 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present study aims to measure the sarcomere lengths in normal broiler muscles and in non-lesion sites of breast muscles focally affected by Wooden Breast (WB). For this purpose, twenty Pectoralis major muscles (10 unaffected and 10 WB-focally affected cases) were sampled and used to measure sarcomere length by laser diffraction method. When compared with their unaffected counterpart, WB cases exhibited 13% longer sarcomeres (1.91 vs. 1.69 μm; p < .001) measured within the non-lesioned site of the muscle. Although it is not simple to draw conclusions about the lesion properties based on the non-lesion area, but as the fibres are bound to each other, it may be reasonable to anticipate that the hardened consistency observed in WB is not ascribable to a more intense contraction of the sarcomeres. In addition, considering the current knowledge concerning this condition, it might be assumed that the longer sarcomeres observed in WB are not triggering the development of this condition but are rather a consequence of the profound alteration in the muscular structure resulting from it. Indeed, despite the outstanding improvements in the live and slaughtering traits, the selection programmes carried out in the past years have resulted in a reduced capillarization and impaired oxygen supply to the Pectoralis major of fast-growing hybrids thus affecting the physiology of its constituting fibres as well as maybe impairing their ability to synthetise new sarcomeres. This may result in a skeletal muscle injury, which would ultimately lead to necrosis and fibrosis.
Wooden Breast is not likely associated to muscle hypercontraction but likely a result of an increased muscular strain;
Similar traits observed in myopathic muscles in human support the hypothesis of a similar underlying mechanism.
HIGHLIGHTS
Introduction
Wooden Breast (WB) condition is commonly observed the Pectoralis major muscles of the fast-growing genotypes developed to obtain high growth rate and breast yield. Macroscopically, the WB-affected muscles exhibit a hardened consistency and pale colour whereas their distinctive microscopic traits include myodegeneration, up to necrosis, and the presence of occasional regenerative processes (Sihvo et al. Citation2014; Petracci et al. Citation2019). In addition, in severe WB cases, connective tissue proliferates within the endomysial and perimysial compartments, ultimately resulting in fibrosis (Sihvo et al. Citation2014; Velleman and Clark Citation2015).
Although raw WB fillets exhibit an increased hardness assessed by both palpation and texture analyses (Sihvo et al. Citation2014), a tendency for a progressive tenderisation process was observed during storage (Soglia et al. Citation2017; Gratta et al. Citation2019). This suggests a possible contribution of the fibrillar structure to the distinctive hardness of WB muscles. In this regard, a couple of studies have been recently carried out by Tijare et al. (Citation2016), Sun et al. (Citation2018) and Velleman et al. (Citation2018) to evaluate whether the occurrence of WB affects the sarcomeric structure and verify if an eventual contraction of sarcomeres may be responsible for the hardened consistency of WB-affected muscles. Those studies have been carried out taking into consideration only the cranial portion of the P. major that is more severely affected by this condition. Thus, the present study aims to assess the sarcomere lengths in both unaffected P. major muscles and in the non-lesion site of focally WB-affected P. major muscles. This would allow us to get information concerning the relationship of focal WB with the sarcomeric structure in those areas of the muscle that did not exhibit any macroscopic features of WB, and hence try to understand whether the longer sarcomeres found in WB are a consequence of this condition or rather involved in its development.
Materials and methods
At a commercial slaughterhouse, 39-day old Ross 508 broiler chickens belonging to the same flock intensively farmed, were slaughtered, chilled to 2 °C and the breast fillets cut off. At 3 h post-mortem, two experienced people were responsible for selecting in total 20 P. major muscles, according to their visual appearance and manual palpation: 10 WB cases, exhibiting focally hardened consistency and pale colour, and 10 unaffected muscles of normal consistency and without any macroscopic features associated with WB. In addition, in order to avoid any interference, muscles affected by other defects (i.e. white striping, abnormal colour) were not included in this study. The muscles were kept at 0–2 °C until sampling in the medial area of each fillet (macroscopically the non-lesion site of the focally lesioned WB muscles) at 10 h post-mortem. All samples, 1 × 1 × 1 cm in size, were excised from the ventral surface of the fillet (facing the skin), excluding the most superficial 5 mm.
