Abstract
The aim of this study was to investigate the effects of fermented Caragana korshinskii feed on meat quality in three muscle tissues (longissimus dorsi m., triceps brachii and biceps femoris) in Tan sheep. Sheep were randomly divided into two groups: the control group was fed a basic diet, and the experimental group was fed the basic diet supplemented with 10% fermented Caragana korshinskii. The results for the meat quality characteristics showed that the fermented Caragana korshinskii group had significantly greater more crude protein in all three muscles and more ash in the longissimus dorsi m. and triceps brachii than the control group. The fermented Caragana korshinskii group had a significantly higher ultimate pH in the biceps femoris and triceps brachii than the control group. The fermented Caragana korshinskii group had greater inosinic acid content in the longissimus dorsi m. and triceps brachii than the control group. The fermented Caragana korshinskii group had significantly higher levels of Asp, Thr, Glu and Ser in the triceps brachii than the control group. The fermented Caragana korshinskii group had significantly higher levels of C12:0 in the triceps brachii, of C15:0 and C16:0 in the biceps femoris, of C16:1 in the longissimus dorsi m. and triceps brachii, of C18:0 in the triceps brachii than the control group. Therefore, adding 10% fermented Caragana korshinskii to the diet of the sheep significantly improved the crude protein, ash, inosinic acid, partial amino acid and fatty acid contents as well as the ultimate pH and L* values in Tan sheep muscles.
Fermentative caragana korshinskii was partially substituted for corn stalk to feed Tan sheep could significantly improve crude protein, ash, ultimate pH, L* value, inosinic acid, partial amino acid and fat acid species and content of Tan sheep.
It was provided scientific basis for the rational utilization of caragana korshinskii resources by studying its effect on meat quality of Tan sheep.
Fermented Caragana korshinskii solves the problem of lack of forage resources in China and the imbalance of forage supply in winter and spring in China.
HIGHLIGHTS
Keywords:
Introduction
In recent years, the cultivation of Caragana korshinskii in a large area of China has played an extremely significant ecological role in wind protection, sand fixation, and water conservation (Zhao et al. Citation2018). Caragana korshinskii has high value as forage, because of its high protein content and because it is rich in a variety of amino acids and trace elements (Zhong et al. Citation2014). However, Caragana korshinskii, which affects its consumption, digestion and absorption by animals. Microbial fermentation can increase the levels of crude protein and other nutrients, and reduce the mycotoxin content in plants and can help to maintain the intestinal microbial balance (McSweeney et al. Citation2001; Hong et al. Citation2004; Soest Citation2018). Fermented plants showed significantly improved palatability and increased the production and enhanced the quality of milk and meat after being fed to cattle, sheep and other ruminants (Williams et al. Citation1991; Nolan et al. Citation2010). It is reported that 60% of the forage in Western Europe is processed and preserved as silage (Wilkinson and Stark Citation1987). Silaging Caragana korshinskii can reduce its content of harmful substances, improve its palatability, and improve weight gain when it is fed to sheep (Zhang et al. Citation2009). Recently, many researchers have carried out feeding experiments on cows, beef cattle, sheep, rabbits and pigs with Caragana korshinskii feed. The dairy cows in the trial gained a significant amount of weight when part of their corn straw diet was replaced with Caragana korshinskii (Zhang et al. Citation2009; Fang et al. Citation2011). However, the effect and quality of the Caragana korshinskii fermentation were still poor. Complex microbial fermentation technology can reduce the cellulose content and increase the protein content of raw materials (Li et al. Citation2008). Many beneficial substances can be produced through the growth and metabolism of beneficial microorganisms. The production of these beneficial substances can effectively improve the digestibility and absorption of animal feed, enhance the disease resistance of animals, promote animal growth and development, realise the conversion of waste into useful resource, and create value for businesses. Therefore, Caragana korshinskii powder was fermented with Saccharomyces cerevisiae, a tannin-degrading bacteria and a cellulase producing bacteria and partially replaced coarse fodder in a feeding experiment with Tan sheep. Our trial will provide a scientific basis for the efficient utilisation of Caragana korshinskii resources by exploring its effect on the meat quality of Tan sheep.
