Abstract
High-intensity ultrasound has been reported as a novel technology applied to improve tenderness in beef, pork, lamb and poultry. Its potential benefit has been studied mostly in individual muscles. This study aimed to evaluate the effect of high-intensity ultrasound (HIU, 24 kHz, 12 W/cm2 for 15 min) on quality traits of whole rabbit carcases. Twenty rabbit carcases were longitudinally split into two sides along the vertebral column. One side of the carcase was kept as Control and the opposed was vacuum packed and ultrasonicated with a probe Hielscher® UP400St. After treatment and 7 d (2 °C) in vacuum packs, m. L. dorsi (LD), Semimembranosus and Semitendinosus were dissected from the carcases to evaluate water holding capacity (WHC), shear force, pH, and colour (L*, a* and b*). Promising results were observed in LD after ultrasonication. LD shear force was reduced (p < .05) from 13.04 to 11.18 N (control vs. sonicated, respectively). The colour was also modified (p < .05), increasing L* from 46.70 to 48.30 units and increasing b* from 4.70 to 6.10 units (control vs. sonicated, respectively). WHC, was reduced (p < .05) by HIU only in loin. The pH, and a* from LD were not modified by ultrasonication (p > .05). In conclusion, HIU applied to rabbit carcases positively influenced LD characteristics, but no changes were observed in leg muscles under this study conditions. The data suggest that the effect of the HIU treatment on the whole carcase may differ according to the muscles.
High intensity ultrasound has been applied to rabbit carcases as a small-scale model for other species.
Ultrasonicating vacuum packed carcases of rabbit may lead to different changes of physicochemical characteristics in particular muscles.
High-intensity ultrasonication increases L*, a* and tenderness in L. dorsi but it does not affect Semimembranosus-Semitendinosus.
High-intensity ultrasound applied to carcases does not have the same effect in all muscles.
Highlights
Introduction
Ultrasound is a form of energy generated by waves with a vibration frequency above the audible limit for humans (20 kHz). The high intensity ultrasound (HIU) is pressure waves and/or stressed waves. They are time-dependent and range between 20 KHz and 1 GHz in the acoustic spectrum. HIU has been reported to improve quality traits of beef, lamb, pork and poultry (Alarcon-Rojo et al. Citation2019). The HIU substantially reduces microbial loads because acoustic cavitation disrupt cell walls, resulting in the destruction of living cells and thereby contributing to food preservation (Dacheng Kang et al. Citation2017). Tenderness is one of the most important characteristic of meat improved by HIU, either applied in fresh meat (Gómez-Salazar et al. Citation2018) or during cooking (Zou et al. Citation2018). It has been observed that the application of HIU of meat after storage affects textural and microstructural properties and accelerates aging without negative impacts on other technological and sensory attributes (Peña-Gonzalez et al. Citation2019). HIU has demonstrated to regulate calpain activation and structural protein degradation of muscle in post-mortem stages (Wang et al. Citation2018). The majority of the studies on HIU have been developed ultrasonicating isolated muscles of different species. Information about the effect of HIU on primary cuts, sections of meat involving different muscles, or whole carcases, is limited.
Rabbit (Oryctolagus cuniculus) meat is considered one of the ‘healthy’ meats, even a functional food, since it is valued for its great nutritional and dietetic properties. It is a lean meat with a low fat content, and specifically, a lower content of saturated fatty acids and cholesterol when compared to other meats (Dalle Zotte and Szendrő Citation2011; Cullere and Dalle Zotte Citation2018).
This study intends to be a starting point to evaluate the effect of ultrasonication on rabbit meat as a small model. Because the use of HIU on whole carcases is scarce, the present design was regarded as an exploratory study of in-line ultrasonication processing of carcases and its effect on different muscles (regions). This study was conceived as an approach for future in-line implementation for carcases of bigger animal species. Based on previous results an ultrasound frequency and intensity of 24 kHz and 12 W/cm2 seem to be promising parameters to exert positive modifications on meat (Jayasooriya et al. Citation2007). Therefore, the objective of this study was to evaluate the high-intensity ultrasonication (24 kHz, 12 W/cm2) in whole rabbit carcases to determine the effect on the quality characteristics of loin and leg meat, stored for 7 d at 2 °C.
