening using commercial tube tests containing Bacillus stearothermophilus var. calidolactis as the indicator organism revealed that 18 samples (4.19%) of samples including 6 chicken livers, 2 hearts, 3 fats/skins, 2 spleens, 2 gizzards; 1 porcine muscle and 2 eggs were positive and 6 samples (1.40%) including 3 chicken livers and 3 eggs were dubious on the Premi®Test, On the EXP Ampulle test, 31 samples (7.21%) including 9 chicken livers, 1 heart, 1 kidneys, 3 fats/skins, 2 spleens, 2 gizzards and 13 eggs were positive and 2 samples (0.47%) including 1 chicken liver and 1 kidneys dubious. On the Milchtest, 15 milk samples (3.49%) were positive and 12 milk samples (2.79%) dubious. Applying the Chi-square statistics to the results of the Premi®Test and the EXP Ampulle tube tests, we found that the p-value was .6590 (p > .05) with the Yates of correction 0.8. This indicates that the two tests do not exhibit any significant differences.
On the STAR method, 62 samples (14.42%) including 10 chicken livers, 2 kidneys, 2 hearts 3 fats/skins, 2 spleens, 2 gizzards; 1 porcine muscle, 13 egg and 27 milk samples were positive on the Bacillus stearothermophilus var. calidolactis ATCC 10149 plates specific for beta-lactams and sulphonamides, 4 samples (0.93%) including 1 chicken kidneys, 2 muscles and 1 milk sample were positive on the Bacillus subtilis BGA test plates specific for aminoglycosides, 8 samples (1.86%) including egg samples were positive on the Kocuria rhizophila ATCC 9341 test plates specific for macrolides and beta-lactams and 7 samples (1.63%) including milk samples were positive on the Bacillus cereus ATCC 11788 test plates specific for tetracyclines. No inhibition zones were observed on the Escherichia coli ATCC 11303 test plates specific for quinolones. Among all positive food samples, milk and egg samples showed the formation of inhibition zones on more test plates as follows: 7 milk samples on Bacillus stearothermophilus var. calidolactis ATCC 10149 and Bacillus cereus ATCC 11788 test plates, 1 milk sample on the Bacillus stearothermophilus var. calidolactis ATCC 10149, Bacillus cereus ATCC 11788 and Bacillus subtilis BGA test plates, and finally, 8 egg samples on the Bacillus stearothermophilus var. calidolactis ATCC 10149 and Kocuria rhizophila ATCC 9341 test plates. Based on the sensitivity of the STAR test strains, the positive samples were deemed suspect for the presence of beta-lactams or sulphonamides followed by macrolides, tetracyclines and aminoglycosides, respectively.
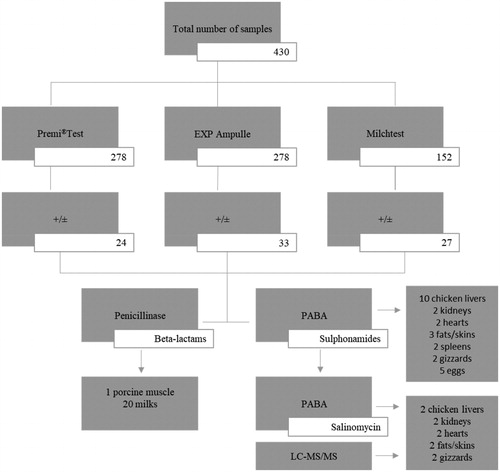
Summarising the results of all positive results of all tube tests and the STAR method, 62 samples were positive (13 of which were dubious) by at least one tube test and 65 samples were positive as determined by the STAR method. The additional post-screening analysis of beta-lactams or sulphonamides in the screen-positive samples using penicillinase and PABA revealed the following: 1 porcine muscle sample and 20 milk samples were positive for beta-lactam antibiotics; and all chicken livers (10), 2 kidneys, 2 hearts, 3 fats/skins, 2 spleens and 2 gizzards and 5 eggs were positive for sulphonamides (Figure ). Based on the screening and post-screening results obtained, we can conclude that positive chicken livers, heart, fats/skins, spleens and gizzards are suspected for the presence of sulphonamides, chicken kidneys for the presence of sulphonamides and aminoglycosides, chicken muscle for the presence of aminoglycosides, porcine muscle for the presence of beta-lactams, egg samples for the presence of sulphonamides and macrolides, and milk samples for the presence of beta-lactams and tetracyclines.
Figure 1. Post-screening identification of beta-lactams and sulphonamides in the positive samples with penicillinase and PABA by using the Premi®Test, EXP Ampulle test and Milchtest.
+/±, positive/dubious result; PABA, p-aminobenzoic acid; LC-MS/MS, Liquid Chromatography Tandem Mass Spectrometry.