Materials and methods
Experimental diet and animal management
Twenty four local male Tan sheep at the age of 9 months ± 10 days, with average initial body weights and similar genetic backgrounds, from the Chunhao Grass Industry Specialised Cooperatives, Ningxia, China, were randomly divided into two equal groups for 60 days of the feeding trial prior to slaughter. The sheep were housed under the same management conditions and were fed a mixed-grain diet (control group: 15% CP and 11.09 MJ kg−1 DE; Fermented Caragana korshinskii group: 15% CP and 11.56 MJ−1 kg DE). The basal diet (Table ) was formulated to meet the nutritional requirements for growing sheep for 100 g weight gain per day, and the fed amount was adjusted according to the sheep body weight, as described by the National Research Council (NRC) (Citation2007). The concentration of metabolisable energy was calculated from the ingredient values based on the feeding standard for meat producing sheep and goats (NY/T816, 2004).
Table 1. Ingredients and chemical composition of the basal diet (on a dry matter basis).
Muscle sampling and storage of meat
The sheep were fasted for 12 h with free access to water and were weighed immediately before slaughter. The live weight before slaughter was 24.6 ± 1.06 kg. The experiment was conducted in accordance with the Chinese guidelines for animal welfare and was approved by the animal welfare committee of the Animal Science College, North Minzu University. Each muscle sample (longissimus dorsi m., triceps brachii and biceps femoris) was cut into five pieces; four pieces were stored at 4 °C for the analyses of pH, drip loss, cooking loss, cooking percentage, shear force and meat colour. The remaining pieces of meat were immediately stored at −20 °C for subsequent chemical analyses of the inosinic acid, amino acid and fatty acid contents.
Chemical composition
The chemical composition of homogenised raw meat samples (250 g) from the different muscle samples was analysed. The moisture, crude protein, fat and ash content in each sample were determined according to ASPA procedures (Citation1996).
pH measurement
The pH values of the different muscle samples were measured at 24 h post-mortem using a portable pH metre after calibration with two buffers (Testo AG, Lenzkirch, Testo 205, Germany). The pH metre had a plastic body and a spear-tipped probe coupled with a temperature probe. Each measurement for each sample had three replicates.
Drip loss
Drip loss was evaluated using the method described by Honikel (Citation1998). Samples were weighed, hung on s-hooks and put in a plastic bags to ensure that the plastic bag did not touch the sample. After 24 h, the samples were removed from the plastic bags and reweighed. The change in sample weight (before versus after storage) divided by the sample weight before storage multiplied by 100 provided the drip loss percentage.
Cooking loss
Cooking loss was evaluated using the method described by Honikel (Citation1998). For cooking loss determination, meat samples of different muscles were weighed (Wi), held in plastic bags and heated to an internal temperature of 70 °C in a 75 °C water bath. Then, the bags were cooled under running tap water for 30 min and blotted dry with paper towels. The cooked meat was weighed again (final weight, Wf), and the cooking loss (%) was calculated as (Wi−Wf) Wi−1×100.
Cooking percentage
For the cooking percentage determination, meat samples from the different muscles were weighed (Wi) and heated to an internal temperature of in a boiling water bath for 30 minutes. Then, the meat samples were cooled for 20 min and weighed again (final weight, Wf), and the cooking percentage (%) was calculated as Wf Wi−1×100.
Shear force
Meat tenderness was measured using a texture analyser (TMS-Pro, Food Technology Corporation) with samples at room temperature. Twelve muscle slices of 20 × 20 × 20 mm were placed on the platform of the analyser, and tenderness was measured by piercing each sample to a depth of 10 mm using a penetration test fixture of 5 mm diameter at a speed of 100 mm min−1. The thrust of the material was assessed using Warner-Bratzler shear force and expressed in Newton (N) units.
