Abstract
The objective of this study was to evaluate the effects of lactic acid bacteria (LAB) inoculants on fermentation quality and subsequent in vitro gas production, ruminal fermentation parameters, cellulolytic bacteria and their activities of alfalfa silage. Primary growth of alfalfa (Medicago sativa L.) was harvested at 50% flowering stage, inoculated without (control) or with Lactobacillus plantarum, Enterococcus mundtii and Enterococcus faecalis at 1.0 × 106 cfu/g of fresh weight (FW) in quadruplicate laboratory silos for 45 d. The silage inoculated with LAB were well preserved, indicated by the lower (p < .05) pH and ammonia-N content and the higher (p < .05) dry matter (DM), organic matter (OM), crude protein and lactic acid contents than the control silage. In vitro asymptotic gas and total volatile fatty acids production were higher in all LAB-treated silages (p < .05). All inoculants increased carboxymethyl-cellulase and β-glycosidase activities, and obtained higher DM and neutral detergent fibre degradability (p < .05) except E. mundtii. Similarly, L. plantarum and E. faecalis inoculants had higher (p < .05) Ruminococcus albus and Fibrobacter succinogenes relative proportions than the control. However, L. plantarum inoculants had lower (p < .05) percentage of methane (CH4) in 72 h gas production than the control and E. faecalis inoculants. These results suggested that L. plantarum were more effective in enhancing alfalfa silage utilisation by promoting forage digestibility and reducing ruminal CH4 emission than E. mundtii and E. faecalis.
Lactic acid bacteria (LAB) inoculants improved alfalfa silage quality.
Silage treated with Lactobacillus plantarum or Enterococcus mundtii increased gas production but reduced the percentage of methane in vitro.
L. plantarum and Enterococcus faecalis promoted neutral detergent fibre digestibility by increased rumen cellulolytic bacteria proportion and cellulase activity.
HIGHLIGHTS
Introduction
Alfalfa (Medicago sativa L.) is one of the most important forages, which is widely cultivated and used as a major source of protein for ruminants in the form of hay or silage in the world (Zhang et al. Citation2017). Harvesting alfalfa as hay has a high risk, because its harvest season is usually rainy in northern China. So ensiling is currently increasingly popular in China. Alfalfa has high buffering capacity and low water-soluble carbohydrates (WSC), and especially a few epiphytic lactic acid bacteria (LAB), consequently, silage pH decline is often not as rapid in alfalfa as other grasses (Cai et al. Citation1999). Application of additives in alfalfa silage has become the consensus of researchers and producers. Homofermentative and facultative heterofermentative LAB are the important silage additives, which not only promote silage fermentation and reduce the nutrition loss of forage (Yuan et al. Citation2015; Zhang et al. Citation2016; Zheng et al. Citation2017), but also improve livestock performance (Kung et al. Citation1993; Chen et al. Citation1994).
Some researchers considered that the improvement in ruminant performance is due to more nutrients preserved in silage by inoculated with homofermentative or facultative heterofermentative LAB (Keady et al. Citation1994; Keady and Steen Citation1995; Keles and Demirci Citation2011). However, based on a meta-analysis, these inoculants did not change the fermentation quality and nutritional value of silage but still improved ruminant performance (Oliveria et al. 2017). Weinberg et al. (Citation2004) considered the improvement of animal performance is related to the interactions between LAB inoculants and rumen microorganism, but the mechanisms are still unclear.
For silage, improving the DM digestibility, especially the digestibility of fibre, is important for enhancing animal productivity. In rumen, the fibre degradation is positively correlated with predominant cellulolytic bacteria, such as Fibrobacter succinogenes, Ruminococcus albus, Ruminococcus flavefaciens and Butyrivibrio fibrisolvens. These cellulose bacteria activities are usually evaluated by cellulase activities, including carboxymethyle-cellulase, β-glycosidase and xylanase (Hobson and Stewart Citation1997; Agarwal et al. Citation2002). Therefore, the objective of this study was to evaluate the effects of the three selected LAB inoculants (Lactobacillus plantarum, Enterococcus mundtii and Enterococcus faecalis) on fermentation quality, in vitro gas production, ruminal fermentation parameters, especially the ruminal cellulolytic bacteria and cellulase activities of alfalfa silage.