Having the muscles and giblets available from two partially eviscerated broiler chicken carcases fed commercially-produced feed containing 70 mg/kg of salinomycin in complete feed, this led us to question whether these chicken samples would prove positive for the coccidiostat salinomycin. The samples from both of these carcases were subjected to chemical confirmation for coccidiostat residues by using the liquid chromatography (LC) coupled with electrospray ionisation tandem mass spectrometry (MS/MS) according to the procedure reported by Tkáčiková et al. (Citation2010, Citation2012). Analysis by LC-MS/MS confirmed the presence of salinomycin residues in the chicken liver (8.42 ± 1.94 µg/kg), kidneys (31.2 ± 7.18 µg/kg), fats/skin (76.3 ± 17.55 µg/kg), heart (11.83 ± 2.72 µg/kg), gizzard (30.31 ± 6.97 µg/kg) and also in the muscle (3.33 ± 0.77 µg/kg).
Taking into account the outcomes of the confirmatory analysis determining the presence of salinomycin in the examined chicken tissue samples and given the sulphonamide positive results after post-screening analysis with PABA, the effect of PABA on salinomycin was evaluated by testing the working solutions of salinomycin standard (S 46729, Sigma-Aldrich, St. Louis, USA) at the concentration of 50 and 100 µg/L. The stock solution of the salinomycin standard was prepared by dissolving 10 mg of salinomycin in 1 ml of 5% methanol (Merck, Darmstadt, Germany) and supplemented with sterile demineralised water to a concentration of 1000 µg/L. The working solutions of salinomycin were prepared by serial dilutions with sterile demineralised water to the final concentration of 50 µg/L. A total of 100 µL of respective salinomycin working solutions were transferred to the ampoules of the Premi®Test and EXP Ampulle test previously fortified with PABA according to the procedure used for the post-screening identification of sulphonamides and further tested using both tube tests screening procedures mentioned above. To investigate the detection capability of the Premi®Test, EXP Ampulle test and the STAR method, the stock solution was further diluted with sterile demineralised water to reach the concentration of 10 µg/L in view of the MRL currently established for salinomycin in liver (150 µg/kg), kidney (40 µg/kg), muscle (15 µg/kg) and skin/fat (150 µg/kg) of chickens reared for fattening and chickens reared for laying by the Commission Implementing Regulation (EU) 2017/1914 (European Commission Citation2014).
PABA completely reversed the inhibitory activity of salinomycin at the concentration of 50 and 100 µg/L on the test organism Bacillus stearothermophilus var. calidolactis by visible decolourization of the agar medium from purple to yellow and thereby confirmed the presence of salinomycin in the positive chicken tissues. This means that PABA seems to be a suitable neutralisation solution for presumptive identification of salinomycin residues in screening-positive samples (Figure ). Therefore, 5 eggs detected positive for sulphonamides could also be positive for salinomycin. Based on the code of the originating farm stamped on these eggs we found that all these eggs were paradoxically organic eggs produced in country in which coccidiostats are still approved for laying hens.
By testing the salinomycin standard solutions with the Premi®Test, EXP Ampulle test and the STAR method, the test organism Bacillus stearothermophilus var. calidolactis (in the STAR method Bacillus stearothermophilus var. calidolactis ATCC 10149) appeared to be sensitive to salinomycin at the level of the concern. The detection capability of the Premi®Test, EXP Ampulle test and the STAR method for salinomycin was as follows: Premi®Test 10 µg/L, EXP Ampulle test 50 µg/L (10 and 20 µg/L dubious) and STAR 75 µg/L. Salinomycin also inhibited the growth and multiplication of the test organism Bacillus cereus ATCC 11778 of the STAR method. However, the detection capability of this test organism for salinomycin was 500 µg/L. A comparison of the detection capability of the Premi®Test, EXP Ampulle test and the STAR method for salinomycin is presented in Table .
Table 2. Comparison of the detection capability of the STAR, Premi®Test and EXP Ampulle test for salinomycin determined by testing the working solutions of salinomycin standard.
Discussion
A wide range of antimicrobial substances with different mechanisms of action are being used in food-producing animals as therapeutic agents to treat diseases or as preventive agents when diseases cannot be eliminated by other means. The endangering of public health associated with the administration of these substances to food-producing animals has increased the need to monitor the residues of these substances in live animals and animal products. The measures to monitor certain substances and residues in live animals and animal products are governed by Council Directive 96/23/EC. These substances are included in Group A as substances having anabolic effect and unauthorised substances and in Group B as veterinary drugs and contaminants. Veterinary drugs are further divided into Group B1 as antibacterials (beta-lactams, tetracyclines, macrolides, aminoglycosides, sulphonamides, quinolones) and Group B2 as other veterinary drugs (antihelmintics/B2a/, anticoccidials including nitroimidazoles/B2b/, carbamates and pyrethroids/B2c/, sedatives/B2d/, non-steroidal anti-inflammatory drugs/B2e/, and other pharmacologically active substances/B2f/) (Council of the European Union Citation1996; Kožárová Citation2018).
It is important to mention that in some EU Member States there are specific control programmes which use microbiological tests (inhibitor tests). In some cases, a positive result in a microbiological test is sufficient to reject the sample. This may mean that no confirmation by a physico-chemical method is carried out and thus there is no conclusive identification of the substance concerned. In other cases, a positive result in the screening test is confirmed by means of an immunochemical or physico-chemical test and it is then possible to identify the substance and establish whether its concentration is above the MRL or not (European Food Safety Authority Citation2019).
