Abstract
In our experiment we studied the effect of dietary orange peel (OP) and grapefruit peel (GP) on broilers health and thigh meat quality. The experiment was conducted on 126 Cobb 500 broiler chicks, assigned to three dietary treatments with 42 broilers per group. Each treatment was conducted in 3 experimental growth boxes, corresponding to 6 replicate pens per treatment and 7 broilers per pen. The growth boxes had identical size with a capacity of 3.5 m2 per box. Experimental dietary treatments differed from control diet (C) by addition of 2% OP or 2% GP supplement. A significant increase in body weight was exhibited in OP group compared to GP group (p < .05). Serum energetic profile (glucose, cholesterol and triglyceride) were lowered in both OP and GP supplemented groups compared to C birds (p < .05). Among fatty acids in thigh meat, OP group has increased the sum of PUFA compared to GP group. Dietary OP and GP have effectively reduced the oxidation process occurring during storage measured by the thiobarbituric acid–reactive substances (TBARS) values in thigh meat. Therefore, lipid oxidation was delayed by incorporating the two citrus peels in broiler diet. Monitoring the antimicrobial effect of OP and GP on intestinal and caecal microbiota population, the supplements reduced the growth of pathogenic Escherichia coli and Staphylococcus spp., proving their antimicrobial effect, while the beneficial bacteria, Lactobacillus spp. was significantly improved.
The use of vegetable residues in the animal diets is an important economic and environmental factor.
The positive effects were observed on thigh meat lipid oxidation, serum energetic profile and intestinal microflora of broiler chickens.
Citrus peel in broilers feeding can act as natural antioxidant and feed additive.
Highlights
Introduction
Poultry meat is a popular, but also very versatile protein food that is consumed in large quantities compared to other meats (Moumeni Citation2001). Management and optimal nutrition are considered today to be the most important issues in the poultry industry. They can reduce costs, save production and simultaneously allow the market to provide consumers with high quality products (Ebrahimi et al. Citation2013). Concerns about bacterial resistance were increased and the use of antibiotics as growth promoters have been banned, prompting researchers and producers to find alternatives for animal feed (Dibaji et al. Citation2012) by the simultaneous or intermittent use of different natural supplements with phytogenic action. Waste from plants, vegetables and fruits are increasingly used as growth promoters in the broiler diet (Mousavi et al. Citation2012).
Citrus fruits, especially oranges (Citrus sinensis) and grapefruit (Citrus paradisi) are some of the most important and oldest horticultural products in many tropical and sub-tropical areas. Orange and grapefruit peels are primary by-products produced by the fruit processing industry, leaving behind tons of waste that can negatively impact the soil and the environment (Yang et al. Citation2011). The European Union countries are a major producer of citrus fruits (oranges, lemons, grapefruit and mandarins), after Brazil, United States and China, which led to higher amounts of citrus wastes after processing them. These wastes are a valuable source of energy, rich in numerous nutrients and bioactive compounds such as: soluble sugars, fibre, organic acids, amino acids, proteins, minerals, oils and lipids (Fernandez-Lopez et al. Citation2005). Orange and grapefruit peels contain high concentrations of phenols, flavonoids (Vlaicu et al. Citation2020) and vitamins, especially vitamin C (Manthey Citation2004). Vitamin C and polyphenols help increase antioxidant enzymes in red blood cells (Dragsted et al. Citation2001; Hasin et al. Citation2006). Previous studies have shown that citrus fruits are effective in lowering the cholesterol level in the blood (Parmar and Kar Citation2008). Oluremi et al. (Citation2006) reported that orange waste up to a level of 15%, have positively impacted the growth performance in broiler production. The effect of vegetable waste, plant extracts, meals, seeds and/or oils on production performance, carcase development, meat quality, health and intestinal microflora of chickens is currently a topic of great scientific and practical importance, due to the tendency to reduce the use of antibiotics in animal feed (Yang et al. Citation2009). Studies on the effectiveness of plant products have yielded promising results (Huyghebaert et al. Citation2011), and this is a stimulus for the implementation of research activities in this direction. While orange wastes have been used and studied by many researchers, grapefruit wastes have been left behind, even if they contains higher concentrations of bioactive compounds. Considering this aspect, grapefruit peels could represent a novelty subject among future researches. The objective of this study was to test and investigate the possible effects of dietary orange and grapefruit peels as supplements incorporated into broilers diets on growth performances, energetic blood profile, thigh meat fatty acids, TBARS and gut microflora of broilers.
Materials and methods
Experimental procedures were approved by the Ethical Committee of the National Research Development Institute for Biology and Animal Nutrition, in accordance with the Romanian legislation (Law 206/2004, ordinance 28/31.08.2011, law 43/11.04.2014, Directive 2010/63/EU).