Colour measurements
The meat surface colour was determined with an HP 2132 chroma metre (HP, Shanghai, China) using the lightness (L*), redness (a*), and yellowness (b*) system, with D65 as the light source.
Inosinic acid analysis
The concentrations of inosinic acid in the different muscle samples were determined by using a reversed-phase high-performance liquid chromatography (RP-HPLC) method. The concentration was determined by HPLC (Waters, USA), with a 2487 ultraviolet detector (λ = 254 nm) with sample injection equipped with a separation column (YWG-C18, 10 µm). The mobile phase flow rate was 0.8 mL min−1 with phosphoric acid: triethylamine: water (3.5:7.2:200, v:v:v) as the developing solvent system. The inosinic acid content was expressed as µg of inosinic acid g−1 of meat and as a percentage of the total meat weight.
Amino acid analyses
The concentrations of amino acids in the different muscle samples were determined by using an external standard method. The concentrations were determined on an L-8900 automatic amino acid analyser (Hitachi, Japan) with a visible photometer detector (λ = 570 nm) and sample injection that was equipped with a chromatographic column (4.6 mm ID × 60 mm). The mobile phase flow rate was 0.45 mL min−1 with citrate buffer solution as the developing solvent system. The amino acid contents were expressed as mg amino acid g−1 of meat and as a percentage of the total meat weight.
Fatty acid analyses
The fatty acid compositions was determined according to the method described by Bhuiyan et al. The composition was determined on a gas chromatograph (Agilent/GC7890, USA) with a flame ionisation detector (FID), automatic sample injection and a capillary column (30 m length; 0.32 mm internal diameter; 0.25 μm film thickness). The injector and detector temperatures were 225 °C and 215 °C, respectively. The carrier gas (nitrogen) flow rate was 22 mL min−1 with chloroform: methanol: water (45:35:10, v:v:v) as the developing solvent system. The saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), omega-3 fatty acid (ω3) and omega-6 fatty acid (ω6) levels as well as the PUFA/SFA and ω6/ω3 ratios were calculated. Selected abbreviations are SFA, no double bonds (C12:0∼C20:0); MUFA, all fatty acids with a single bond (C14:1∼C20:1); PUFA, all fatty acids with 2 or more double bonds (including C18:2n6c, C20:3n3, C20:4n6, C20:5n3 and C22:6n3); eicosapentaenoic acid (EPA); docosahexaenoic acid (DHA); the sum of the content of C18:3n3, C20:3n3, C20:5n3 and C20:6n3 (ω3); and the sum of the content of C18:2n6c and C20:4n6 (ω6). The contents of each type of fatty acid were expressed as mg 100 g−1 of the total fatty acids and as a percentage of the total fatty acids by weight.
Statistical analysis
The data were analysed with t-tests to determine the differences in the different muscles of Tan sheep between the control and fermented Caragana korshinskii groups using SPSS 16.0 software (SPSS Inc., Michigan Avenue, Chicago, IL, USA). The results are reported as the mean ± SD of twenty-four measurements. Statistical significance was set at p < .05 and p < .01. The visuals were generated using GraphPad Prism 5.0a (GraphPad Software, Inc.).
Results
Chemical composition
The chemical compositions of the longissimus dorsi m., triceps brachii and biceps femoris are shown in Table . The fermented Caragana korshinskii group had significantly lower (p < .01) moisture and fat than the control group in the three muscles. Conversely, the fermented Caragana korshinskii group had significantly higher (p < .01) levels of crude protein than the control group in the three muscles. The fermented Caragana korshinskii group had significantly higher (p < .05) ash levels than the control group in the longissimus dorsi m. and triceps brachii.
Table 2. Effects of fermented Caragana korshinskii on the chemical composition of different muscles of Tan sheep.
Physical parameters
The physical parameters of the longissimus dorsi m., triceps brachii and biceps femoris are shown in Table . The fermented Caragana korshinskii group showed significantly higher (p < .01) ultimate pH in the biceps femoris and significantly higher (p < .05) ultimate pH in the triceps brachii than the control group. The control group showed significantly greater (p < .05) cooking loss than the fermented Caragana korshinskii group in the longissimus dorsi m. The fermented Caragana korshinskii group had a significantly greater (p < .05) L* value than the control group in the biceps femoris.