The use of the microbial screening tests or methods within the residue detection is a very cost-effective way of reducing the number of samples that need to be analysed with an expensive physico-chemical confirmation. Another important advantage, compared for example to the confirmatory LC-MS systems, is that the microbial inhibition tests can detect any antibiotic or metabolite with antibacterial activity, whereas LC-MS systems are commonly applied to the compounds previously selected as targets, so that any other antibiotics present would pass undetected (Picó and Barceló Citation2008; Cháfer-Pericás et al. Citation2010).
There is a whole range of such tests or methods with various test organisms used worldwide. Their development dates back to the second half of the 20th century and in recent years, significant progress has been seen in this area. The current tests or methods have the capability for a high sample throughput and are used to sift large numbers of samples for the potential non-compliant results. The suitable selection of a respective microbial inhibition test or the use of appropriate tube and plate test combination models constitutes an efficient mean of controlling of a wide range of antimicrobial residues in animal products and foodstuffs. A feasible post-screening confirmatory test using a substance which selectively reserves the inhibitory activity of a respective class of antimicrobials significantly reduces the efforts devoted to the identification and quantitation of the residue by the physico-chemical methods and generates considerable savings of time and resources (Myllyniemi Citation2004; Pikkemaat Citation2009; Cháfer-Pericás et al. Citation2010; Sanz et al. Citation2015).
Many authors, based on the evaluation of the sensitivity and specificity of microbial inhibition tests, confirmed their suitability and practical applicability for the screening of antimicrobial residues in foods of animal origin at the first stage of the residue monitoring and control strategy. A few examples are given bellow. Pikkemaat et al. (Citation2009, Citation2011), in two similar studies, evaluated and compared the performance of a commercial tube Premi®Test and three multi-plate tests, the Four-Plate Test (FPT; Bogaerts and Wolf Citation1980), the STAR method and the Nouws antibiotic test (NAT; Pikkemaat et al. Citation2008) for the screening of antimicrobial residues in meat and kidneys of slaughter animals taken as the part of the national monitoring programme. The authors found that the FPT lacks sufficient sensitivity to be used in the routine monitoring, the Premi®Test showed a slightly better result; however, it exhibits a very high false-positive rate and the STAR method and the NAT appeared to be the most sensitive tests; this reduces the confirmatory efforts. The number of suspect samples detectable by the STAR method and the NAT were comparable. Based on the antibiotic group identification, the samples tested positive were deemed suspect for the presence of tetracyclines, sulphonamides, macrolides, aminoglycosides and beta-lactams. In all samples for which the presence of residues were confirmed, the antibiotic group identification using the STAR method and the NAT appeared correct. In both studies, the suspect samples were always re-tested in the presence and absence of penicillinase for the confirmation of the presence of beta-lactam antibiotics.
The incidence of antimicrobial residues in market muscle samples from different animal species (bovine, ovine, poultry and porcine) was evaluated with a screening strategy that combined two other commercial tube tests, a broad spectrum test Explorer (Zeulab, Zaragoza, Spain) and a specific test for quinolones detection Equinox (Zeulab, Zaragoza, Spain) by Sanz et al. (Citation2015). The authors declared that a combination of the Explorer and the Equinox tests appears to be a useful tool since it would enable a broad screening of antimicrobials, especially quinolones, in the muscle samples. The supplementary tests performed to obtain additional information about the nature of antimicrobials in positive muscle samples confirmed the presence of tetracyclines, aminoglycosides, sulphonamides and quinolones. Similar results were obtained by Gaudin, Hedou, Rault, and Verdon (2009). They reported that the Explorer was able to detect compounds belonging to different antimicrobial families (penicillins, cephalosporins, tetracyclines, sulphonamides and macrolides) in the muscle samples from different species (bovine, porcine, ovine and poultry) with the detection capabilities around the MRL level for the tested antimicrobials.
Gaudin, Hedou, Rault, Sanders, et al. (Citation2009) compared the Explorer and the Premi®Test for the detection of antimicrobial and sulphonamide residues in eggs. The sensitivity of the Premi®Test was better than that of Explorer test for all the tested sulphonamides and the other tested antimicrobials, probably because of the dilution of the eggs before Explorer test, as recommended by the manufacturer. In spite of this finding, the authors recommended to use both tests as the wide screening tests allowing the detection of most of the antimicrobial families in eggs. El Nasri et al. (Citation2012) used the disc assay method with Bacillus stearothermophilus (Fagbamila et al. Citation2010) and the Premi®Test for the detection of antibiotic residues in table eggs. They showed a poor hygiene status of the poultry farms with the concomitant use of antibiotics for the treatment of diseases and the lack of the knowledge regarding the use of antibiotics resulted in the high percentage of positive samples. The Premi®Test detected lower positive percentage of the positive samples while the disc assay correlated well with this excessive use of antibiotics. Shahbazi et al. (Citation2016) assessed the prevalence of drug residues in eggs using the FPT. According to the results of this study, the highest contamination rate of antibiotic residues was related to penicillin and tetracycline groups following aminoglycosides.