Animals, diets, and experimental design
By using a completely randomised design, a six-week trial was performed on 126 unsexed Cobb 500 broiler chickens, in a hall divided into 3 experimental spaces as growth boxes (3.5 m2/growth box) of identical size. Each experimental space was considered an experimental growth box with 6 replicate pens per treatment and 7 broilers per pen, totalling 42 broilers per group. The experimental growth box provided the necessary space for development up to 42 days (a capacity of 16 broilers/m2), according to Romanian Law no. 205/2004 (regarding animal protection) and Directive CE no 43/2007 (regarding establishment of minimum protection of broiler chickens). The broiler chicks were purchased from a commercial hatchery and were housed in an experimental hall equipped with Big Dutchman operating equipment. The broilers were reared on the floor on permanent litter of wood shaves (10–12 cm thick), simulating the semi intensive system conditions, under controlled microclimate (59.08 ± 7.91% humidity and internal air temperature 25.05 ± 2.56°C). At the beginning room temperature was maintained at 33°C from day 0 to 3 and was reduced gradually to 24°C until the end of the experiment. The light exposure program was as follows: 0 to 7 days: 23 h; 8–36 days: 20 h; 37 days till the end: 23 h, according to the broiler commercial management guide. One-day-old chickens were fed during the starter period (1–14 days) with a compound feed based on corn, soybean meal and corn gluten, having 23% crude protein and 3039.79 kcal/kg metabolisable energy. At 14 days of age, birds were weighed individually (average weight 440.77 g) and randomly divided into three equal treatments as follows: commercial control diet (C), a diet supplemented with 2% orange peel (OP), and a diet supplemented with 2% grapefruit peel (GP), as natural antioxidant. Ingredient and chemical composition of the basal diet are shown in Table . The experimental feed and clean drinking water were available ad libitum throughout the experimental period. No medical care program was applied during the experimental period.
Table 1. Ingredient and nutrient composition of basal diets.
At the end of the trial, according to the experimental procedures, 6 broiler chicks per treatment, one from each replicate were randomly selected for blood collection. After that, the same chickens were slaughtered and samples of thigh meat were collected in order to determine the proximate composition, fatty acids composition and TBARS. For microbiological determinations, content from caecum and small intestine (duodenum, jejunum and ileum) was collected.
Plant materials
Fresh citrus fruits (oranges and grapefruits) of eating quality and without blemishes, or damage were purchased from a local market in Bucharest, Romania. After washing them with warm water, the juice was manually extracted with a press and the remained peels were chopped with a knife and spread in thin layers on a wooden floor for drying one week, to obtain the dry weight, as described by Alzawqari et al. (Citation2016). After drying, the peels were milled to the powder in a hammer mill with a 1-mm screen and stored in paper bags at room temperature until used.
Chemical analysis in citrus peels
Proximate composition
The basic chemical composition analyses were determined on samples dried at 65°C. Standardised methods were used to determine the nutrient concentration. The crude protein (CP) was determined by the Kjeldahl method according to Regulation (CE) nr. 152/2009 and standard SR EN ISO 5983-2:2009 (Kjeltec auto 1030 - Tecator Instruments, Hoganas, Sweden). Crude fat (EE) was determined by extraction in organic solvents - the method complies with Regulation (CE) nr. 152/2009 and standard SR EN ISO 6492:2001 (Soxtec 2055 - Foss Tecator, Sweden). Crude fibre (CF) was determined by the method with intermediary filtration, according to Regulation (CE) nr. Citation152/2009 and standard SR EN ISO 6865:2002 (Fibertec 2010 System - Foss Tecator, Sweden), (Olteanu et al. Citation2017).
Antioxidant compounds from citrus peels
Lutein and zeaxanthin determination
Analysis of dietary lutein and zeaxanthin concentration was carried out as described by Varzaru et al. (Citation2015). Briefly, the samples were hydrolysed with ethanolic potassium hydroxide solution, and extracted with petroleum ether 4 times. The combined extracts were washed with water, in order to remove the alkaline traces. The washed extract was passed through a filter with anhydrous sodium sulphate in order to remove any suspended water, and evaporated under vacuum until dry. The residue was dissolved in ethanol and analysed using a high-performance liquid chromatograph (Perkin Elmer 200 series, Shelton, CT, USA) with a UV detector (445 nm), and a C18 reversed-phase column (250 × 4.60 mm i.d.) (Nucleodur, Macherey-Nagel,Germany). Chromatographic analysis was carried with a mobile phase of 13% water and 87% acetone, and a flow rate of 1.0 mL/min.
Vitamin E determination
Analysis of vitamin E was performed according to the method described in EC Regulation no 152/2009, using a high-performance liquid chromatograph with PDA-UV detector (HPLC Finningan Surveyor Plus, Thermo-Electron Corporation, Waltham, MA) and detection at 292 nm. A HyperSil BDS C18 column 5 μm (Thermo-Electron Corporation, Waltham, MA) was used. Chromatographic analysis was carried out at a flow rate of 1.5 mL/min, and a mobile phase consisting in 4% water and 96% methanol.
Total antioxidant capacity (TAC) by the phosphomolybdenum method
The total antioxidant capacity of the vegetal extracts was based by the reaction between the sample solution with reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) and the absorbance recorded at 695 nm. The method used for determination was described by Untea et al. (Citation2020).
Zinc determination
Zinc concentrations was determined in citrus peel samples using flame atomic absorption spectrometry (FAAS) as described by Untea et al. (Citation2012). After microwave digestion (Berghof, Eningen, Germany), the zinc concentrations from sample solutions was determined by using a Thermo Electron – SOLAAR M6 Dual Zeeman Comfort (Cambridge, UK) equipment.
Content of total polyphenols
The total polyphenols content of plants methanolic extract (1 g of dried powder in 10 mL of methanol 80%) was measured spectrophotometrically according to the Folin-Ciocalteu’s method, as described by Untea et al. Citation2020. Gallic acid was used for calibration curve and the results were expressed as mg Gallic acid equivalents per gram sample (mg GAE/g).
Performance measurement
Throughout the experimental period (14–42 days) bodyweight (BW, g) was recorded weekly by weighing every chick individually. Daily weight gain (DWG, g) was calculated dividing total broiler weight by the number of experimental days. Average daily feed intake (ADFI, g CF/chick/day) was calculated on a daily basis by subtracting the amount of rejected feed from the offered feed at the beginning and end of every 24 hours (1 day). The feed conversion ratio (FCR, kg) values were calculated by dividing total feed consumed (kg) at total live weight (kg).