Materials and methods
Silage preparation
Alfalfa was harvested at the 50% flowering stage with hand clippers in the experimental field of Shanxi Agriculture University (37°25′08″ N, 112°35′25″E, elevation 783 m, Shanxi province, China), and immediately chopped into approximately 2 cm length with a fodder chopper. After mixing, alfalfa was divided into 16 equal parts and any 4 parts were randomly added with one of the 4 additions: L. plantarum (KC479667), E. mundtii (KC479665), E. faecalis (KC479663) or equal volume of distilled water (Control). All inoculants were applied at a rate of 1.0 × 106 cfu/g fresh weight (FW). Approximately 680 g alfalfa was ensiled in a 1 L plastic laboratory silo (Guo et al. Citation2015), sealed immediately and stored at ambient temperature for 45 d for subsequent tests. Chemical composition and microbial population of fresh material are shown in Table .
Table 1. Chemical composition and microbial population of fresh alfalfa.
In vitro ruminal fermentation
Incubation fluid consisted of rumen fluid and artificial buffer solution at a proportion of 1:2 (v/v). Rumen fluid was obtained from three rumen-cannulated Jinnan cattle before the morning feeding. The experimental protocol was approved by the Animal Care and Use Committee of Shanxi Agriculture University. The cattle were fed with TMR that consisted of corn silage (300 g/kg DM), alfalfa hay (50 g/kg DM), alfalfa silage (100 g/kg DM), corn grain (320 g/kg DM), soybean meal (100 g/kg DM), whole cottonseed (60 g/kg DM), wet brewer grain (50 g/kg DM) and a supplement with vitamin and minerals (20 g/kg DM). The mixture of the rumen fluid was filtered through four layers of gauze, mixed with buffer solution and kept at 39 °C in a water bath while continually flushed with CO2. The artificial buffer solution was prepared by the method of Menke and Steingass (Citation1988) and kept in water bath at 39 °C.
After 45 d ensiling, a portion of the fresh silage samples was wet-ground in a mixer (FJ200S, Hangzhou Qiwei Instrument Co., Ltd., China) to a particle size of approximately 1–4 mm. Approximately 0.5 g fresh ground silage samples were weighed in triplicate into calibrated 100 mL glass syringes (Häberle Labortechnik, Lonsee, Germany). Syringes were pre-warmed to 39 °C, and then 30 mL of the incubation fluid was inhaled. A total of 51 syringes (Four treatments × four individual samples × three glass syringes per sample, with three syringes as blanks without substrate) were prepared. The gas production was recorded at 0, 4, 8, 12, 24, 48 and 72 h incubation, and at each time point gases were collected with gas sampling bags (E-Switch, volume 500 mL, Shanghai ShenYuan Scientific Instrument Co., Ltd., China) for determining methane production. Cumulative gas production data were fitted to the exponential equation (Ørskov and McDonald Citation1979): Y=b (1−e−ct), where Y is the gas production at time t, b is the asymptotic gas volume (mL), c is the gas production rate constant, and t is the incubation time (h). After incubation, each incubation fluid was immediately measured for pH, then collected into a falcon tube (10 mL) and stored at −80 °C for the following tests with ammonia nitrogen (NH3-N), volatile fatty acid (VFA), enzyme activity and DNA extraction. The residue was collected into a nylon bag, washed with distilled water, dried at 60 °C for 48 h and used to determine the DM and NDF contents.
Chemical and microbial quantitative analysis
The fresh material and silage were dried in an oven at 65 °C for 48 h, and ground to pass a 1-mm screen with a mill (FZ102, Shanghai Hong Ji instrument Co., Ltd., Shanghai, China) for chemical analysis. Analytical DM, ether extracts (EE), ash and total nitrogen (TN) contents of each sample were determined according to the AOAC (Citation2012). Organic matter (OM) content was calculated as the difference between DM and ash contents. The WSC content was determined according to method of Kim and Adesogan (Citation2006). Neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL) contents were determined according to method of Van Soest et al. (Citation1991). Thirty grams of each silage sample was blended with 60 mL of distilled water for 24 h and filtered through two layers of cheesecloth (Guo et al. Citation2019). The filtrate was used for determining pH, lactic acid, NH3-N and VFA contents. Lactic acid and NH3-N contents were determined colorimetry (Kleinschmit et al. Citation2005), and VFA contents were determined by gas chromatography (Thermo T1300; Guo et al. Citation2015). The methane (CH4) content in gas samples was determined by gas chromatography with flame ionisation detector (Thermo T1300, USA; Kougias et al. Citation2014). Enumeration of aerobic bacteria, LAB, moulds and yeasts was performed by the method of Guo et al. (Citation2015), and these data were log10 transformed. The incubation fluid samples were used to analyse the cellulase activities (carboxymethyl-cellulase, β-glycosidase, xylanase and pectase) as described by Agarwal et al. (Citation2002).