There are also a lot of studies evaluating the Bacillus stearothermophilus based tube tests for the routine screening of antibiotic residues in cow, sheep and goat milk (Navrátilová Citation2009; Pikkemaat Citation2009; Sierra et al. Citation2009; Sýkorová Goffová et al. Citation2012; Wu, Zhu, et al. Citation2019). In the case of the milk, the primary control begins on the farm and all food business operators must initiate procedures to ensure that raw milk is not placed on the market if it contains antibiotic residues above the level of EU MRL (European Commission Citation2004). Recently, Wu, Peng, et al. (Citation2019) introduced a novel broad-spectrum microbiological inhibition method for the rapid screening of different kinds of antibiotics such as β-lactam, aminoglycosides, tetracyclines, sulphonamides, macrolides, lincosamides and quinolones in milk, chicken egg and honey by using the microbiological system in microtiter plates with test bacteria Geobacillus stearothermophilus var. C 953. It was observed that the limit of the detection of the kit used in this study for all kinds of antibiotics in milk was lower than or close to the maximum residue limits determined by EU. For chicken egg and honey, the detection capability of the kit was similar to that determined in milk. Moreover, it was revealed that the kit in the present study was more sensitive to aminoglycosides, macrolides and quinolones in various matrixes than internationally available commercial kits. In spite of the fact that the microbial inhibition tests or methods are preferential methods for the screening of antimicrobial residues in foods of animal origin, many authors are convinced that there is still a need for rapid screening methods with a wider spectrum of detection and improved detection capabilities.
All the microbial inhibition tests used in our study characterised the samples as positive or negative. Evaluation of the results of the Premi®Test and the EXP Ampulle tube tests based on the presence or absence of colour change, both tests yielded a comparable number of the positive/dubious results except for 2 chicken kidneys and table eggs mainly in which a higher number of positive results were detected by the EXP Ampulle test. The most positive/dubious results were found in the chicken tissues and eggs. All bovine, ovine and porcine tissue samples with exception of 1 porcine muscle sample yielded negative results. From all chicken matrices examined, the most positive results were found in chicken livers, followed by fats/skins, spleens, gizzards, heart, and finally, kidneys. No positive results were detected in chicken muscles.
Evaluating the results of the STAR method based on the formation of the inhibition zone around the animal post-slaughter matrices exceeding 2 or 4 mm in width dependent on the test organism, the most positive results were found also in chicken matrices. From all chicken matrices, the largest inhibition zones were detected around the chicken spleens followed by livers, kidneys, hearts, fats/skins, gizzards, and finally, muscle (2.37 ± 0.61 mm –12.22 ± 0.12 mm). No inhibition zones were detected around the bovine, ovine and porcine tissue samples with exception of 1 porcine muscle sample, in which the size of the inhibition zone was 4.51 ± 0.54 mm. Evaluating the results of the positive egg and milk samples, the mean diameters of the inhibition zones ranged from 4.29 ± 0.42 to 15.73 ± 0.48 mm and 2.19 ± 0.97 to 6.82 ± 0.69 mm, respectively.
The positive samples were deemed suspect for the presence of beta-lactams or sulphonamides followed by macrolides, tetracyclines and aminoglycosides, respectively. As the highest number of positive results were detected with the Bacillus stearothermophilus var. calidolactis ATCC 10149 test organism very sensitive to beta-lactam antibiotics and sulphonamides, it was deemed necessary to perform the post-screening confirmatory analysis of all these positive samples to make a distinction between beta-lactam antibiotics and sulphonamides by using all tube tests with the use of penicillinase and PABA. The post-screening analysis found a clear distinction between both main classes of antimicrobial substances: beta-lactam antibiotics and sulphonamides; with sulphonamides confirmed only in chicken tissues and eggs.
Taking into account the outcomes of the confirmatory analysis determining the presence of salinomycin in the examined chicken tissue samples and given the sulphonamide positive results after post-screening analysis with PABA, this led us to conduct further analysis to verify whether PABA could actually block the action of salinomycin. Salinomycin is a monocarboxylic polyether ionophore used as an anticoccidial agent in the poultry industries. Salinomycin also exhibits antibacterial activity, especially against Gram-positive bacteria, including various antibiotic-resistant species of Streptomyces. Antifungal, antiparasitic, antiviral, anti-inflammatory, and most recently, anticancer activities have also been reported for salinomycin (Dewangan et al. Citation2017). Our results confirmed the antibacterial activity of salinomycin on Gram-positive bacteria (Bacillus stearothermophilus var. calidolactis, Bacillus cereus ATCC 11778) used as the test organisms of the microbial inhibition tests, which are used as the main method for the screening of antibiotic residues in products of animal origin. Interestingly the results also revealed the unexpected finding that PABA antagonises the antibacterial action of salinomycin. As, until now, there is no existing literature on this fact and no other studies are published for comparison of our results in this subject area, further experimental studies are needed to build on this finding, to understand the principle of the specific interaction between salinomycin and PABA and to verify the effect of PABA on the antibacterial (anticoccidial) activity of the other coccidiostats authorised for food-producing animals.