Sampling and collection of blood, thigh, intestinal and caecal samples
At the end of the trial, before slaughter, 6 birds were randomly selected from each treatment (one from each replicate) for blood sample collection. Blood samples were aseptically collected from the wing veins into 9-mL anticoagulant-free Vacutainers containing 14.3 U/mL of lithium heparin (Vacutest®, Arzergrande, Italy). These samples were used to determine the serum biochemical parameters. After blood sampling, same birds were slaughtered by sectioning the jugular vein and carotid artery, followed by 2 min exsanguination period, birds were scalded in a water tank (Tehno MS-70 70 L ms-70), at 65–70 °C for 20 s and defeathered in an automatic feather picker (professional MDP 1500 W- WQ-50) for 30 s. The thigh meat was then excised from the carcase by removing the skin, fat, bones and connective tissue. After that, the samples were divided into two parts, one for the oxidative stability analysis (TBARS) and another for the fatty acid composition analysis, then vacuum-packed into plastic sample bags, after which those for TBARS analysis were refrigerated at 4 °C and samples for other analyses were stored at −20 °C. Small intestinal (duodenum, jejunum and ileum) and caecal contents were collected aseptically in sterilised plastic tubes and preserved at −20 °C until the microbiological analyses of Escherichia coli, Enterobacteriacae, Staphylococcus spp., Lactobacillus spp., and Salmonella spp., were performed.
Analyses of serum biochemical parameters
Blood samples were centrifuged to separate the serum (centrifugation for 10 min at 3000 g). Then, the serum samples were carefully transferred to plastic vials and stored at −20 °C until the biochemical markers (glucose, cholesterol and triglyceride) analysis was performed on an automatic BS-130 chemistry analyser (Bio-Medical Electronics Co., Ltd, China).
Fatty acid composition
The fatty acids (FA) were determined by gas chromatography method as follows: the fatty acids from the sample are transformed in methyl esters of the fatty acids (FAME) by methylation, followed by component separation in capillary column, identification by comparison with standard chromatograms and quantitative determination of the fatty acids according to the method described by Turcu et al. (Citation2019), using Perkin Elmer Clarus 500 gas chromatograph, with capillary column injection system, high polarity stationary phase (BPX70:60m × 0.25 mm inner diameter and 0.25 µm thick film).
Lipid peroxidation evaluation (TBARS method)
The TBARS values were measured by third derivative spectrophotometry using a method described by Botsoglou et al. (Citation1994) and expressed as milligrams of malondialdehyde (MDA) per kg of muscle (mg MDA kg − 1). The mixed sample containing 5 g minced meat; 10 mL trichloroacetic acid (7.5%) and 5 mL butyrate hydroxytoluene was centrifuged at 3000 g for 3 min. Aliquots of supernatant reacted with 1.5 mL of 0.8% aqueous thiobarbituric acid solution at 80 °C for 50 min. and the absorbance was read at 540 nm (sp 3).
Microbiological analyses
Approximately 1 g of GIT content (ileum and caecum) per capita from six broilers per treatment were homogenised with 9 ml Brain Heart Infusion, Oxoid (BHI) broth and immediately stored at − 20 °C, in presence of glycerol 20% until further testing (not more than three months). After defrost, one mL from each sample (ileum and caecum) was supposed to ten-fold dilutions with Phosphate-Buffered Saline (PBS; Oxoid LTD, England). The bacteria determined from intestinal microflora were incubated aerobically at 37 °C for 48 h. Escherichia coli (E. coli; biotype β-hemolytic) and Salmonella spp. was analysed as reported by Dumitru et al. (Citation2019). The viable counts of Lactobacillus spp. were determined by plating serial ten-fold dilutions on MRS selective medium (Man, Rogosa and Sharpe agar, Oxoid CM0361). Staphylococcus spp. were enumerated on Baird-Parker Agar (BPA; Oxoid LTD, England) supplemented with egg yolk tellurite emulsion. Enterobacteriaceae were determined as previously described by Vlaicu et al. (Citation2020). The results were expressed as log base 10 colony-forming units (CFU) per gram of intestinal and caecal content.
Statistical analysis
The analytical data were compared by variance analysis (ANOVA) using Stat View for Windows (SAS, version 6.0), which included the effects of dietary treatments (OP and GP), and their interactions. The results of bioproductive performances are presented as mean values of each experimental growth box and data regarding the serum parameters, meat quality determinations and microbiological analysis were based on individual broilers (n = 6). Tukey’s post-hoc test was used to separate means when interactive effects significantly differed. The difference between the means was considered significant at p < .05.
Results
Chemical composition of dietary supplements
Proximate composition of the supplements used in the broiler diets is presented in Table . Our results showed that the OP and GP had close values regarding the crude protein and crude fat concentration while crude fibre was with 17.93% higher in GP compared to OP. Antioxidant compounds (Vitamin E, lutein and zeaxanthin) were presented in higher concentration in OP compared to GP, being with 18.69% respectively 55.27% higher. On the other hand, GP had highest concentration of total polyphenols (with 33.93% higher) and zinc. The total antioxidant capacity (TAC) of the citrus peel extracts expressed as mmoli per kg equivalent ascorbic acid (the antioxidant potential of hydrophilic compounds) registered similar values, while the TAC expressed as mmoli per kg equivalent vitamin E (the antioxidant potential of lypophilic compounds) was lower in GP compared to OP.
Table 2. Chemical composition of the supplements used in the experiment.
Table 3. Productive performance (14–42 days).