Ruminal microbial DNA was extracted from 1 mL of incubation fluid using TIANamp Stool DNA Kit (DP302-02, Tiangen, Beijing, China). The relative abundance of R. albus, R. flavefaciens, Ruminobacter amylophilus, Prevotella ruminicola, F. succinogenes and B. fibrisolvens was determined by qPCR, and calculated by a proportion of total bacterial 16SrDNA according to the formula: Relative quantification = 2–ΔΔCt, where Ct was threshold cycle (Pei et al. Citation2013), and the heat map was made by GraphPad Prism 7 software. The primers used for qPCR were forward primer 5′-CCC TAA AAGCAG TCT TAG TTC G-3′ and reverse primer 5′-CCT CCTTGC GGT TAG AAC A-3′ for R. albus (Koike and Kobayashi Citation2001), forward primer 5′-TAA CAT GAG AGT TTG ATC CTG GCT C-3′ and reverse primer 5′-CGT TAC TCA CCC GTC CGC-3′ for B. fibrisolvens (Ma et al. Citation2016), forward primer 5′-CTG GGG AGC TGC CTG AAT G −3′ and reverse primer 5′-GCA TCT GAA TGC GAC TGG TTG-3′ for R. amylophilus, forward primer 5′-GAA AGT CGG ATT AAT GCT CTA TGT TG −3′ and reverse primer 5′-CAT CCT ATA GCG GTA AAC CTT TGG-3′ for P. ruminicola (Stevenson and Weimer Citation2007), forward primer 5′-CGA ACG GAG ATA ATT TGA GTT TAC TTA GG-3′ and reverse primer 5′-CGG TCT CTG TAT GTT ATG AGG TAT TAC C-3′ for R. flavefaciens, forward primer 5′-GTT CGG AAT TAC TGG GCG TAA A-3′ and reverse primer 5′-CGC CTG CCC CTG AAC TAT C-3′ for F. succinogenes, forward primer 5′-CGG CAA CGA GCG CAA CCC-3′ and reverse primer 5′-CCA TTG TAG CAC GTG TGT AGC C-3′ for total bacteria (Denman and McSweeney Citation2006). qPCR was carried out on an Applied Biosystems stepone plus Fast Real-Time PCR System (Applied Biosystems Co., Foster City, CA). The reaction mixture and PCR programs referred to the literature of Denman and McSweeney (Citation2006).
Statistical analyses
Analyses were performed using the GLM procedure and correlation procedure of SAS version 9.1 (SAS Institute, Cary, NC). Data on the silage fermentation quality and in vitro ruminal fermentation characters were analysed by one-way analysis of variance (ANOVA) with treatments as main effect. Each replicate served as an experimental unit. The means were compared by Duncan’s multiple test, and differences were considered significant when p < .05. The relationship of rumen cellulolytic bacteria population and cellulase activity was evaluated by Pearson correlation coefficient.
Result
Silage fermentation characteristics
Fermentation characteristics of alfalfa silage are presented in Table . LAB treated silage had higher (p < .05) DM, OM, CP, lactic acid and acetic acid contents, lower (p < .05) LAB counts and similar (p > .05) ADL, EE and WSC contents compared with those of control silage. In addition, the butyric acid content in control silage was 1.26 g/kg DM, but it was not detected in all LAB-treated silages. Alfalfa silage inoculated with L. plantarum had lower (p<.05) NDF and ADF contents compared with the control silage.
Table 2. Fermentation characteristics of alfalfa silage inoculated with or without lactic acid bacteria.