Materials and methods
Sample preparation
All rabbits (N = 20 males, New Zealand x Rex) were born and grown at the Universidad de Chihuahua premises. At the age of 35 d they were weaned and kept in individual cages feeding with a commercial diet until 70 d old. They were slaughtered according to the ‘Bioethical code and Regulations of animal welfare’ of the Facultad de Zootecnia y Ecologia, UACH. Those regulations are based on the Official Mexican Regulations for slaughtering domestic and wild animals (NOM-033-SAG/ZOO-2014). Animal were slaughtered under commercial facilities at the educational slaughterhouse in the meat technology complex at the university. After 48 h post-mortem, the twenty rabbit carcases (1.78 ± 0.17 kg) were divided longitudinally in two sides along the spinal column, and then vacuum packed. Halves were vacuum packed to avoid microbial contamination before or during treatment by direct contact with the water for sonication. One side of the carcases was assigned to the control treatment, and the opposite side was ultrasonicated (24 kHz, 400 W).
Ultrasound application
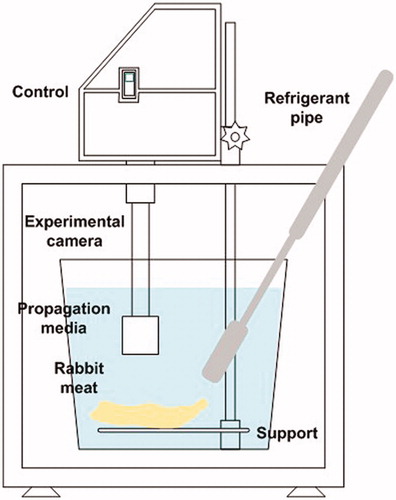
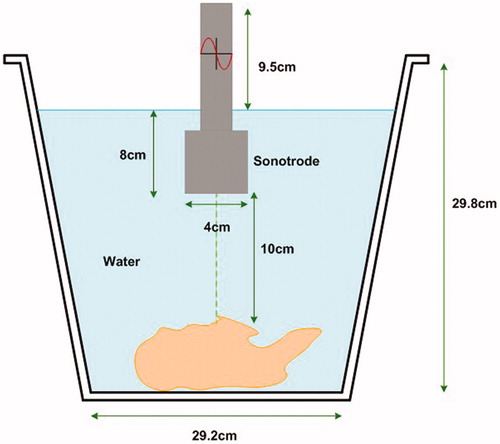
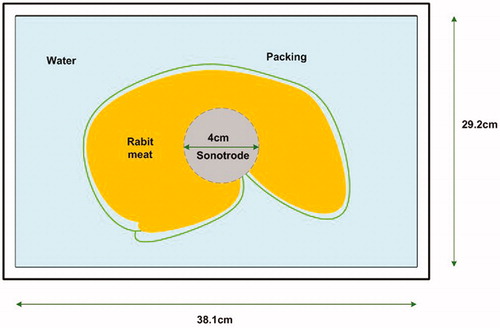
All samples were immersed in deionised water after vacuum packing. Control halves were kept immersed in the water for 15 min, simulating treatment. Ultrasonicated samples were treated with an ultrasonic processor (UP400St, Hielscher®) and a probe (H22, Hielscher®) with an intensity of 12 W/cm2, at a distance of 10 cm for 15 min (5 °C; Figures and ). All ultrasonicated carcase halves were kept in the same location of the supporting platform during treatment, seeking to locate the probe hit on the centre of the carcase (Figure ). After treatment or simulation, all samples were kept for 7 d (2 °C) in vacuum packs. After storage, the loin (m. L. dorsi, LD) and two leg muscles (Semitendinosus, ST and Semimembranosus, SM) were dissected from the carcases to evaluate pH, water holding capacity, shear force, and colour (L* = lightness, a* = redness, and b* = yellowness).