The samples were prepared following the procedure described by Cross et al. (Citation1981) with minor modifications (Liu et al. Citation2014). Briefly, 10 ml of formalin solution (35 g/L formaldehyde in 85 mM phosphate buffer) were added to 0.6 g of P. major muscle and homogenised for 30 s with a Potter-Elvehjem-Type Tissue Grinder with PTFE Pestle (Tomas Scientific, Swedesboro, NJ). A drop of the homogenate was placed under a Novette 1570-0 Helium-neon gas laser (Uniphase, Manteca, CA) and the projected laser diffraction band was measured. A total of 20 measurements were performed per sample (4 drops, 5 measurements/drop). Then, sarcomere length was calculated by the means of the following equation:
where SL represents sarcomere length (μm), D the distance from the specimen-holding device to the screen (mm), and T the width between the diffraction bands (mm) (Cross et al. Citation1981).
The data were analysed by the Shapiro-Wilk test for normality. The difference in sarcomere length between the unaffected and WB cases was tested with the Student’s t-test. All the statistical tests were performed using SAS Software (SAS Institute Inc., Cary, NY).
Results and discussion
When compared with their unaffected counterpart, WB cases exhibited 13% longer sarcomeres (1.91 vs. 1.69 μm; p < .001) measured within the non-lesion site of the muscles (Figure ). In previous studies the mean sarcomere lengths in broiler breast muscles have varied between 1.61 and 1.80 μm, and hence the sarcomere lengths we found in the unaffected muscles are in agreement with those observed earlier (Papinaho et al. Citation1996; Wattanachant et al. Citation2005; An et al. Citation2010). Thus, although it is not simple to draw conclusions about the lesion properties based on the non-lesion area, as the fibres are bound to each other, it may be reasonable to anticipate that the hardened consistency observed in WB is not ascribable to a more intense contraction of the sarcomeres. Overall, this is in agreement with the results of Tijare et al. (Citation2016) and Sun et al. (Citation2018) on muscles affected by WB, but on the contrary, Velleman et al. (Citation2018) did not find significant differences in sarcomere lengths between normal and WB muscles. Tijare et al. (Citation2016) and Sun et al. (Citation2018) speculated that the longer sarcomeres observed in WB might be the result of the increased collagen content and/or of the necrosis found in these muscles, which would prevent their post-mortem shortening. Overall, the longer sarcomeres in WB suggest that the mechanism responsible for the hardening and stiffening associated with the development of this condition differs from the traditional rigour state of the muscle, in which the hardness is induced by contraction (Marsh and Leet Citation1966; Wheeler and Koohmaraie Citation1994; Marsh and Carse Citation2007). Within this context, the longer sarcomeres we observed in the non-lesion site of WB suggest that the mechanism responsible for their development may be partly associated to the degenerative processes taking place within the cranial portion of the muscle (more severely and primarily affected by this condition), and partly related to factors involving the physiology of the whole muscle itself. Therefore, trying to explain the longer sarcomeres found in WB, different aspects including both the posture of the birds and the position of the wings as well as the physiology of the WB muscle should be carefully considered. In detail, the impressive development of the pectoral muscles obtained through the selection programmes carried out during the past fifty years remarkably affected the posture of the birds as well as the architecture and metabolism of their P. major muscles (Petracci et al. Citation2019). Within this context, Kawasaki et al. (Citation2016) investigated the standing position of both unaffected and WB-affected birds belonging to the fast-growing genotypes. These authors demonstrated that WB-affected broilers exhibit a bent-forward posture with the wings being outstretched to achieve the balance. Thus, as the P. major represents the main adductor muscle of the humerus, when the birds outstretch their wings to adjust the centre of gravity indirectly induce the development of longer sarcomeres. This stretching of the sarcomeric structure may be induced by the position of the wings and may contribute to explain the longer sarcomeres we observed in WB. In addition, the position of the wings after cooling will also have an effect on the sarcomere lengths measured. Finally, the passive tension of the damaged muscle may contribute the longer sarcomere lengths to some extent as well.