In vitro ruminal fermentation characteristics
The inoculants significantly (p < .05) increased the asymptotic gas production, total VFA and acetate concentrations, but did not have effects (p> .05) on the rate of gas production, pH, NH3-N, propionate and butyrate concentrations of incubation fluid (Table ). Alfalfa silage inoculated with L. plantarum or E. mundtii had lower (p < .05) percentage of CH4 in 72 h gas production, and L. plantarum or E. faecalis groups had higher (p < .05) dry matter (DM) degradability (DM-D) and neutral detergent fibre degradability (NDF-D) than those of the control in vitro. The rate of ratio of acetate to propionate did not show a significant difference among all silages.
Table 3. In vitro gas production parameters, degradability and fermentation parameters after 72 h in vitro incubation of alfalfa silage inoculated with or without lactic acid bacteria.
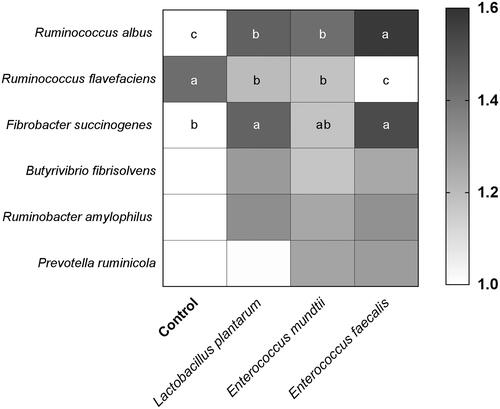
The activities of carboxymethyl-cellulase and β-glycosidase, and relative proportion of F. succinogenes and R. albus were higher (p < .05), but the relative population of R. flavefaciens was lower (p < .05) for silage inoculated with L. plantarum or E. faecalis as compared to those of control silage (Figures and ). Compared with the control, inoculants slightly increased the relative population of B. fibrisolvens and R. amylophilus. Moreover, the two strains of enterococci had a slightly higher relative population of P. ruminicola than that of L. plantarum and control.
Figure 1. Cellulase activity of incubation fluid after 72 h in vitro incubation of alfalfa silage. (A) carboxymethyl-cellulase. (B) xylanase. (C) β-glycosidase. The error bars represent standard error of the mean (n = 4). Different letters in each figure panel indicate significant difference (p < 0.05).
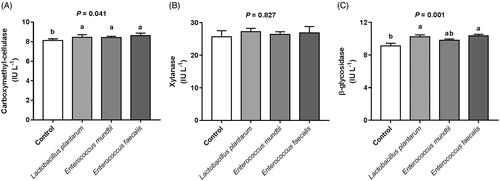
Figure 2. Relative proportion of ruminal microbes after 72 h in vitro incubation of alfalfa silage. Different letters in each row indicate significant difference (p < .05).
As shown in Table , the activity of carboxymethyl-cellulase had positive correlation (p < .05) with R. albus and F. succinogenes populations in incubation fluid. The activity of β-glycosidase had positive (p < .05) correlation with populations of R. albus, F. succinogenes and B. fibrisolvens and had negative (p < .05) correlation with R. flavefaciens population.
Table 4. Correlation analysis between rumen cellulolytic bacteria population and cellulase activity after 72 h in vitro incubation of alfalfa silage.
Discussion
The main purpose of using LAB inoculants is to improve silage fermentation, such as rapid accumulation of lactic acid, reduction in pH values, inhibition on undesirable microbiological activity and preservation of more nutrients of forage (Kung et al. Citation1984). In this study, silages inoculated with LAB had better fermentation quality, indicated by significantly lower pH and NH3-N contents, and higher lactic acid contents compared with those of the control. This indicated that L. plantarum and the two enterococci strains effectively improved the fermentation quality of alfalfa silage. During ensiling, ammonia is produced by proteolysis and subsequent amino acids decomposition. Proteolysis is mainly the result of plant enzyme activity, but the further degradation of the amino acids is mainly caused by harmful microbial activity, such as clostridium in anaerobic state (McDonald et al. Citation1991). Therefore, in order to obtain good silage fermentation attributes, it is particularly important to limit the activity of harmful microorganisms during ensiling. Effective inhibition of harmful microorganisms and plant enzyme activities mainly depends on the rapid accumulation of lactic acid and low silage pH (Zhang et al. Citation2010). E. mundtii and E. faecalis are homofermentative LAB, and L. plantarum is facultative heterofermentative LAB (Buxton et al. Citation2003). Oliveira et al. (Citation2017) concluded that alfalfa silage inoculated with homofermentative and facultative heterofermentative LAB reduced the pH and NH3-N content, and increased lactic acid content. This study has obtained similar results.