Physic-chemical parameters analysis
The pH was determined instrumentally with a digital pH metre (Hanna instruments, HI99163, Romania) before and after ultrasound treatment or simulation. The electrode was placed at 1.5 cm depth into the muscle to read the value.
Water holding capacity (WHC) was measured following the methodology described by Tsai and Ockerman (Citation1981). After storage, a 3 g lean muscle sample was collected to perform the test in triplicate. 1.0 ± 0.1 g of each sample was placed between 2 layers of filter paper (Whaltam #1, Buckinghamshire, UK) and deposited between two plexiglass plates (20 × 20 cm), compressed at 10 kg constant weight for 5 min. The weight difference of the sample was calculated after compression to determine WHC percentage.
After ageing and dissection, surface colour of all muscles was measured in triplicate with a Minolta Chromameter (Konica Minolta Camera, UK Aperture, 8 mm. Illuminant C, D65. Standard observer, C: Y = 94.2, x=.3130 and y=.3190). Following the CIE Lab methodology (Roufs Citation1978), L* (lightness), a* (redness) and b* (yellowness) coordinates were measured. Colour was determined at 3 positions (repetitions) on every piece of dissected muscle. The values for leg are means of the two muscles (Semimembranosus and Semitendinosus).
Meat shear force analysis was performed using the Warner-Bratzler method with adaptations for small rabbit muscles. Ten whole carcases (10 ultrasonicated halves and 10 control halves) were subsampled for the shear force analysis. After dissection, pH and colour analysis, whole intact muscles (ø ≈ 2.0 cm; length ≈ 8.0 cm) were cooked on a George Foreman® grill (GR2080R) until reaching an internal temperature of 71 °C (4 min approx.). Samples were stored for 24 h (1 °C) until shear force analysis (AMSA Citation2016). The test was performed on a TA.XT2i texturometer (Stable Micro Systems, Surrey, UK) with a V-shaped blade (Warner Bratzler meat shear-compression), attached to a 50 N load cell and a cross head speed of 2 mm/s. In all tests, samples were cut transversely to the muscle fibres. Average values of at least 6 replicates/sample were calculated, and the shear force peak (maximum force to cut the meat) and the total shear force (area under the curve, representing the total force used to cut the meat) were reported as N.
Statistical analysis
Meat quality traits were analysed using a t-test for paired samples (control half vs. treatment half) to compare means. Loin values are exclusively from the Longissimus dorsi. However, due to size issues, leg values are the mean of both muscles; Semimembranosus and Semitendinosus. In addition, Pearson tests were used to calculate correlation coefficients between variables of colour, WHC and shear force (p < .05). Data was analysed in software SPSS, version 17 (IBM SPSS Citation2008).
Results and discussion
All pH values were considered in the normal range (Table ). The pH was not different (p > .5) between treatment. WHC of LD decreased (p < .05) after ultrasonication (61.63 vs. 54.50%, for control and ultrasonicated meat, respectively). Ultrasonication as well, affected tri-stimuli coordinates of LD (Table ). Lightness was fairly increased around 1.53 units (p < .05). Yellowness was increased (p < .05) by 1.4 units when carcases were ultrasonicated. Redness (a*) did not differ (p > .5) between control and ultrasonication. The most remarkable benefit of HIU on vacuum packed carcases of rabbits was observed for muscle tenderness. LD shear force peak was reduced (p < .05) from 13.04 to 11.18 N, when the carcase was ultrasonicated for 15 min.
Table 1. Quality parameters (± SE) of loin and leg muscles (mean of Semimembranosus and Semitendinosus) in rabbit carcases vacuum packed and ultrasonicated (24 kHz, 12 W/cm2, 15 min) or kept as controls.
In contrast to the loin, no changes were observed on WHC of leg muscles (p > .05; Table ). Ultrasonication of the carcases affected tri-stimuli coordinates of leg muscles (Table ). Lightness was reduced around 4.34 units after sonication (p < .05). No differences (p > .05) were observed in yellowness or redness by ultrasonication. Shear force peak of leg muscles (ST and SM) was neither affected (p > .05). Thus, different muscles in the same carcase may responded differently to the application of HIU, despite the same conditions of treatment.