Effect of dietary supplements on bioproductive parameters of broilers
To explore the effect of dietary supplementation of OP and GP in broiler diets, chickens were fed experimental diets for 28 days (14 days grower period followed by 14 days finisher period). No significant differences were recorded between OP and C groups regarding any bioproductive parameter (Table 3). Dietary supplementation with 2% OP resulted in a significantly (p < .05) higher final BW (g) and DWG (g) compared with GP group. The GP supplement in broiler diets led to a significant decreased BW and DWG compared to C group. The group supplemented with GP registered a lower ADFI by 3.76% than the C group and 2.75% lower than the group supplemented with OP, but the differences were not statistically sustained (P > 0.05). Furthermore, the FCR (kg CF/kg gain) registered in group OP, was 5.38% lower than group GP and with 2.46% lower than group C.
Effect of dietary supplements on serum energetic profile
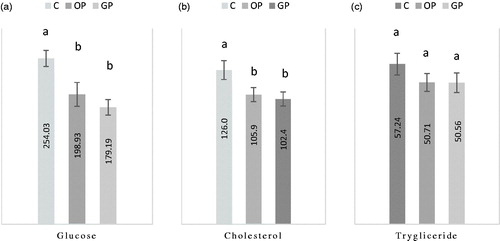
The effect of feeding OP and GP on serum energetic components in broiler chickens is presented in Figure . Serum glucose (a) and cholesterol (b) significantly (p < .05) decreased in both treated groups compared with C group. The triglyceride had a tendency of decreasing in both experimental groups, but not significantly. Comparing the effect of the two dietary citrus peels it was noticed that GP had the best effects on serum glucose and cholesterol.
Figure 1. a,b,c. Effect of dietary OP and GP supplements on energetic parameters from blood serum (ml/dl). Where: a, bMeans within a row with no common superscript differ (P< 0.05). C = control diet; OP = control diet supplemented with orange peel; GP= control diet supplemented with grapefruit peel.
Effect of dietary supplements on thigh meat fatty acids composition
The fatty acid (FA) composition for the thigh meat is presented in Table . It was observed that, sum of saturated fatty acids (SFA) and n-6/n-3 concentration did not differ in OP and GP in comparison to the C group; however, the sum of monounsaturated fatty acids (MUFA) was significantly improved in experimental groups compared to C group. On the other hand, the total of polyunsaturated fatty acids (PUFA) were significantly (p < .05) lower in GP group compared to OP and C groups. The sum of n-3 fatty acids was higher in OP and C groups compared to GP, while the sum of n-6 was significantly (p < .05) lower in both experimental groups compared to C group.
Table 4. Fatty acids composition in thigh meat.
Effect of dietary supplements on thigh meat lipid peroxidation products
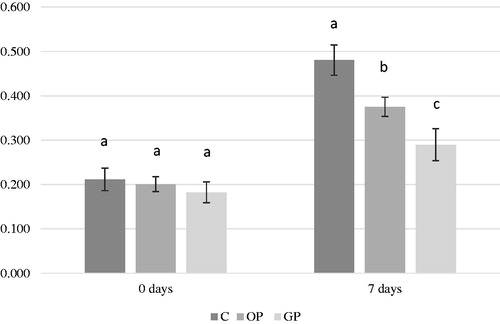
Deterioration in the quality of meat occurs mainly due to the oxidation of meat fats, and the TBARS test is the most used determination method of the extent of oxidation in fats. The data in Figure show that the TBARS value of the meat was reduced for both groups supplemented with citrus peels. Although at the end of the experiment, the TBARS values were lower in the experimental groups compared to those from C group, but the differences were not significant. After 7 days of refrigeration, the oxidative processes were significantly slowed down under the influence of the supplements administered, the TBARS values being 21.8% for the OP group and 39% for the GP group lower than in the case of group C. As it can be noticed from the Figure , the MDA levels of refrigerated samples increased in control meat, indicating higher deposition of MDA in in vivo tissue. The results showed that incorporation of citrus peels (orange and grapefruit) into broiler diets at levels of 2 g/kg delayed lipid oxidation in the refrigerated stored thigh samples.
Figure 2. Effect of dietary OP and GP supplements on TBARS concentration evolution in time (0 and 7 days). Where: a, b, cMeans within a row with no common superscript significantly differ (p < 0.05). C = control diet; OP = control diet supplemented with orange peel; GP = control diet supplemented with grapefruit peel.
Effect of dietary supplements on broilers intestinal health parameters
In Table are presented the results of the microbiological determinations on intestinal and caecal contents. The effect of our dietary supplements had a positive impact on the intestinal microbiological population. The colony forming-units (CFU) of Enterobacteriaceae and Escherichia coli significantly (p < .05) decreased in intestine of both experimental groups compared with C. The group supplemented with GP had the lowest CFU of Staphylococcus spp. compared with OP and C group in both intestine and caecum. Lactobacillus spp., the beneficial bacteria was significantly higher (p < .05) in the intestinal content of both OP and GP groups. Regarding the microbiological population in the caecal content Escherichia coli was significantly lower in both experimental groups, while the CFU of Lactobacillus spp. was significantly higher in the caecal content of GP group compared with both OP and C group. Salmonella spp. was absent in all cases.
Table 5. Effect of OP and GP on broilers intestinal microbial population from small intestine and caecum (log 10).