The residue control in the European Union pursues the primary goal to protect consumers from intolerable health hazards which may be associated with the residues of veterinary drugs or non-licensed or forbidden substances in animal products (Sterk Citation2015). The latest European Food Safety Authority (EFSA) report summarises the monitoring data collected from EU Member States in 2017 (European Food Safety Authority Citation2019). Overall, the percentage of non-compliant samples in 2017 (0.35%) was comparable to the previous 10 years (0.25%–0.37%). 109,260 targeted samples were analysed for substances in the group B1 (antibacterials) of which 284 samples (0.26%) were non-compliant. 111,029 targeted samples were analysed for substances in the group B2 (other veterinary drugs) of which 182 samples (0.16%) were non-compliant. Of a total of samples analysed for substances in the Group B2, 33,151 targeted samples were analysed for substances in the subgroup B2b (anticoccidials) of which 49 samples (0.15%) were non-compliant. Non-compliant samples for antibacterials were reported in bovines (0.31%), ovine/caprine (0.24%), swine (0.3%), poultry (0.1%), milk (0.18%), eqine (0.39%), rabbits (0.44%) and eggs (0.25%). Non-compliant samples for anticoccidials were reported in equine (0.85%), swine (0.01%), poultry (0.21%), rabbits (0.65%) and eggs (0.47%). Salinomycin was e.g. detected in poultry (12), eggs (2) and rabbits (2). Since 2009, an important decrease has been observed in the frequency of non-compliant samples for anticoccidials in poultry. This decrease is most likely the result of increased awareness and the measures that followed as a result of the implementation of the Commission Directive 2009/8/EC which set up maximum levels of unavoidable carry-over of coccidiostats in non-target feed (European Commission Citation2009).
The EU requires by law that foodstuffs such as meat, milk or eggs must not contain residue levels of veterinary medicines or biocidal products that might present a hazard to the health of the consumer (European Medicines Agency Citation2019). The results of the monitoring of residues of antimicrobial substances in live animals and animal products submitted by the Member States to the European Commission every year, the results of research papers and scientific studies published by authors in this field, and finally, the results of our study clearly indicate that monitoring and control of residues of antibiotics and coccidiostats are still necessary in order to verify the safety of products of animal origin and to protect the public health.
Conclusions
Residue control is very important to ensure food safety. Microbial inhibition tests are methods of choice for in the initial screening of antibiotic residues in food of animal origin. In spite of the fact that microbial inhibition tests do not provide the data on sensitivity to coccidiostats, the antibacterial activity of salinomycin allows us to confirm our previous findings and contribute additional evidence to the fact that the microbial inhibition tests which use the test organism Bacillus stearothermophilus var. calidolactis as their test organism are suitable for the effective detection of salinomycin residues in food of animal origin. The spectrum of antimicrobials detectable by the screening methods described is not exhaustive and there is still scope for its further expansion and improvement. The outcomes of our study may also be promising with respect to the unexpected finding that PABA counteracted the inhibition of salinomycin on the test organism Bacillus stearothermophilus var. calidolactis. This means that in addition to beta-lactams and sulphonamides, the presence of coccidiostats should also be taken into account. The two-tier testing system based on the microbial screening and more specific post-screening is proven to be an effective tool in controlling residues of antimicrobial substances in food of animal origin and, equally, is an effective tool in the initial screening of coccidiostat residues in poultry meat and eggs.
Ethics statement
This study does not contain any studies with animals performed by the authors. All procedures used for the sample collection were routine non-invasive procedures.
Disclosure statement
The authors declare that there is no conflict of interest associated with the paper.
Additional information
Funding
References
- Bacanli M, Başaran N. 2019. Importance of antibiotic residues in animal food. Food Chem Toxicol. 125:462–466.
- Barreto F, Ribeiro C, Hoff RB, Dalla Costa T. 2017. A simple and high-throughput method for determination and confirmation of 14 coccidiostats in poultry muscle and eggs using liquid chromatography–quadrupole linear ion trap-tandem mass spectrometry (HPLC–QqLIT-MS/MS): validation according to European Union 2002/657/EC. Talanta. 168:43–51.
- Beltrán MC, Althaus RL, Molina A, Berruga MI, Molina MP. 2015. Analytical strategy for the detection of antibiotic residues in sheep and goat’s milk. Span J Agric Res. 13(1):e0501–e0509.
- Bogaerts R, Wolf F. 1980. A standardised method for the detection of residues of antibacterial substances in fresh meat. Fleischwirtschaft. 60:672–673.
- Cháfer-Pericás C, MTQuieira Á, Puchades R. 2010. Fast screening methods to detect antibiotic residues in food samples. Trends Anal Chem. 29(9):1038–1049.