Figure 1. Sarcomere length measured in the unaffected and the non-lesioned site of the Wooden Breast-affected (WB) muscles by laser diffraction method (N = 20; 10 muscles/group).
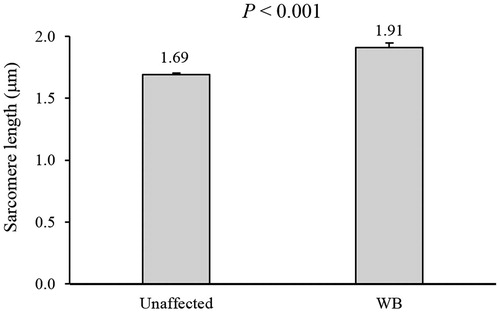
Our results on WB are also in accordance with the electron microscopic study of Ashmore et al. (Citation1988) on Patagialis muscle dystrophy in chickens in which the affected cases exhibited 22% and 25% longer sarcomeres than the controls at 2 and 8 weeks of age, respectively. These authors suggested that in dystrophic muscles the alteration of sarcomere lengths might be the result of an increased passive tension in the myofibers during the growth period. Indeed, since the addition of new sarcomeres at the end of an existing myofiber is stimulated by the passive tension arising from the longitudinal growth of bones, this phenomenon exerts an important role in normal muscle physiology (Goldspink Citation1971). Based on the results obtained in the present study, we hypothesise that the distinctive histological features of WB might also contribute to explain their longer sarcomeres. In detail, the intense degenerative processes taking place in WB induce profound modifications of the muscular architecture including the detachment of the fibres and a remarkable proliferation of connective tissue, up to fibrosis (Sihvo et al. Citation2014; Sihvo et al. Citation2017; Papah et al. Citation2017). Although the exact mechanism and the sequence of events responsible for the development of WB are not fully understood, its microscopic features are hypothesised to be a direct consequence of the hypertrophic growth of the fibres which represents a distinctive trait in the fast-growing genotypes (Petracci et al. Citation2019). This phenomenon, resulting in a reduced capillarization, as recently demonstrated by Sihvo et al. (Citation2018), and impaired oxygen supply to the P. major muscle may lead to an overall altered physiology of the fibres as well as to an impaired ability of the muscle to synthetise new sarcomeres. This would mean that the sarcomeres of the WB fibres are under strain and thus longer. The same hypothesis has been previously proposed by Smith et al. (Citation2011) to explain the longer sarcomeres observed in gracilis and semitendinosus muscles of children affected by cerebral palsy. In detail, these affected muscles having longer sarcomeres concomitantly exhibited a distinctive stiffening and an increased collagen content and extracellular matrix (ECM) development (Smith et al. Citation2011). Intriguingly these macroscopic and microscopic features overlap with those observed in WB-affected muscles thus suggesting that a similar mechanism might underlie the development of the longer sarcomeres in WB. In detail, in stretched muscles, an increased deposition of collagen and ECM has been considered as a compensatory mechanism to reinforce their weakened muscular structure (Smith et al. Citation2011). Thus, the longer sarcomeres measured in WB might suggest an increased strain which has been previously shown to associate to the development of skeletal muscle injury and necrosis of the fibres ultimately resulting in increased collagen content and fibrosis (Patel et al. Citation2004; Stauber Citation2004).