Table 3. Effects of fermented Caragana korshinskii on the physical parameters in different muscles of Tan sheep.
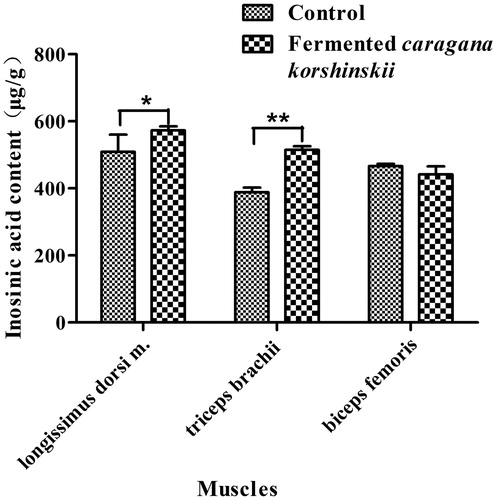
Inosinic acid content
The inosinic acid content in the longissimus dorsi m., triceps brachii and biceps femoris is shown in Figure . The fermented Caragana korshinskii group had higher inosinic acid levels in the longissimus dorsi m. (p < .05), and in the triceps brachii (p < .01) than control group. There were no significant differences in inosinic acid content (p > .05) between the fermented Caragana korshinskii group and the control group.
Figure 1. Effects of fermented Caragana korshinskii on the inosinic acid content in different muscles of Tan sheep. *Indicates a significant difference (p < .05) between the different tested diet types; **indicates an extremely significant difference (p < .01) between the different tested diet types according to the t-test.
Amino acid composition
The amino acid composition of the longissimus dorsi m., triceps brachii and biceps femoris is shown in Table . We detected a total of 17 amino acids in the muscle samples. The fermented Caragana korshinskii group had significantly higher (p < .05) levels of Asp, Thr and Ser in the triceps brachii but significantly lower (p < .05) levels of Asp, Thr and Ser in the biceps femoris than the control group. The fermented Caragana korshinskii group had significantly higher (p < .05) levels of Glu in the triceps brachii than the control group. The fermented Caragana korshinskii group had significantly lower (p < .05) levels of Ile and Leu in the longissimus dorsi m. than the control group.
Table 4. Effects of fermented Caragana korshinskii on the amino acids in different muscles of Tan sheep.
Fatty acid composition
The fatty acid composition of the longissimus dorsi m., triceps brachii and biceps femoris are shown in Table . We detected a total of 17 fatty acids in the muscle samples. The fermented Caragana korshinskii group had significantly higher (p < .01) levels of C12:0 in the triceps brachii than the control group. The fermented Caragana korshinskii group had significantly higher levels of C15:0 (p < .05) and C16:0 (p < .01) in the biceps femoris, of C16:1 (p < .05) in the longissimus dorsi m. and triceps brachii, and of C18:0 (p < .05) in the triceps brachii than the control group. The fermented Caragana korshinskii group had significantly higher levels of C18:1n9t in the longissimus dorsi m. (p < .05), and in the triceps brachii and biceps femoris (p < .01), of C18:2n6c in the longissimus dorsi m. and triceps brachii (p < .01), of C18:3n3 in the three muscles (p < .05), of C20:0 in the longissimus dorsi m. (p < .01) and in the triceps brachii (p < .05), and of C20:4n6 in the triceps brachii (p < .01) than the control group. The fermented Caragana korshinskii group had significantly lower levels of C20:5n3 and C22:6n3 in the longissimus dorsi m. (p < .05) but higher levels of C20:5n3 in the biceps femoris (p < .05) than the control group. The fermented Caragana korshinskii group had significantly higher total SFAs in the longissimus dorsi m. (p < .01), total MUFAs in the longissimus dorsi m. and the triceps brachii (p < .01), in the biceps femoris (p < .05), total PUFA in the triceps brachii (p < .05), but lower total PUFAs in the longissimus dorsi m. (p < .05) than the control group. The fermented Caragana korshinskii group had significantly lower total ω3 in the longissimus dorsi m. (p < .01) and a significantly lower ratio of ω6:ω3 in the biceps femoris (p < .05) than the control group. The fermented Caragana korshinskii group had but higher total ω3 levels in the biceps femoris (p < .05) and total ω6 in the triceps brachii (p < .01) as well as higher ω6:ω3 in the longissimus dorsi m. and the triceps brachii than the control group.