The microflora metabolise WSC to obtain energy for growth. Epiphytic LAB on grass material is often low in number, about 1 × 104 cfu/g FW in this study. LAB inoculants can become dominant bacteria in silage, and reduce the consumption of WSC by harmful bacteria and retain more WSC (Queiroz et al. Citation2013; Santos et al. Citation2013). However, the WSC content did not show a significant difference among all silages in this study. This probably because the WSC content in alfalfa material was too low (only 19.8 g/kg FW) to meet the minimum requirement for successful silage preservation (Zhang et al. Citation2010), inoculants improved fermentation efficiency by producing more lactic acid instead of preserving more WSC. The nutrition value of silage was also improved by inoculants, especially L. plantarum, which reduced NDF and ADF in inoculated silage when compared to the control.
The main intention of this study was to determine the further effect of LAB inoculants on nutrient digestibility and the ruminal fermentation of alfalfa silage in vitro. Menke and Steingass (Citation1988) found that gas production is an indicator of feed value, which depends on the amount of fermentable OM and the activity of the ruminal microorganisms. Asymptotic gas production was higher for silage inoculated with LAB compared with control silage after 72 h of incubation, which was related to the improvement in nutrition value (Trabi et al. Citation2017). In LAB inoculated silages, more DM, OM and CP were preserved, which ensured the variety of rumen bacteria proliferation, and increased gas production as well as total VFA production. Blümmel et al. (Citation1997) have demonstrated that VFA produced by rumen microorganisms during in vitro fermentation is positively related to gas production. In this study, the concentration of acetate and isobutyrate in all LAB inoculation treatments was higher than control. This was mainly due to the silage inoculated with LAB had higher NDF-D than the control. In rumen, the main products of fibre degradation are acetic acid and butyric acid (Dijkstra et al. Citation1993).
It is interesting to note that silage inoculated with and L. plantarum and E. mundtii reduced the percentage of CH4 in gas production, but E. faecium did not, although it increased silage DM-D and the ruminal VFA production. These results were related to rumen microbial community. In the rumen, large amounts of hydrogen are mainly produced by cellulolytic Ruminococci, while the cellulolytic bacterium F. succinogenes is the non-hydrogen producing bacteria (Holdeman et al. Citation1977; Mitsumori et al. Citation2012). Hydrogen is the major precursor for methanogens to produce CH4 (Hobson and Stewart Citation1997). Although the highest ruminal F. succinogenes population was found in silage inoculated with E. faecium, the enhanced ruminal R. albus might result in large amounts of hydrogen production, and thus resulted in no significant difference in percentage of CH4 in gas production compared with that of control. However, L. plantarum and E. mundtii inoculants had lower ruminal R. albus population and similar ruminal F. succinogenes population. Some research found that the increased of DM-D and the ruminal VFA production was beneficial to enhance energy efficiency of alfalfa silage by inoculants, whether they decreased (Cao et al. Citation2010, Citation2011) or did not change (Contreras-Govea et al. Citation2011) the ruminal CH4 production.
This study showed that the higher ruminal F. succinogenes and R. albus populations were observed in alfalfa silage inoculated with E. faecium and L. plantarum. Of the selected cellulolytic bacteria, F. succinogenes has a strong ability to degrade the structure of tough cellulose by carboxymethyl-cellulase and β-glycosidase (Wanapat et al. Citation2014). The results of this study confirmed that ruminal carboxymethyl-cellulase and β-glycosidase activity had positive correlation with F. succinogenes population. The high DM-D and NDF-D were observed in E. faecium and L. plantarum groups, which were consistent with the results reported by Weinberg et al. (Citation2007). Furthermore, unlike the proliferation of ruminal R. albus, the population of ruminal R. flavefaciens decreased in LAB-treated silage. On the contrary, the maximum ruminal R. flavefaciens population and the least ruminal R. albus population were observed in the control group. This may be due to some strains of R. albus can produce bacteriocins and thus inhibit R. flavefaciens, and R. albus is usually more numerous than R. flavefaciens in the rumen (Odenyo et al. Citation1994; Hobson and Stewart Citation1997). In this study, with the increase of CP content in silage, the relative proportions of ruminal major proteolytic bacteria B. fibrisolvens and R. amylophilus slightly increased. In addition, slightly lower P. ruminicola proportion was observed in alfalfa silage inoculated with L. plantarum compared to the enterococci. Although it is unable to reveal the mechanism of inoculants effect on the rumen microbiota, the increased proportions of ruminal cellulolytic bacteria and their activities by silage inoculated with LAB were identified. In some reports, inoculants could promote the ruminal microbial biomass (Muck et al. Citation2007; Contreras-Govea et al. Citation2011). In the future, it is worthwhile to investigate the interactions between different types of LAB and selected rumen bacterial strains.