The regenerative processes should restore the necrotic fibres by inducing satellite cells proliferation and differentiation (Velleman Citation2019). However, a significant reduction in satellite cells’ number and an impairment of their ability to proliferate and differentiate has been demonstrated in fast-growing broilers (Clark and Velleman Citation2016), thus suggesting an inhibition of the repair process. This hypothesis is further corroborated by the increased expression of myostatin and TGF-β measured in WB-affected muscles if compared with the macroscopically unaffected cases (Velleman and Clark Citation2015; Brothers et al. Citation2019). Indeed, myostatin and TGF-β exert a relevant role in stimulating the proliferation of muscle fibroblasts, in inducing the shift of the satellite cells from a myogenic lineage to fibroblasts and thus influence the release of ECM proteins (Li et al. Citation2004; Li et al. Citation2008). Thus, within this context, it might be speculated that the hardened consistency, which represents the distinctive trait of WB, might be the result of fewer numbers of sarcomeres that are overly stretched rather than contracted. The sarcomere lengths of the lesion sites are probably less relevant, as the fibres are variably loose and thus rather free to contract if still able to function normally. The WB phenomenon is usually accompanied by an increased deposition of collagen and ECM (to reinforce the muscular structure) that is the main load-bearing structure within the muscle (Purslow Citation1989). Increased collagen and ECM might be responsible for the distinctive hardening associated with the development of this condition, especially in later period of the syndrome.
Conclusions
In conclusion, focal WB muscles exhibited in their non-lesion site longer sarcomeres when compared with their unaffected counterparts. It might be assumed that the longer sarcomeres observed in WB are not triggering the development of this condition but are rather a consequence of the profound alteration in the muscular structure resulting from it. Indeed, despite the outstanding improvements in the live and slaughtering performances, especially the selection programmes for high breast yield in modern hybrids carried out in the past years has resulted in a reduced capillarization and impaired oxygen supply to the P. major thus affecting the physiology of its constituting fibres as well as maybe impairing their ability to synthetise new sarcomeres.
Ethics approval
All farming procedures followed the Council Directive 98/58/EC concerning the protection of animals kept for farming purposes, Council Directive 2008/120/EC and Council Directive 43/2007/EC laying down minimum rules for the protection of chickens kept for meat production. Animal transport was performed according to Council Regulation (EC) No 1/2005, slaughter was performed in accomplishment with the Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing as indicated in the Regulation (EU) 2017/625 of the European Parliament. The samples used for the present study were gathered from carcases intended for meat consumption. The research did not involve any experiment on animals and for this reason, no ethics approval was necessary.
Author contributions
FS, MP and EP conceived and designed the experiment. FS performed the experiment, FS and analysed the data. FS and EP wrote the paper. MP and EP edited and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank Dr. Vet. Med. Hanna-Kaisa Sihvo for her help in the design and methodological aspects of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
All data generated or analysed are available from the corresponding author on request.
References
- An JY, Zheng JX, Li JY, Zeng D, Qu LJ, Xu YG, Yang N. 2010. Effect of myofiber characteristics and thickness on meat tenderness of chickens. Poult Sci. 89(8):1750–1754.
- Ashmore CR, Mechling K, Lee YB. 1988. Sarcomere length in normal and dystrophic chick muscles. Exp Neurol. 101(2):221–227.
- Brothers B, Zhu Z, Papah MB, Abasht B. 2019. RNA-Seq analysis reveals spatial and sex differences in pectoralis major muscle of broiler chickens contributing to difference in susceptibility to wooden breast disease. Front Physiol. 10:764.
- Clark DL, Velleman SG. 2016. Spatial influence on breast muscle morphological structure, myofiber size and gene expression associated with the wooden breast myopathy in broilers. Poult Sci. 95(12):2930–2945.
- Cross HR, West RL, Dutson TR. 1981. Comparison of methods for measuring sarcomere length in beef semitendinosus muscle. Meat Sci. 5(4):261–266.
- Goldspink G. 1971. Changes in striated muscle fibers during contraction and growth with particular reference to myofibril splitting. J Cell Sci. 9:123–129.
- Gratta F, Fasolato L, Birolo M, Zomeño C, Novelli E, Petracci M, Pascual A, Xiccato G, Trocino A. 2019. Effect of breast myopathies on quality and microbial shelf life of broiler meat. Poult Sci. 98(6):2641–2651.