Table 5. Effects of fermented Caragana korshinskii on fatty acids in different muscles of Tan sheep.
Discussion
Caragana korshinskii rich in crude fibre and protein. As Caragana korshinskii ages, the content of crude fibre increases obviously at first and then stabilises, the content of crude protein and crude ash remains stable, the content of crude fat generally on the declines, and the nitrogen-free extract content decreases in the branches (Wu and Ao Citation2014). Therefore, Caragana korshinskii feed can play a prominent role as a “green protein”. Caragana korshinskii contains a large amount of lignin and silicate. Livestock are incapable of directly utilising either substance, and they adversely affect the digestion of other nutrients, thus reducing the nutritional value of the feed as a whole. After ensiling, the cellulose and lignin contents of Caragana korshinskii decreased by 9.97% and 10.22%, respectively; the nitrogen-free extract and crude protein did not significantly change; and the crude fat and crude ash contents increased by 10.16% and 36.10%, respectively, indicating that the loss of nutrients from Caragana korshinskii after ensiling is minor (Ren et al. Citation2014). This also indicated that the palatability and consumption of Caragana korshinskii after ensiling could be obviously improved. It is feasible to partially substitute fermented Caragana korshinskii for coarse material in sheep feed.
Prior study found that the addition of 20% fermented feed in a test group could significantly improve the growth performance in terms of average daily weight gain, average daily feed intake, and consumption weight gain ratio compared with that of a control group. In our experiment, it was revealed that the average daily feed intake and average daily weight gain of the experimental group with 10% fermented Caragana korshinskii feed increased. This result is basically consistent with previous reports. In addition to live probiotics, fermented feed contains various digestive enzymes such as amylase, protease and cellulase, that are produced by its metabolic process. The feed fermentation process partially decomposes the feed protein into small peptides and free amino acids, which endows the feeds with a pleasant acidic fragrance, improves palatability and digestibility, elevates feed digestibility and finally modifies the growth performance of the animals.
Sensory evaluation indexes of mutton quality include colour, tenderness and smell. The internal indexes of meat quality evaluation cannot be determined by sensory perception alone. The related parameters of mutton quality, including mutton drip loss, cooking loss, cooking percentage, shear force, colour measurements, pH value and so on, must be evaluated by instruments and equipment. In our research, the meat quality changed significantly under the conditions of concentrate: coarse material (60:40) and with 10% fermented Caragana korshinskii instead of corn straw. Compared with those in the control group, the ultimate pH and cooking loss in the fermented Caragana korshinskii group were significantly lower in the biceps femoris and longissimus dorsi m. Conversely, the cooking percentage increased. This is likely due to the damage to the lining of the muscle caused by the low pH, which causes water loss within muscles, resulting in increased loss during cooking. Cooking loss reflects the degree of water lost in meat cooking and processing. Generally, the cooking loss from cooked smaller muscles is higher, the water retention of the meat is better, and the meat quality is better than those of larger muscles. Muscle tenderness is usually measured by shear force, and meat tenderness is one of the traits used to evaluate food quality. The smaller the intermuscular shear force is, the finer the muscle fibre is, the better the meat quality is, and the better the taste is. The greater the shear force is, the lower the tenderness. Muscle tenderness is affected by many factors, such as the connective tissues, the intramuscular fat content, and the muscle fibre structure (Watanabe et al. Citation1996). In our research, the moisture and fat contents of the fermented Caragana korshinskii group were significantly lower in the longissimus dorsi m., triceps brachii and biceps femoris than those of the control group. Conversely, the shear force increased in the longissimus dorsi m. and biceps femoris. The loss of tenderness was also due to the decreases in water and fat in the muscle.