Discussions
Chemical composition of dietary supplements
Our studied supplements, OP and GP presented low percentages of crude fat and ash, while the crude fibre was higher in both dietary supplements, especially in GP. This property is interesting from a nutritional point of view because fibre, preferably from fruits, is utilised in food enrichment, which makes them a good candidate for possible food enrichment as a good dietary fibre source. The fibre content in our studied peels (9.70% in OP and 11.82% in GP) was higher than that from soybean (6.64%), corn (5.19%) and watermelon rind (2.98%) (Egbuonu Citation2015). Citrus peels have better quality than other dietary supplements due to the presence of associated bioactive compounds, such as antioxidants, polyphenols, carotenes and flavonoids. These metabolites are known to have antibacterial activity against Escherichia coli, Staphylococci spp. and Bacillus. The results of our investigation show that the two varieties of citrus peel possess a high concentration of antioxidants (Vitamin E, lutein and zeaxanthin) and phenolic compounds when compared with those reported by Wang et al. (Citation2008) who found that citrus peels carotenoids concentrations, lutein and zeaxanthin concentrations ranged between 1.31 and 72.8 µg/g dried base. Regarding the phenolic compounds from OP and GP, according to Lagha-Benamrouche and Madani (Citation2013) the values varies significantly (p < .05) from 9.61-31.62 (mg GAE/g DM) in OP and 161.60 (mg GAE/g DM) depending on the variety of fruits. Ghasemi et al. (Citation2009) determined the total phenol content in OP at 132.9 mg gallic acid equivalent/g of extract powder and a total of 222.22 mg gallic acid equivalent/g of extract powder in GP. This research team found a low direct correlation between total phenol contents of citrus peels methanolic extracts and radical scavenging activity proving that only antioxidant compounds with particularly hydroxyl position can donate protons and act like a radical scavenger. Regarding the zinc concentration, Martín et al. (Citation2010) found that OP had 4.5 mg/kg. De Moraes Barros et al. (Citation2012) considered that trace elements are important compounds from antioxidant defence system, Mn, Cu and Zn being components of superoxide dismutase.
Effect of dietary supplements on bioproductive parameters of broilers
In our study, the highest BW of chickens and the lowest trend of FCR (1.59, kg CF/kg gain) were recorded for the diet of chickens supplemented with 2% OP, while the reduction in ADFI was recorded in the group whose diet was supplemented with 2% GP. The results of studies on the supplementation of broiler chickens diets with citrus peels indicate that in some cases, they may negatively influence bioproductive performances (weight, average daily consumption and feed conversion ratio) due to anti-nutrients (such as oxalate, saponins, tannins, phytates) present in citrus peels (Oluremi et al. Citation2006). Another cause of the decrease in bioproductive performance is the substances with antibiotic/phytoadditives properties present in grapefruit peels, which affect the increase in body weight through various mechanisms by reducing the presence of pathogens in the gastrointestinal tract, allowing nutrients to be absorbed by birds (Nannapaneni et al. Citation2008). These pathogenic microorganisms stimulate the immune system of birds and therefore nutrients, instead of being used to grow protein and muscle are used to strengthen the immune system of animals (Apata Citation2009). Growth performances and animal health are significantly affected by the gut microflora which uses the nutrients for gut system development (Barrow Citation1992). This complex interaction is depending on the composition and activity of the gut microflora, which can have either positive or negative effects on the growth performances of birds (Neish Citation2002). The effects of experimental diets on BW, DWG ADFI and FCR for broilers were significantly different between the treatments used. As it is well known, the FCR is strictly related to the daily growth in body weight and the total feed consumption. Therefore, the difference obtained in the present study can associated to these two factors. Moreover, physiological factors can affect the performance of birds. Among these factors, we can point out that, although birds have a less developed sense of smell and taste compared to other species, the compounds present in diets supplemented with citrus have the ability to change the flavour of the feed (Perdok et al. Citation2003; Isabel and Santos Citation2009).
Effect of dietary supplements on serum energetic profile
Feeding diets supplemented with OP, has been previously reported (Abbasi et al. Citation2015; Alzawqari et al. Citation2016) to positively affect the serum profile of broiler chickens, for example significantly reducing the LDL cholesterol, triglyceride and glucose but not all studies have reported these positive findings (Ojabo et al. Citation2013). Our results show that the dietary OP and GP not only modulated the serum profile of the broilers, but significantly (p < .05) decreased the LDL cholesterol and glucose, especially in GP group, while triglycerides only had a tendency to decrease compared to C group. The ability of GP and OP to decrease the serum biochemical parameters may differ depending on the mechanism action of vitamin C and other components present in the citrus fruits that may be responsible for the altered blood metabolites. Also, this beneficial effect could be due to the desirable effects on the reduction of blood cholesterol (LDL) because, citrus fruit is a rich source of pectin, different polyphenols and flavonoid compounds present in the tested citrus fruits, which confirm the reported results made by Hong et al. (Citation2012).