- Clarke L, Fodey TL, Crooks SRH, Moloney M, O'Mahony J, Delahaut P, O'Kennedy R, Danaher M. 2014. A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci. 97(3):358–374.
- Council of the European Union. 1996. Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products. Off J Eur Union. L125:10–32.
- Danaher M, Shanahan C, Butler F, Evans R, O’Sullivan D, Glynn D, Camon T, Lawlor P, O’Keeffe M. 2016. Risk based approach to developing a national residue sampling plan for testing under European Union regulation for veterinary medicinal products and coccidiostat feed additives in domestic animal production. Food Addit Contam Part A. 33(7):1155–1165.
- Dasenaki ME, Thomaidis NS. 2019. Multi-residue methodology for the determination of 16 coccidiostats in animal tissues and eggs by hydrophilic interaction liquid chromatography–Tandem mass spectrometry. Food Chem. 275:668–680.
- Dewangan J, Srivastava S, Rath SK. 2017. Salinomycin: a new paradigm in cancer therapy. Tumour Biol. 39(3):101042831769503–101042831769512.
- El Nasri HA, Salman AM, Osman IA. 2012. Detection of antibiotic residues in table eggs using disc assay and Premi test in Khartoum state, Sudan. J Vet Med Anim Prod. 3:16–27.
- European Commission. 2002. Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Union. L221:8–36.
- European Commission. 2004. Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off J Eur Union. L139:14–74.
- European Commission. 2009. Commission Directive 2009/8/EC of 10 February 2009 amending Annex I to Directive 202/32/EC of the European Parliament and of the Council as regards maximum levels of unavoidable carry-over of coccidiostats or histomonostats in non-target feed. Off J Eur Union. L40:19–25.
- European Commission. 2010. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union. L15:1–72.
- European Commission. 2014. Commission Implementing Regulation (EU) 2017/1914 of 19 October 2017 concerning the authorisation of salinomycin sodium (Sacox 120 microGranulate and Sacox 200 microGranulate) as a feed additive for chickens for fattening and chickens reared for laying and repealing Regulations (EC) No 1852/2003 and (EC) No 1463/2004. Off J Eur Union. L271:1–6.
- European Food Safety Authority. 2019. Report for 2017 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA supporting publication 2019:EN-1578. p. 88.
- European Medicines Agency. 2019. Maximum residue limits. [accessed 2019 December 5]. https://www.ema.europa.eu/en/veterinary-regulatory/research-development/maximum-residue-limits-mrl.
- European Parliament and the Council of the European Union. 2009. Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No. 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council Regulation (EC) No. 726/2004 of the European Parliament and the Council. Off J Eur Union. L152:11–22.
- Ezenduka EV, Ike OS, Anaelom NJ. 2014. Rapid detection of antimicrobial residues in poultry: a consequence of non-prudent use of antimicrobials. Health. 6(2):149–152.
- Fagbamila I, Kabir J, Abdu P, Omeiza G, Ankeli P, Ngulukun S, Muhammad M, Umoh J. 2010. Antimicrobial screening of commercial eggs and determination of tetracycline residue using two microbiological methods. Int J Poult Sci. 10:959–962.
- Ferrini AM, Mannoni V, Aureli P. 2006. Combined Plate Microbial Assay (CPMA): a 6-plate-method for simultaneous first and second level screening of antibacterial residues in meat. Food Addit Contam. 23(1):16–24.
- Gaudin V, Hedou C, Rault A, Sanders P, Verdon E. 2009. Comparative study of three screening tests, two microbiological tube tests, and a multi-sulphonamide ELISA kit for the detection of antimicrobial and sulphonamide residues in eggs. Food Addit Contam Part A. 26(4):427–440.
- Gaudin V, Hedou C, Rault A, Verdon E. 2010. Validation of Five Plate Tets, the STAR protocol, for the screening of antibiotic residues in muscle from different animal species according to European Decision 2002/657/EC. Food Addit Contam Part A. 27(7):935–952.
- Gaudin V, Hedou C, Verdon E. 2009. Validation of a wide-spectrum microbiological tube test, the Explorer® test, for the detection of antimicrobials in muscle from different animal species. Food Addit Contam Part A. 26(8):1162–1171.
- Gaudin V, Juhel-Gaugain M, Morétain JP, Sanders P. 2008. AFNOR validation of Premi®Test, a microbiological-based screening tube-test for the detection of antimicrobial residues in animal muscle tissue. Food Addit Contam. 25(12):1451–1464.
- Gaudin V, Maris P, Fuselier R, Ribouchon JL, Cadie N, Rault A. 2004. Validation of a microbiological method: the STAR protocol, a five-plate test, for the screening of antibiotics residues in milk. Food Addit. Contam. 21(5):422–433.
- Gaudin V, Rault A, Hedou C, Soumet C, Verdon E. 2017. Strategies for the screening of antibiotic residues in eggs: comparison of the validation of the classical microbiological method with an immunobiosensor method. Food Addit Contam Part A. 34:510–1527.