Compared with those of the control group, the crude protein and ash content of the fermented Caragana korshinskii group increased significantly. The content of crude protein was also higher by 12%, 44% and 42% in the longissimus dorsi m., triceps brachii and biceps femoris, respectively, in the Caragana korshinskii group than in the control group. This is consistent with Zhang’s findings. According to Zhang’s research, animals fed a higher-protein diet will reduce their subcutaneous fat rate and muscle fat content, but the flesh colour and hydraulic system will not be not affected (Zhang et al. Citation2002). This may be due to the increased protein content in the Caragana korshinskii seeds and the increased ash content after fermentation. This result shows that fermented feed can increase the protein content of Tan mutton. The reason may be that probiotics enter the gastrointestinal tract of the animals, produce digestive enzymes, promote the absorption of nutrients, and can change the nutritional composition of muscles, thereby improving the nutritional value of lambs.
Compared with that in the control group, the L* value of the fermented Caragana korshinskii group was increased in the biceps femoris. Ayeb et al. (Citation2019) found that the meat colour parameters of the same breed of mutton, such as muscle redness (a value) and muscle yellowness (b value), were close to the average values, but the muscle brightness (L value) of lambs provided with supplementary feed was higher than that of lambs fed with the normal feeding method. Shen et al. (Citation2011) mainly studied the effects of biofermented feed on the growth and meat quality of Lulai black pigs at fattening. The water retention capacity, lean meat rate, and pH of the pork in the experimental group were significantly higher than that those in the control group, but the intramuscular fat value in the experimental group was lower than that in the control group. This is consistent with our findings.
The variety and content of amino acids are closely related to the flavour of muscles. Among them, the umami amino acids in muscles are important substances for enhancing the flavour of muscles, and the essential amino acids contained in muscles are also important sources of amino acids for human beings. The umami amino acids include aspartic acid, glutamic acid, alanine and glycine (Huang et al. Citation2019). Research indicates that mutton contains 17 kinds of amino acids and is abundant in amino acids that are essential and semiessential for the human body. Mutton is rich in 8 essential amino acids: lysine, tryptophan, phenylalanine, methionine, threonine, isoleucine, leucine and valine. Mutton is a particularly good source of methionine and lysine for human beings (Lindsay et al. Citation1980). We determined the contents of 17 amino acids, among which aspartic acid (Asp), threonine (Thr), serine (Ser) and glutamic acid (Glu) in the fermented Caragana korshinskii group were significantly increased in the triceps brachii compared with those in the control group. However aspartic acid (Asp), threonine (Thr) and serine (Ser) in the fermented Caragana korshinskii group decreased in the biceps femoris. Studies have shown that serine, glutamate, glycine, isoleucine, leucine, alanine and proline are essential precursor amino acids for determining the smell of meat, especially glutamate, which is the main umami substance in meat (Yang and Zhao Citation2004). In terms of essential amino acids for adults, essential amino acids and umami amino acids, both the control and fermented Caragana korshinskii groups had high levels. Fermented feed can improve the quality of meat, which may be closely related to changes caused by fermentation in the composition of taste-related amino acids and in the content of small peptides in the feed. Caragana korshinskii is rich in crude protein and essential amino acids. In particular, lysine, isoleucine, threonine and valine are abundant (Zhang et al. Citation2011). In this test, the muscle protein content of the test group was higher than that of the control group. It can be inferred that the increase in the proportion of amino acids leads to higher protein levels. Therefore, different dietary protein levels will lead to different ratios of amino acids in mutton, but the results of studies on this topic are inconsistent. This may be due to the differences in the dietary protein levels chosen by the researchers, so further investigation is needed.