In agreement with this study, Gómez-Salazar et al. (Citation2018), reported that WHC and colour (L*, a*, and b*) of rabbit meat was affected by HIU (40 kHz and 110 W). Slices of rabbit meat were sonicated (120 m, 4 °C) into different marinating solutions, and meat had a higher loss of water independent of the salt concentration. HIU increased L* and b* (from 7 to 10 and 11 to 24 units, respectively), independent of salt concentration. Further, redness was increased up to twice the control value.
Early reports of HIU application in beef describe an important reduction of WHC by HIU (Stadnik et al. Citation2008). When HIU (40 kHz and 1500 W, for 10–60 min) was applied to beef ST, water loss rates gradually increased after 20 min of ultrasonication, going from 30 up to 40% of total loss. The increase of water loss by HIU was attributed to a possible disruption in the cellular structure of meat (Chang et al. Citation2015). In contrast, HIU may decrease water loss of beef (5 to 10 added %) by moderated induction of myosin oxidation which causes polymerisation, contributing to a higher capacity to retain water (Da-cheng Kang et al. Citation2017). Alternatively, due to the destruction of the myofibrils integrity by HIU, more water can be accommodated in the tissue (Zou et al. Citation2018). Furthermore, ultrasonication of beef for 40 min (16 or 28 W/cm2), resulted in no differences on WHC. Changes in WHC may take place during the ultrasonication process, but those changes were not detected because the evaluation was after treatment and 6 d of storage (Garcia-Galicia et al. Citation2020). In the present study, it is also possible to hypothesise that the 7 d of storage after treatment caused chemical alterations while the effect of HIU on WHC were not detected.
Lightness intensification by ultrasonication may be considered detrimental, because it can be associated with water release in the surface, reflecting more light (Warriss Citation2010). The increase in lightness in ultrasonicated loins may be related to a reduced WHC described previously. A significant Pearson correlation can be observed between loin shear force peak and lightness (r = 0.747, p < .001). This confirms that a reduced WHC, results in more water released. An increase of b* may grant the rabbit meat a more intense colour, since yellowness contribute to the chroma of colour (C*= √a*2 + b*2) (CIELab Citation2004). Hence, it is expected that as higher yellowness/redness as higher chroma of the colour. However, yellowness has been related to brown (O’Sullivan et al. Citation2003), and it is highly influenced by factors affecting redox state of pigment in meat (Lindahl et al. Citation2001). There are at least two potential mechanisms for HIU inducing colour changes in meat; a) an instability of heme-pigments resulting from oxidation, promoted by free-radicals production when HIU is applied (Kang et al. Citation2016), or 2) a denaturation of myoglobin and haemoglobin colour pigments, either by a thermal or by an acoustic HIU effect on chemical structures of the pigment (Suslick and Flannigan Citation2008).
Despite tenderness may not be as an important issue in rabbit meat, these small-scale model results, may be important to understand the implications to apply HIU to bigger species carcases on their tenderness. HIU can modify physical, chemical and biological properties of the subject to study by the acoustic cavitation and other physical forces such as micro-jets (Flint and Suslick Citation1991). There is a vast research in this phenomenon. Nevertheless, it is still a current exploration area, where description of the concept in areas such as sono-luminescence, sono-physic and sono-chemistry is still discussed.
When HIU (40 kHz, 1500 W, 10–60 min) was applied to bovine m. ST, hardness was reduced from 5500 g in control samples to 1000 g in sonicated meat (Chang et al. Citation2012). In bovine m. LD, HIU (11 W/cm2/60 min) reduced the shear force values by around 20 N, when compared to the control treatment, after 7 and 14 d of retail display (Peña-Gonzalez et al. Citation2019). In rabbit meat, contrasting results have been reported on tenderness. When applying HIU (40 kHz and 110 W for 120 min) to marinated rabbit meat slices (length 50 mm, width 30 mm, and thickness 10 mm), meat hardness was increased in comparison to non-ultrasonicated meat (Gómez-Salazar et al. Citation2018). It has been proposed that HIU potentially can oxidise meat proteins as a result of generation of free radicals. Protein oxidation can lead to crosslinking, which consequently can result in meat toughening (Zhang et al. Citation2013; Moczkowska et al. Citation2017).