- Gondová Z, Kožárová I, Poláková Z, Maďarová M. 2014. Comparison of four microbiological inhibition tests for the screening of antimicrobial residues in the tissues of food-producing animals. Ital J Anim Sci. 13:728–734.
- Gondová Z, Kožárová I. 2012. The NAT test – screening for antibiotic residues in the tissues of food-producing animals. Maso Int. 4:200–202.
- Granados-Chinchilla F, Rodríguez C. 2017. Tetracyclines in food and feedingstuffs: From regulation to analytical methods, bacterial resistance, and environmental and health implications. J Anal Methods Chem. 2017:1–24.
- Hagren V. 2009. Food safety testing: rapid molecular methods for chemical and biological hazards [Thesis]. Turku: Painosalama Oy; [accessed 2019 August 28]. https://pdfs.semanticscholar.org/cbe8/fc1d23cab61e8cdeab4ef5bc30127358fc34.pdf.
- Kožárová I, Janošová J, Máté D, Tkáčiková S. 2009. Evaluation of different microbial inhibition tests for the detection of sulphamethazine residues in the edible tissues of rabbits. Food Addit Contam Part A. 26(7):978–987.
- Kožárová I, Mačanga J, Goldová M, Major P, Koréneková B. 2009. Comparative study of detection of the presence of selected coccidiostats in the tissues of chickens and pheasants. Potravinarstvo. 2:40–44.
- Kožárová I, Mačanga J, Goldová M, Major P, Tkáčiková S. 2011. Detection of maduramycin residues in the tissues of chickens and pheasants by screening test for antibiotic residues (STAR). Food Addit Contam Part A. 28(5):608–618.
- Kožárová I, Máté D, Cabadaj R, Różańska H, Hussein K, Laciaková A. 2002. An evaluation of the microbiological diffusion methods as a tool for screening monensin residues in the tissues of broiler chickens. Folia Veterinaria. 46:27–33.
- Kožárová I, Máté D. 2000. Evaluation of the sensitivity of individual test organisms to residual concentrations of selected types of anticoccidial drugs. B Vet I Pulawy. 44:187–192.
- Kožárová I, Šimková J, Mártonová M, Mačanga J, Levkut M. 2011. Detection of lasalocid residues in the tissues of broiler chickens by a new screening test Total antibiotics. Potr. 5(2):45–48.
- Kožárová I. 2018. Antibiotic residues in poultry products – the current state of the results of residue monitoring in the European Union. Proceedings of Lectures and Posters of the International Scientific Conference “Hygiena Alimentorum XXXVI” Safety and quality of poultry meat, eggs, fishery products and game meat, May 16–18, 2018. Košice: UVMP in Košice; p. 176–180.
- Myllyniemi AL. 2004. Development of microbiological methods for the detection and identification of antimicrobial residues in meat [Dissertation].Helsinki: Fakulty of Veterinary Medicine, p. 87.
- Navrátilová P. 2009. Screening methods used for the detection of veterinary drug residues in raw cow milk–a review. Czech J Food Sci. 26(6):393–401.
- Olejnik M, Szprengier-Juszkiewicz T, Jedziniak P, Śledzińska E, Szymanek-Bany I, Korycińska B, Pietruk K, Zmudzki J. 2011. Residue control of coccidiostats in food of animal origin in Poland during 2007–2010. Food Addit Contam. Part B. 4(4):259–267.
- Olejnik M, Szprengier-Juszkiewicz T, Jedziniak P. 2009. Multi-residue confirmatory method for the determination of twelve coccidiostats in chicken liver using liquid chromatography tandem mass spectrometry. J Chromatogr A. 1216(46):8141–8148.
- Picó Y, Barceló D. 2008. The expanding role of LC-MS in analyzing metabolites and degradation products of food contaminants. Trends Anal Chem. 27(10):821–835.
- Pietruk K, Olejnik M, Posyniak A. 2018. Coccidiostats in milk: development of a multi-residue method and transfer of salinomycin and lasalocid from contaminated feed. Food Addit Contam Part A. 35(8):1508–1518.
- Pikkemaat MG, Dijk SO, Schouten J, Rapallini M, Egmond HJ. 2008. A new microbial screening method for the detection of antimicrobial residues in slaughter animals: the Nouws antibiotic test (NAT-screening). Food Control. 19(8):781–789.
- Pikkemaat MG, Rapallini MLBA, Dijk S. O-v, Elferink JWA. 2009. Comparison of three microbial screening methods for antibiotics using routine monitoring samples. Anal Chim Acta. 637(1–2):298–304.
- Pikkemaat MG, Rapallini M, Zuidema T, Elferink JWA, Oostra-Van Dijk S, Driessen-Van Lankveld W. 2011. Screening methods for the detection of antibiotic residues in slaughter animals: comparison of the European Union Four- Plate Test, the Nouws Antibiotic Test and the Premi®Test (applied to muscle and kidney. Food Addit. Contam Part A. 28(1):26–34.
- Pikkemaat MG. 2009. Microbial screening methods for detection of antibiotic residues in slaughter animals. Anal Bioanal Chem. 395(4):893–905.