Adipose tissue and muscle tissue as well as the fat content and fatty acid composition have important effects on meat quality. It is important to study the mechanisms of action of fatty acids and control the composition and content of fatty acids in order to maintain the quality of meat. From the perspective of nutrition, fatty acids can be divided into nonessential fatty acids and essential fatty acids. Essential fatty acids that are necessary for human health and life activities are n-3 and n-6 polyunsaturated fatty acids (Astorg Citation2004). Essential fatty acids are not only necessary for nutrition but are also closely related to the growth, development and health of the body. The main source of SFAs in the human diet is animal meat products. SFAs are closely related to many diseases of modern life, such as various cancers and coronary heart disease. Studies have found that the occurrence of cardiovascular and cerebrovascular diseases, tumours and obesity is due to an imbalance in the n-6 PUFA/n-3 PUFA ratio ( Wijendran and Hayes Citation2004). The type and composition of fatty acids are important factors that determine the physical and chemical properties of adipose tissue and the quality of meat. In our research, the fermented Caragana korshinskii group had significantly higher levels of C18:0 in the triceps brachii, of C18:1n9t in the longissimus dorsi m., of C18:2n6c in the longissimus dorsi m. and triceps brachii, of C18:3n3 in all three muscles than the control group. The fermented Caragana korshinskii group had lower ratios of ω6:ω3 in the biceps femoris, longissimus dorsi m. and triceps brachii than the control group. The main fatty acid of the n-6 series of polyunsaturated fatty acids is linoleic acid, which lowers human cholesterol levels more noticeably than oleic acid. Oleic acid is transformed into other polyunsaturated fatty acids in the body and has positive nutritional and health maintenance functions (Eduardo Citation2010). Compared with those in the control group, the contents of c18:2n6c and c18:3n3 in the fermented Caragana korshinskii group were significantly higher, indicating that Caragana korshinskii, when used as feed for feeding Tan sheep, could play an active role in lowering cholesterol levels.
Conclusions
Caragana korshinskii maintains its nutrient content well after fermentation. In addition, it has acupuncture softening qualities, a soft texture, a sour smell, good palatability and high digestibility. The processing method is simple and inexpensive, and the product is easy to store. Caragana korshinskii has a wide range of applications, a balanced supply can be ensured throughout the year. Fermented Caragana korshinskii was partially substituted for corn stalks in feed for Tan sheep and significantly improved the crude protein, ash, ultimate pH, L* value, inosinic acid, partial amino acid and fatty acid species and contents of the Tan sheep. In addition, the cooking loss from the sheep fed fermented Caragana korshinskii was significantly lower than that from the sheep in the control group. These findings are of great value in the development of grain-saving animal husbandry and the production of high-quality mutton.
Ethical approval
The experimental method was approved by the National Committee for Ethics in Biomedical Research of China.
Acknowledgments
We are grateful to the Yanchi County, Ningxia Chunhao Grass Industry Specialized Cooperatives, for their kind help and cooperation during the sample collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are included within the article and in the supplementary information file.
Additional information
Funding
References
- Astorg P. 2004. Dietary n-6 and n-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Control. 15(4):367–386.
- ASPA 1996. Metodiche per la determinazione delle caratteristiche qualitative della carne. Perugia (Italy): Universita degli Studi di Perugia ed.
- Ayeb N, Addis M, Fiori M, Atti N, Barmat A, Hammadi M, Boukhris H, Damergi C, Khorchani T. 2019. Effect of local diets on nutritional and sensory quality of meat of indigenous goats in Tunisian arid regions. J Anim Physiol Anim Nutr (Berl). 103(6):1637–1645.
- Eduardo LH. 2010. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol Res. 61:200–207.
- Fang SJ, Zheng C, Li FD, Han XM, Hao ZL, Zhang PK. 2011. Effects of different Caragana korshinskii level in milking cow ration on milk yield and quality. J Gansu Agric Univ. 46:18–21.
- Hong KJ, Lee CH, Kim SW. 2004. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 7(4):430–435.
- Honikel KO. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49(4):447–457.