In the present study, there were no observed tenderness changes in leg muscles as a result of HIU. Since the ultrasonication hit of the probe was in the centre of every carcase halve with a homogenous distribution of cavitation around the packed carcase, the difference in effect between muscles or regions of the same animal may be attributed to the difference in fibres within muscles. Probably, an abundance of oxidative fibres with smaller diameters and more condensed structure in the leg muscles (Klont et al. Citation1998), may offer a physical resistance for ultrasonic waves to penetrate the tissue. In other meat species, a lack of an effect of HIU (600 kHz at 48 kPa and 65 kPa acoustic pressure, 40% or 100%) on tenderness of beef strip-loin steaks has also been reported. However, neck muscle seems to respond positively to HIU, reducing their peak force (Sikes et al. Citation2014). When comparing the effect of ultrasound (16 or 28 W/cm2, for 40 min) application on vacuum packed beef muscles L. lumborum and ST, different effects were observed. Under the same conditions, texture of m. L. lumborum responded negatively to HIU application, with an increase of shear force, whereas m. ST did not change in tenderness (Garcia-Galicia et al. Citation2020).
In this study, since meat quality was not evaluated immediately after sonication, it is possible that alterations in leg muscles on shear force, colour or WHC, were not detected after the storage. In other words, the differences in fibres discussed previously, may interact with the 7 d of storage, hiding some effects of the ultrasonication on leg muscles. Further investigation may be recommended in this regard, evaluating the quality of meat at different moments after the ultrasonication of the whole carcases.
Conclusions
Under the conditions of the present study, HIU applied for 15 min to vacuum packed halved rabbit carcases, affected colour and shear force peak of LD, modifying its tenderness. Furthermore, HIU reduced WHC of the same muscle and no important effects on leg were observed. This suggests that in a single carcase that is ultrasonicated under the same conditions, the intrinsic variation among the muscles may lead to variation in the effects of HIU on quality parameters of the muscles under evaluation. Despite a reduced effect of HIU on leg muscles, the reduction of LD shear force by HIU is a promising result, because it opens a possibility for applying the process to bigger carcases, with potential benefits on particular muscles.
Ethical Approval Statement
All animals were risen and slaughtered according to the Bioethical code and Regulations of animal welfare of the Facultad de Zootecnia y Ecologia, UACH. The code is aligned to the Official Mexican Regulations. No ethical committee permission for the experiment was required as the samples were collected post-mortem.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Alarcon-Rojo AD, Carrillo-Lopez LM, Reyes-Villagrana R, Huerta-Jiménez M, Garcia-Galicia IA. 2019. Ultrasound and meat quality: a review. Ultrason Sonochem. 55:369–382.
- AMSA. 2016. Research guidelines for cookery, sensory evaluation, and instrumental tenderness measurements of meat. AMSA: Champaign (IL).
- Chang HJ, Wang Q, Tang CH, Zhou GH. 2015. Effects of ultrasound treatment on connective tissue collagen and meat quality of beef semitendinosus muscle. J Food Qual. 38:256–267.
- Chang HJ, Xu XL, Zhou GH, Li CB, Huang M. 2012. Effects of characteristics changes of collagen on meat physicochemical properties of beef semitendinosus muscle during ultrasonic processing. Food Bioprocess Technol. 5:285–297.
- CIELab. 2004. Colorimetry. 3th ed. Vienna (Austria): CIE.
- Cullere M, Dalle Zotte A. 2018. Rabbit meat production and consumption: State of knowledge and future perspectives. Meat Sci. 143:137–146.
- Dalle Zotte A, Szendrő Z. 2011. The role of rabbit meat as functional food. Meat Sci. 88:319–331.
- Flint EB, Suslick KS. 1991. The temperature of cavitation. Science. 253:1397–1399.