- Kawasaki T, Yoshida T, Watanabe T. 2016. Simple method for screening the affected birds with remarkably hardened pectoralis major muscles among broiler chickens. Jpn Poult Sci. 53(4):291–297.
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. 2004. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 164(3):1007–1019.
- Li ZB, Kollias HD, Wagner KR. 2008. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 283(28):19371–19378.
- Liu J, Ruusunen M, Puolanne E, Ertbjerg P. 2014. Effect of pre-rigor temperature incubation on sarcoplasmic protein solubility, calpain activity and meat properties in porcine muscle. LWT - Food Sci Technol. 55(2):483–489.
- Marsh BB, Carse WA. 2007. Meat tenderness and the sliding-filament hypothesis. J Food Technol. 9(2):129–139.
- Marsh BB, Leet NG. 1966. Studies in meat tenderness. III. The effects of cold shortening on tenderness. J Food Science. 31(3):450–459.
- Papah MB, Brannick EM, Schmidt CJ, Abasht B. 2017. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of Wooden Breast Disease in modern broiler chickens. Avian Pathol. 46(6):623–643.
- Papinaho PA, Ruusunen M, Suuronen T, Fletcher DL. 1996. Relationship between muscle biochemical and meat quality properties of early deboned broiler breasts. J Appl Poult Res. 5(2):126–133.
- Patel TJ, Das R, Friden J, Lutz GJ, Lieber RL. 2004. Sarcomere strain and heterogeneity correlate with injury to frog skeletal muscle fiber bundles. J Appl Physiol. 97(5):1803–1813.
- Petracci M, Soglia F, Madruga M, Carvalho L, Elza I, Estévez M. 2019. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr Rev F Sci F Saf. 18(2):565–583.
- Purslow PP. 1989. Strain-induced reorientation of an intramuscular connective tissue network: implications for passive muscle elasticity. J Biomech. 22(1):21–31.
- Sihvo HK, Airas N, Lindén J, Puolanne E. 2018. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy. J Comp Pathol. 161:1–10.
- Sihvo HK, Immonen K, Puolanne E. 2014. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet Pathol. 51(3):619–623.
- Sihvo HK, Linden J, Airas N, Immonen K, Valaja J, Puolanne E. 2017. Wooden breast myodegeneration of the Pectoralis major muscle over the growth period in broilers. Vet Pathol. 54(1):119–128.
- Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. 2011. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 589(10):2625–2639.
- Soglia F, Gao J, Mazzoni M, Puolanne E, Cavani C, Petracci M, Ertbjerg P. 2017. Superficial and deep changes of histology, texture and particle size distribution in broiler wooden breast muscle during refrigerated storage. Poult Sci. 96(9):3465–3472.
- Stauber WT. 2004. Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposures. J Electromyogr Kinesiol. 14(1):61–70.
- Sun X, Koltes DA, Coon CN, Chen K, Owens CM. 2018. Instrumental compression force and meat attribute changes in woody broiler breast fillets during short-term storage. Poult Sci. 97(7):2600–2606.
- Tijare VV, Yang FL, Kuttappan VA, Alvarado CZ, Coon CN, Owens CM. 2016. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult Sci. 95(9):2167–2173.
- Velleman S, Clark DL. 2015. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 59(3):410–418.
- Velleman SG, Clark DL, Tonniges JR. 2018. The effect of wooden breast myopathy on sarcomere structure and organization. Avian Dis. 62(1):28–35.
- Velleman SG. 2019. Recent developments in breast muscle myopathies associated with growth in poultry. Annual Rev Anim Biosci. 7:1–20.
- Wattanachant S, Benjakul S, Ledward DA. 2005. Microstructure and thermal characteristics of Thai indigenous and broiler chicken muscles. Poult Sci. 84(2):328–336.
- Wheeler TL, Koohmaraie M. 1994. Prerigor and postrigor changes in tenderness of ovine Longissimus muscle. J Anim Sci. 72(5):1232–1238.