Effect of dietary supplements on thigh meat fatty acids composition
Fatty acids play a major and important role in human nutrition, having a different and important function in the metabolism of plants, mammals and animals (Kim et al. Citation2017). The profile of FA in food has a direct impact on human health, according to epidemiological studies (Lopez-Huertas Citation2010; Solfrizzi et al. Citation2005). It is well known that unsaturated fats have a hypocholesterolemic effect, while saturated fats tend to proliferate the level of total cholesterol and low-density lipoproteins (LDL) (Torres-Moreno et al. Citation2015). The reduction of unsaturated fatty acids results in the compositional change of membrane lipids, which have an impact on the transition of the membrane lipid phase (Jin et al. Citation2014). Bostami et al. (Citation2017) reported that supplementation with plant by-products may decrease FA concentrations due to the presence of phenolic compounds. In the present study, an impact was found on the amounts of PUFA, n-6 and n-3 in the meat thigh, especially in the case of the GP group where the amount of PUFA decreased significantly compared to group C. However, the amount of the PUFA of OP group was maintained in relation to C group, which is indicated, considering the meat quality and health risk. Usually, the variation of SFA, MUFA and PUFA occurs due to the phenomena of conversion of one fatty acid into another, such as stearic acid in oleic acid, but also due to the action of the enzyme in the formation and depletion of FAs (Bruce and Salter Citation1996). In our study, GP supplementation resulted in decreased PUFA and increased MUFA, which is similar with the results obtained previously from breast meat (Vlaicu et al. Citation2020). PUFA reduction could be caused by the action of flavonoids, tannins, and polyphenols from citrus peels in chicken meat (Mourão et al. Citation2008). Kamboh and Zhu (Citation2013) reported that the key enzyme associated with the process of converting and decreasing FA growth is the enzyme 9-desaturase. Nuernberg et al. (Citation2005) stated that the phenomena of enlargement and reduction of n-3 and n-6 PUFAs could be attributed to the competition for similar enzymes during elongation and desaturation of FA metabolism in the physiological system. Metabolites derived from the combination of plant materials together with multi-microbial probiotics from citrus peels could affect the enzyme system, thus influencing the composition of FA in our study. At present, consumers are increasingly interested in products rich in n-3 PUFAs to fight cardiovascular disease. From this reason, eicosapentaenoic (EPA) and docosahexaenoic (DHA) n-3 FA are the most important for human nutrition. They acting as precursors of prostaglandins, tromoboxane, eicosanoids and leukotriene resolutions, which play an important role in preventing cardiac arrest, stroke and has anti-inflammatory function (Schwab et al. Citation2007; Grey and Bolland Citation2014). In our study, the variation of FA concentration could be attributed to the variation of the composition of the chemical constituents and of the dose of bioflavonoids derived from OP and GP (Kamboh and Zhu Citation2013).
Effect of dietary supplements on thigh meat lipid peroxidation products
The broiler diet supplemented with GP had the lowest level of TBARS values in the thigh meat, compared to the group fed with the basic diet. Equally, the broiler diet supplemented with OP had a lower level of TBARS values compared to group C. Including OP and GP in broiler chickens' diets has shown beneficial effects on stored thigh meat quality. This effect is related to their antioxidant properties in the case of the reduction or delaying of lipid oxidation from thigh meat. It is widely acknowledged that supplementing broilers diet with natural phytogenic compounds, contribute to food microbiological safety and quality upon food storage in the raw or cooked stages through their antimicrobial and antioxidant functions (Soltan et al. Citation2008). Citrus waste management helps minimise TBARS levels and improve the status of antioxidants in muscle. In agreement with our results regarding the effect of citrus peels on slowing TBARS values were obtained by Faiz et al. (Citation2017) by using diets supplemented with citrus processing wastes as natural antioxidants. According to this result, we can declare that the antioxidants from the broiler feed suppress the development of MDA in the thigh meat and delayed the lipid oxidation process.
Effect of dietary supplements on broilers intestinal health parameters
Gut microflora is a complex ecosystem that helps the host by serving as a barrier against pathogen colonisation (Akbarian et al. Citation2013). If this protective barrier is altered the host may be susceptible to pathogen colonisation by enteric pathogens (Durant et al. Citation1999). The supplements used in our experiment, acted against this pathogen due to the high polyphenolic content and antioxidants. Besides that, the dietary intake of natural phytogenic feed additives could contribute to food safety through the reduction of pathogens in the gut, thus promoting a healthy gut environment, which in turn could contribute to a reduction of carcase contamination at slaughter. Several observations support the hypothesis that citrus peels as feed additives may favourably affect gut functions (e.g., enzyme activity, microbial eubiosis) in vitro. Also, the phytogenic compounds are responsible with enhancing the mucus production and thickness in the gastrointestinal tract suggesting a potential protective against colonisation by gut pathogens. As it was previously stated by Engberg et al. (Citation2002) the composition and physical structure of the feed have a major influence on bacterial composition and activity in broilers gut microflora. The results obtained in the present study, partly agreed with those of Ebrahimi et al. (Citation2015) who stated that supplementation with orange peel in broiler diets improved the mean of Escherichia coli in caecum but no other gastrointestinal effect was found. Contrary, Pourhossein et al. (Citation2012) found similar results with ours when different levels of citrus peel were used, on the CFU of Lactobacillus spp. and Escherichia coli, on both small intestine and caecum. There was no significant interaction between the two experimental treatments (OP and GP) regarding the effect of dietary supplements on broilers intestinal health parameters.
Conclusions
Supplementing broiler diets with 2% OP or 2% GP as natural phytoadditives might modify the intestinal microflora of broilers and some serum components from energetic profile. Also, could improve meat quality by inhibiting lipid peroxidation of thigh meat, but without beneficial effects on the performances of broiler chickens. Further studies, especially on grapefruit peels are required to fully explore dose-response effects on broiler chickens performance and physiology.
Disclosure statement
The authors declare that there is no conflict of interest associated with the paper. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Abbasi H, Seidavi A, Liu W, Asadpour L. 2015. Investigation on the effect of different levels of dried sweet orange (Citrus sinensis) pulp on performance, carcass characteristics and physiological and biochemical parameters in broiler chicken. Saudi J Biol Sci. 22(2):139–146.
- Akbarian A, Golian A, Gilani A, Kermanshahi H, Zhaleh S, Akhavan A, De Smet S, Michiels J. 2013. Effect of feeding citrus peel extracts on growth performance, serum components, and intestinal morphology of broilers exposed to high ambient temperature during the finisher phase. Livestock Sci. 157(2-3):490–497.