- Huang Y, Duan W, Wang L, Xiao J, Zhang Y. 2019. Orthogonal optimization of beef stir-fried process followed by isolation and identification of the umami peptides by consecutive chromatography and LC-Q-TOF/MS. Int J Food Prop. 22(1):1773–1785.
- Li J, Gao LY, Shen YX. 2008. Effects of lactic acid bacteria and cellulase on the fermentation quality of rice straw silage. J Nanjing Agric Univ. 4:18.
- Lindsay JR, Hogan JP, Donnelly JB. 1980. The digestion of protein from forage diets in the small intestine of the sheep. Aust J Agric Res. 31(3):589–600.
- McSweeney CS, Palmer B, McNeill DM, Krause DO. 2001. Microbial interactions with tannins: nutritional consequences for ruminants. Anim Feed Sci Technol. 91(1-2):83–93.
- Nolan JV, Hegarty RS, Hegarty J, Godwin IR, Woodgate R. 2010. Effects of dietary nitrate on fermentation, methane production and digesta kinetics in sheep. Anim Prod Sci. 50(8):801–806.
- National Research Council. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington (DC): The National Academies Press.
- Ren YY, Wang ZG, Zhang WJ. 2014. Research on feeding value of pea tree as silage. Siliao Gongye. 35:24–26.
- Shen YF, Ruan RB, Sun YX, Liu SS, Sheng QK. 2011. Effect of biological fermented feed on fatting performance of Lulai Black Boars. Feed Review. 9:27–30.
- Soest PJV. 2018. Nutritional ecology of the ruminant. Ithaca (NY): Comstock.
- Watanabe A, Daly CC, Devine CE. 1996. The effects of the ultimate pH of meat on tenderness changes during ageing. Meat Sci. 42(1):67–78.
- Williams PE, Tait CAG, Innes GM, Newbold CJ. 1991. Effects of the inclusion of yeast culture (Saccharomyces cerevisiae plus growth medium) in the diet of dairy cows on milk yield and forage degradation and fermentation patterns in the rumen of steers. J Anim Sci. 69(7):3016–3026.
- Wilkinson JM, Stark BA. 1987. Silage in Western Europe. A survey of 17 countries. Marlow: Chalcombe Publications.
- Wijendran V, Hayes KC. 2004. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 24:597–615.
- Wu XJ, Ao XP. 2014. Analysis on branch feed nutrients of Caragana korshinskii Kom. Shanxi Forestry Sci Technol. 43:36–38.
- Yang FM, Zhao YZ. 2004. Study on the meat quality of Hybrids of Borderdale with local sheep. China Herbivores. 6:28.
- Zhao YN, Zhou YR, Wang HM. 2018. [Spatial heterogeneity of soil water content under introduced shrub (Caragana korshinskii) in desert grassland of the eastern Ningxia, China]. Ying Yong Sheng Tai Xue Bao. 29(11):3577–3586.
- Zhong C, Sun Z, Zhou Z, Jin JM, Tan ZL, Jia SR. 2014. Chemical characterization and nutritional analysis of protein isolates from Caragana korshinskii Kom. J Agric Food Chem. 62(14):3217–3222.
- Zhang GJ, Li Y, Fu YR, Lu C, Hu JY. 2009. Silage quality of Robinia pseudoacacia, Caragana korshinskii and Amorpha fruticosa. J Northwest Forestry Univ. 1:38.
- Zhang X, Ma F, Han XL, Guo WB. 2009. The status quo on processing and utilization of caragana feed and its foreground analyzing in inner Mongolia. J Agric Mech Res. 2:70.
- Zhang KY, Chen DW, Luo XM, Li XW, Hu ZY. 2002. Effects of dietary levels of ideal protein on pig meat quality. J Sichuan Agric Univ. 20:1.
- Zhang HN, Fang XW, Jiang ZR, Feng YH. 2011. Free amino acid content in different tissues of Caragana korshinskii following all shoot removal. Acta Ecol Sin. 31:2454–2460.