- R–25. 2013. Screening test for determination of antibiotic residues using five bacterial strain (STAR method). List of official methods for laboratory diagnostic of food and feed. State Veterinary and Food Administration of the Slovak Republic. [accessed 2017 April 25]. https://www.svps.sk/dokumenty/zakladne_info/R_25.pdf. (Slovak)
- R–26. 2013. Determination of residues of inhibitory substances in meat by the Premi®Test. List of official methods for laboratory diagnostic of food and feed. State Veterinary and Food Administration of the Slovak Republic. [accessed 2017 April 25]. https://www.svps.sk/dokumenty/zakladne_info/R_26.pdf. (Slovak)
- Radko L, Cybulski W. 2019. The decrease of lasalocid residue in the edible tissues by silymarin supplementation of chicken diet. Food Addit Contam Part A. 36(5):722–728.
- Roila R, Branciari R, Pecorelli I, Cristofani E, Carloni C, Ranucci D, Fioroni L. 2019. Occurrence and residue concentration of coccidiostats in feed and food of animal origin. Hum Expo Assess Foods. 8(10):477–416.
- Samandoulougou S, Ilboudo AJ, Bagre TS, Tapsoba FW, Savadogo A, Scippo ML, Traore AS. 2015. Screening of antibiotic residues in beef consumed in Ouagadougou, Burkina Faso. Afr J Food Sci. 9:367–371.
- Sanz D, Razquin P, Condón S, Juan T, Juan T, Herraiz B, Mata L. 2015. Incidence of antimicrobial residues in meat using a broad spectrum screening strategy. EJNFS. 5(3):156–165.
- Serraino A, Giacometti F, Marchetti G, Zambrini AV, Zanirato G, Fustini M, Rosmini R. 2013. Survey on antimicrobial residues in raw milk and antimicrobial use in dairy farms in the Emilia-Romagna region, Italy. Ital J Anim Sci. 12:422–425.
- Shahbazi Y, Hashemi M, Afshari A, Karami N. 2016. A survey of antibiotic residues in commercial eggs in Kermanshah. Iran. Iran J Veterinary Sci Technol. 7:57–62.
- Sierra D, Sánchez A, Contreras A, Luengo C, Corrales JC, Morales CT, de la Fe C, Guirao I, Gonzalo C. 2009. Detection limits of four antimicrobial residue screening tests for β-lactams in goat’s milk. J Dairy Sci. 92(8):3585–3591.
- Sophila JR, Raj GD, Kumanan K, Chandra GS, Vairamuthu S. 2018. Microbial inhibition assay for detection of antibiotic residues in chicken meat using vegetative form of Geobacillus stearothermophilus. Pharma Innov J. 7:753–757.
- Stead S, Sharman M, Tarbin JA, Gibson E, Richmond S, Stark J, Geijp E. 2004. Meeting maximum residue limits: an improved screening technique for the rapid detection of antimicrobial residues in animal food products. Food Addit Contam. 21(3):216–221.
- Stead S, Stark J. 2012. Bioanalytical screening methods. In: Wang J, MacNeil JD, Kay JF, editors. Chemical analysis of antibiotic residues in food. Hoboken, NJ: John Wiley & Sons, Inc.; p. 153–164.
- Sterk SS. 2015. Residue control in the European Union, the present and future challenges: experiences from the Netherlands. Procedia Food Sci. 5:278–281.
- Sýkorová Goffová Z, Kožárová I, Máté D, Marcinčák S, Gondová Z, Sopková D. 2012. Comparison of detection sensitivity of five microbial inhibition tests for the screening of aminoglykozide residues in fortified milk. Czech J Food Sci. 30(4):314–320.
- Tkáčiková S, Kožárová I, Mačanga J, Levkut M. 2012. Determination of lasalocid residues in the tissues of broiler chickens by liquid chromatography tandem mass spectrometry. Food Addit Contam Part A. 29(5):761–769.
- Tkáčiková S, Kožárová I, Máté D. 2010. Liquid chromatography tandem chromatography- tandem mass spectrometry determination of maduramycin residues in the tissues of broiler chickens. Food Addit Contam Part A. 27(9):1226–1232.
- Wang J, MacNeil JD, Kay JF. 2012. Chemical analysis of antibiotic residues in food. Hoboken, NJ: John Wiley & Sons, Inc. p. 384.
- Wu Q, Peng D, Liu Q, Shabbir MAB, Sajid A, Liu Z, Yuan Z. 2019. A novel microbiological method in microtiter plates for screening seven kinds of widely used antibiotics residues in milk, chicken egg and honey. Front Microbiol. 10:1–14.
- Wu Q, Zhu Q, Liu Y, Shabbir MAB, Sattar A, Peng D, Tao Y, Chen D, Wang Y, Yuan Z. 2019. A microbiological inhibition method for the rapid, broad-spectrum, and high-throughput screening of 34 antibiotic residues in milk. J Dairy Sci. 102(12):10825–10837.