- Garcia-Galicia IA, Huerta-Jimenez M, Morales-Piñon C, Diaz-Almanza S, Carrillo-Lopez LM, Reyes-Villagrana R, Estepp C, Alarcon-Rojo AD. 2020. The impact of ultrasound and vacuum pack on quality properties of beef after modified atmosphere on display. J Food Process Eng. 43:1–10.
- Gómez-Salazar JA, Ochoa-Montes DA, Ozuna C, Sosa-Morales ME, Cerón-García A, Ozuna C, Sosa-Morales ME. 2018. Effect of acid marination assisted by power ultrasound on the quality of rabbit meat. J Food Qual. 2018:1–6.
- IBM SPSS. 2008. SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago (IL): SPSS.
- Jayasooriya SD, Torley PJ, D’Arcy BR, Bhandari BR. 2007. Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 75:628–639.
- Kang DC, Gao X, Ge Q, Zhou G, Zhang W. 2017. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason Sonochem. 38:317–325.
- Kang D, Jiang Y, Xing L, Zhou G, Zhang W. 2017. Inactivation of Escherichia coli O157:H7 and Bacillus cereus by power ultrasound during the curing processing in brining liquid and beef. Food Res Int. 102:717–727.
- Kang D, Zou Y, Chen Y, Xing L, Zhou G, Zhang W. 2016. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason Sonochem. 33:47–53.
- Klont RE, Brocks L, Eikelenboom G. 1998. Muscle fibre type and meat quality. Meat Sci. 49:S219–S229.
- Lindahl G, Lundström K, Tornberg E. 2001. Contribution of pigment content, myoglobin forms and internal reflectance to the colour of pork loin and ham from pure breed pigs. Meat Sci. 59:141–151.
- Moczkowska M, Półtorak A, Montowska M, Pospiech E, Wierzbicka A. 2017. The effect of the packaging system and storage time on myofibrillar protein degradation and oxidation process in relation to beef tenderness. Meat Sci. 130:7–15.
- O’Sullivan MG, Byrne DV, Martens M. 2003. Evaluation of pork colour: sensory colour assessment using trained and untrained sensory panellists. Meat Sci. 63:119–129.
- Peña-Gonzalez E, Alarcon-Rojo AD, Garcia-Galicia I, Carrillo-Lopez L, Huerta-Jimenez M. 2019. Ultrasound as a potential process to tenderize beef: sensory and technological parameters. Ultrason Sonochem. 53:134–141.
- Roufs J. 1978. Light as a true visual quantity: principles of measurement. Paris (France): Commission Internationale de l'Éclairage.
- Sikes AL, Mawson R, Stark J, Warner R. 2014. Quality properties of pre- and post-rigor beef muscle after interventions with high frequency ultrasound. Ultrason Sonochem. 21:2138–2143.
- Stadnik J, Dolatowski ZJ, Baranowska HM. 2008. Effect of ultrasound treatment on water holding properties and microstructure of beef (m. semimembranosus) during ageing. LWT Food Sci Technol. 41:2151–2158.
- Suslick KS, Flannigan DJ. 2008. Inside a collapsing bubble: sonoluminescence and the conditions during cavitation. Annu Rev Phys Chem. 59:659–683.
- Tsai TC, Ockerman HW. 1981. Water binding measurement of meat. J Food Science. 46:697–701.
- Wang A, Kang D, Zhang W, Zhang C, Zou Y, Zhou G. 2018. Changes in calpain activity, protein degradation and microstructure of beef M. semitendinosus by the application of ultrasound. Food Chem. 245:724–730.
- Warriss PD. 2010. Meat science: an introductory text. 2nd ed. Wallingford (UK): CABI Publishing.
- Zhang W, Xiao S, Ahn DU. 2013. Protein oxidation: basic principles and implications for meat quality. Crit Rev Food Sci Nutr. 53:1191–1201.
- Zou Y, Zhang W, Kang D, Zhou G. 2018. Improvement of tenderness and water holding capacity of spiced beef by the application of ultrasound during cooking. Int J Food Sci Technol. 53:828–836.