- Alzawqari MH, Al-Baddany AA, Al-Baadani HH, Alhidary IA, Khan RU, Aqil GM, Abdurab A. 2016. Effect of feeding dried sweet orange (Citrus sinensis) peel and lemon grass (Cymbopogon citratus) leaves on growth performance, carcass traits, serum metabolites and antioxidant status in broiler during the finisher phase. Environ Sci Pollut Res. 23(17):17077–17082.
- Apata DF. 2009. Antibiotic resistance in poultry. Inter J Poultry Science. 8(4):404–408.
- Barrow PA. 1992. Probiotics for chickens. In: Fuller R, editor. Probiotics: the scientific basis. London, UK: Chapman and Hall, p. 255–257.
- Bostami ABMR, Sarker MSK, Yang CJ. 2017. Performance and meat fatty acid profile in mixed sex broilers fed diet supplemented with fermented medicinal plant combinations. J Anim Plant Sci. 27:360–372.
- Botsoglou NA, Fletouris DJ, Papageorgiou GE, Vassilopoulos VN, Mantis AJ, Trakatellis AG. 1994. Trakatellis AG. 1994. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J Agric Food Chem. 42(9):1931–1937.
- Bruce JS, Salter AM. 1996. Metabolic fate of oleic acid, palmitic acid and stearic acid in cultured hamster hepatocytes. Biochem J. 316(3):847–852.
- De Moraes Barros HR, de Castro Ferreira TAP, Genovese MI. 2012. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 134(4):1892–1898.
- Dibaji SM, Seidavi A, Asadpour L. 2012. Effect of dietary inclusion of the synbiotic Biomin IMBO on broilers' some blood metabolites. Res Opin Animal Vet Sci. 2(1):10–13.
- Dragsted LO, Young JF, Loft S, Sandström B. 2001. Biomarkers of oxidative stress and of antioxidative defense: relationship to intervention with antioxidant-rich foods. In: Molecular Biological Mechanisms. Copenhagen: AOCS Press, p. 272–278.
- Dumitru M, Hăbeanu M, Tabuc C, Jurcoane S. 2019. Preliminary characterization of the probiotic properties of a bacterial strain for used in monogastric nutrition. BUASVMCN-ASB. 76(2):102–108.
- Durant JA, Corrier DE, Byrd JA, Stanker LH, Ricke SC. 1999. Feed deprivation affects crop environment and modulates Salmonella enteritidis colonization and invasion of leghorn hens. Appl Environ Microbiol. 65(5):1919–1923.
- Ebrahimi A, Qotbi AAA, Seidavi A, Laudadio V, Tufarelli V. 2013. Effect of different levels of dried sweet orange (Citrus sinensis) peel on broiler chickens growth performance. Arch Tierz. 56:11–17.
- Ebrahimi A, Santini A, Alise M, Pourhossein Z, Miraalami N, Seidavi A. 2015. Effect of dried Citrus sinensis peel on gastrointestinal microbiota and immune system traits of broiler chickens. Ital J Anim Sci. 14(4):4194.
- Egbuonu ACC. 2015. Comparative assessment of some mineral, amino acid and vitamin compositions of watermelon (Citrullus lanatus) rind and seed. Asian J Biochemistry. 10(5):230–236.
- Engberg RM, Hedemann MS, Jensen BB. 2002. The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens. Br Poult Sci. 43(4):569–579.
- Faiz F, Khan MI, Butt MS, Nawaz H. 2017. Enhancement of broiler meat oxidative stability through dietary supplementation of citrus processing waste. PAKJAS. 54(04):893–898.
- Fernandez-Lopez J, Zhi N, Aleson-Carbonell L, Perez-Alvarez JA, Kuri V. 2005. Antioxidant and antibacterial activities of natural extracts: application in beef meatballs. Meat Sci. 69(3):371–380.
- Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. 2009. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 22(3):277–281.
- Grey A, Bolland M. 2014. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 174(3):460–462.
- Hasin BM, Ferdaus AJM, Islam MA, Uddin MJ, Islam MS. 2006. Marigold and orange skin as egg yolk color promoting agents. Inter J. of Poultry Sci. 5(10):979–987.
- Hong JC, Steiner T, Aufy A, Lien TF. 2012. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livestock Sci. 144(3):253–262.
- Huyghebaert G, Ducatelle R, Van Immerseel F. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 187(2):182–188.
- Isabel B, Santos Y. 2009. Effects of dietary organic acids and essential oils on growth performance and carcass characteristics of broiler chickens. J Appl Poult Res. 18(3):472–476.
- Jin P, Zhu H, Wang L, Shan T, Zheng Y. 2014. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 161:87–93.
- Kamboh AA, Zhu WY. 2013. Effect of increasing levels of bioflavonoids in broiler feed on plasma anti-oxidative potential, lipid metabolites, and fatty acid composition of meat. Poultr Sci. 92(2):454–461.
- Kim Y-J, Abm RB, Mm I, Mun HS, Ko S-Y, Yang C-J. 2017. Performance, immunity, meat composition and fatty acid pattern in broilers after dietary supplementation of fermented ginkgo biloba and citrus junos. J Nutr Food Sci. 07 (02):2.
- Lagha-Benamrouche S, Madani K. 2013. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind Crops Prod. 50:723–730.
- Lopez-Huertas E. 2010. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol Res. 61(3):200–207.
- Manthey JA. 2004. Fractionation of orange peel phenols in ultrafiltered molasses and mass balance studies of their antioxidant levels. J Agric Food Chem. 52(25):7586–7592.