Conclusions
All LAB inoculants could improve fermentation quality of alfalfa silage and did not show significant difference among them. However, L. plantarum inoculants were more effective in enhancing alfalfa silage utilisation than the two enterococci inoculants. L. plantarum not only increased in vitro digestibility of silage, but also reduced the percentage of CH4 in the ruminal gas emissions. Therefore, it is suggested that L. plantarum be used as inoculants for alfalfa silage making.
Disclosure statement
No potential conflict of interest was reported by the authors. The authors alone are responsible for the content and writing of this paper.
Additional information
Funding
References
- Agarwal N, Kamra DN, Chaudhary LC, Agarwal I, Sahoo A, Pathak NN. 2002. Microbial status and rumen enzyme profile of crossbred calves fed on different microbial feed additives. Lett Appl Microbiol. 34(5):329–336.
- [AOAC] International (Association of Official Analytical Chemists). 2012. Official methods of analysis. 19th ed. Gaithersburg (MD): AOAC International.
- Blümmel M, Makkar HP, Becker K. 1997. In vitro gas production: a technique revisited. J Anim Physiol Anim Nutritio. 77(1–5):24–34.
- Buxton DR, Muck RE, Harrison JH. 2003. Silage science and technology. Madison (WI): ASAS, CSSA, SSSA Inc.
- Cai YM, Kumai S, Zhang JG, Benno Y. 1999. Comparative studies of lactobacilli and enterococci associated with forage crops as silage inoculants. Anim Sci J. 70(4):188–194.
- Cao Y, Cai Y, Takahashi T, Yoshida N, Tohno M, Uegaki R, Nonaka K, Terada F. 2011. Effect of lactic acid bacteria inoculant and beet pulp addition on fermentation characteristics and in vitro ruminal digestion of vegetable residue silage. J Dairy Sci. 94(8):3902–3912.
- Cao Y, Takahashi T, Horiguchi K, Yoshida N. 2010. Effect of adding lactic acid bacteria and molasses on fermentation quality and in vitro ruminal digestion of total mixed ration silage prepared with whole crop rice. Grassland Sci. 56(1):19–25.
- Chen J, Stokes MR, Wallace CR. 1994. Effects of enzyme-inoculant systems on preservation and nutritive value of hay crop and corn silages. J Dairy Sci. 77(2):501–512.
- Contreras-Govea FE, Muck RE, Mertens DR, Weimer PJ. 2011. Microbial inoculant effects on silage and in vitro ruminal fermentation, and microbial biomass estimation for alfalfa, bmr corn, and corn silages. Anim Feed Sci Technol. 163(1):2–10.
- Denman SE, McSweeney CS. 2006. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen . FEMS Microbiol Ecol. 58(3):572–582.
- Dijkstra J, Forbes JM, France J. 1993. Quantitative aspects of ruminant digestion and metabolism. Wallingford, Oxford, England: CAB International; p. 107–121.
- Guo G, Shen C, Liu Q, Zhang SL, Wang C, Chen L, Xu QF, Wang YX, Huo WJ. 2019. Effects of betaine and rumen-protected folic acid supplementation on lactation performance, nutrient digestion, rumen fermentation and blood metabolites in dairy cows. Anim Feed Sci Technol. 257: 114274
- Guo G, Yuan XJ, Wen AY, Liu Q, Zhang SL, Shao T. 2015. Silage fermentation characteristics of Napiergrass (Pennisetum purpureum Sch.) harvested at various times on sunny day. Crop Sci. 55(1):458–464.
- Hobson PN, Stewart CS. 1997. The rumen microbial ecosystem. 2nd ed. London: Chapman and Hall; p. 10–68.
- Holdeman LV, Cato EP, Moore WEC. 1977. Anaerobe laboratory manual. Blacksburg (VI): Virginia Polytechnic Institute.