- Martín MA, Siles JA, Chica AF, Martín A. 2010. Biomethanization of orange peel waste. Bioresour Technol. 101(23):8993–8999.
- Moumeni T. 2001. Herbal extracts, Farhad Reza Publication, 382.
- Mourão JL, Pinheiro VM, Prates JAM, Bessa RJB, Ferreira LMA, Fontes CMGA, Ponte PIP. 2008. Effect of dietary dehydrated pasture and citrus pulp on the performance and meat quality of broiler chickens. Poultr Sci. 87(4):733–743.
- Mousavi SMAA, Seidavi AR, Dadashbeiki M. 2012. Effect of different levels of synbiotics on carcass characteristics of broiler. Res Opin Anim Vet Sci. 2(3):161–165.
- Nannapaneni R, Muthaiyan A, Crandall PG, Johnson MG, O'Bryan CA, Chalova VI, Callaway TR, Carroll JA, Arthington JD, Nisbet DJ, Ricke SC. 2008. Antimicrobial activity of commercial citrus-based natural extracts against Escherichia coli O157:H7 isolates and mutant strains. Foodborne Pathog Dis. 5(5):695–699.
- Neish AS. 2002. The gut microflora and intestinal epithelial cells: a continuing dialogue. Microbes Infect. 4(3):309–317.
- Nuernberg K, Fischer K, Nuernberg G, Kuechenmeister U, Klosowska D, Eliminowska-Wenda G, Fiedler I, Ender K. 2005. Effects of dietary olive and linseed oil on lipid composition, meat quality, sensory characteristics and muscle structure in pigs. Meat Sci. 70(1):63–74.
- Ojabo LD, Oluremi OIA, Carew SN, Uza DV. 2013. Heamatology and serum biochemistry of pullet grower chickens fed sweet orange (Citrus sinensis) fruit peel meal-based diets. Res Opin Anim Vet Sci. 3(8):252–256.
- Olteanu M, Criste RD, Panaite TD, Ropota M, Vlaicu PA, Turcu RP. 2017. Bioproductive parameters and fatty acids profile of the meat from broilers treated with flax meal and grape seeds meal. Sci Papers Anim Sci Biotechnol. 50(1):15–21.
- Oluremi OIA, Ojighen VO, Ejembi EH. 2006. The nutritive potentials of sweet orange (Citrus sinensis) rind in broiler production. Int J Poult Sci. 5(7):613–617.
- Parmar HS, Kar A. 2008. Antiperoxidative, antithyroidal, antihyperglycemic and cardioprotective role of Citrus sinensis peel extract in male mice. Phytother Res. 22(6):791–795.
- Perdok H, Langhout P, van Vugt P. 2003. Stimulating appetite. Feed Mix. 11(3):10–13.
- Pourhossein Z, Qotbi AAA, Seidavi AR. 2012. Does different levels of dried Citrus sinensis peel affect on broilers gastrointestinal microbial population. Ann Biol Res. 3(9):4474–4479.
- Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed, O.J. L 54, 26.02.2009.
- Schwab JM, Chiang N, Arita M, Serhan CN. 2007. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 447(7146):869–874.
- Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Palasciano R, Capurso S, Torres F, Capurso A, Panza F. 2005. Unsaturated fatty acids intake and all-causes mortality: a 8.5-year follow-up of the Italian longitudinal study on aging. Exp Gerontol. 40(4):335–343.
- Soltan MA, Shewita RS and El-Katcha MI. 2008. Effect of dietary anise seeds supplementation on growth performance, immune response, carcass traits and some blood parameters of broiler chickens. Int. J. Poult. Sci. 7 (11): 1078–1088.
- Torres-Moreno M, Torrescasana E, Salas-Salvadó J, Blanch C. 2015. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 166:125–132.
- Turcu RP, Olteanu M, Criste RD, Panaite TD, Ropotă M, Vlaicu PA, Drăgotoiu D. 2019. Grapeseed meal used as natural antioxidant in high fatty acid diets for Hubbard broilers. Braz. J. Poult. Sci. 21(2): 001–012.
- Untea A, Criste RC, Vladescu L. 2012. Development and validation of a microwave digestion–FAAS procedure for Cu, Mn and Zn determination in liver. Rev Chim. 63(4):341–346.
- Untea AE, Varzaru I, Panaite TD, Gavris T, Lupu A, Ropota M. 2020. The effects of dietary inclusion of bilberry and walnut leaves in laying hens’ diets on the antioxidant properties of eggs. Animals. 10(2):191.
- Varzaru I, Untea AE, Van I. 2015. Determination of bioactive compounds with benefic potential on health in several medicinal plants. Roman Biotechnol Lett. 20(5):10773–10783.
- Vlaicu PA, Panaite TD, Turcu RP, Tabuc C. 2020. Dietary Origanum Vulgare oil and powder supplements for broilers (14–42 days). Rom Biotechnol Lett. 25(5):1922–1929.
- Vlaicu PA, Turcu RP, Mironeasa S, Panaite TD. 2020. Meat quality of breast from broilers fed a diet supplemented with orange and red grapefruit dried peel. Sci Papers Series D Anim Sci. 63(1):161–169.
- Wang YC, Chuang YC, Hsu HW. 2008. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 106(1):277–284.
- Yang XY, Xie JX, Wang FF, Zhong J, Liu YZ, Li GH, Peng SA. 2011. Comparison of ascorbate metabolism in fruits of two citrus species with obvious difference in ascorbate content in pulp. J Plant Physiol. 168(18):2196–2205.
- Yang Y, Iji PA, Choct M. 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World's Poult Sci J. 65(1):97–114.