- Keady TWJ, Steen WJ. 1995. The effects of treating low dry matter, low digestibility grass with a bacterial inoculant on the intake and performance of beef cattle, and studies of its mode of action. Grass and Forage Sci. 50(3):217–226.
- Keady TWJ, Steen RWJ, Kilpatrick DJ, Mayne CS. 1994. Effects of inoculant treatment on silage fermentation, digestibility and intake by growing cattle. Grass and Forage Sci. 49(3):284–294.
- Keles G, Demirci U. 2011. The effect of homofermentative and heterofermentative lactic acid bacteria on conservation characteristics of baled triticale-Hungarian vetch silage and lamb performance. Anim Feed Sci Technol. 164(1–2):21–28.
- Kim SC, Adesogan AT. 2006. Influence of ensiling temperature, simulated rainfall, and delayed sealing on fermentation characteristics and aerobic stability of corn silage. J Dairy Sci. 89(8):3122–3132.
- Kleinschmit DH, Schmidt RJ, Kung LJ. 2005. The effects of various antifungal additives on the fermentation and aerobic stability of corn silage. J Dairy Sci. 88(6):2130–2139.
- Koike S, Kobayashi Y. 2001. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol Lett. 204(2):361–366.
- Kougias PG, Boe K, Tsapekos P, Angelidaki I. 2014. Foam suppression in overloaded manure-based biogas reactors using antifoaming agents. Bioresour Technol. 153:198–205.
- Kung L, Chen JH, Kreck EM, Knutsen K. 1993. Effect of microbial inoculants on the nutritive value of corn silage for lactating dairy cows. J Dairy Sci. 76(12):3763–3770.
- Kung L, Grieve DB, Thomas JW, Huber JT. 1984. Added ammonia or microbial inocula for fermentation and nitrogenous compounds of alfalfa ensiled at various percents of dry matter. J Dairy Sci. 67(2):299–306.
- Ma T, Chen DD, Tu Y, Zhang NF, Si BW, Deng KD, Diao QY. 2016. Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes. J Anim Sci Biotechnol. 7:1.
- McDonald P, Henderson AR, Heron SJ. 1991. The biochemistry of silage. 2nd ed. Aberystwyth: Cambrian Printers Ltd.
- Menke KH, Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 28:7–55.
- Mitsumori M, Shinkai T, Takenaka A, Enishi O, Higuchi K, Kobayashi Y, Nonaka I, Asanuma N, Denman SE, McSweeney CS. 2012. Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue. Br J Nutr. 108(3):482–491.
- Muck RE, Filya I, Contreras-Govea FE. 2007. Inoculant effects on alfalfa silage: in vitro gas and volatile fatty acid production. J Dairy Sci. 90(11):5115–5125.
- Odenyo AA, Mackie RI, Stahl DA, White BA. 1994. The use of 16S rRNA-targeted oligonucleotide probes to study competition between ruminal fibrolytic bacteria: development of probes for Ruminococcus species and evidence for bacteriocin production. Appl Environ Microbiol. 60(10):3688–3696.
- Oliveira AS, Weinberg ZG, Ogunade IM, Cervantes AAP, Arriola KG, Jiang Y, Kim D, Li XJ, Goncalves MCM, Vyas D, et al. 2017. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J Dairy Sci. 100(6):4587–4603.
- Ørskov ER, McDonald I. 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 92(2):499–503.
- Pei CX, Liu Q, Dong CS, Li HQ, Jiang JB, Gao WJ. 2013. Microbial community in the forestomachs of alpacas (Lama pacos) and sheep (Ovis aries). J Integr Agric. 12:4–318.
- Queiroz OCM, Arriola KG, Daniel JLP, Adesogan AT. 2013. Effects of 8 chemical and bacterial additives on the quality of corn silage. J Dairy Sci. 96(9):5836–5843.
- Santos AO, Avila CLS, Schwan RF. 2013. Selection of tropical lactic acid bacteria for enhancing the quality of maize silage. J Dairy Sci. 96(12):7777–7789.
- Stevenson DM, Weimer PJ. 2007. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relativequantification real-time PCR. Appl Microbiol Biotechnol. 75(1):165–174.
- Trabi EB, Yuan XJ, Li JF, Dong ZH, Shah AA, Shao T. 2017. Effect of glucose and lactic acid bacteria on the fermentation quality, chemical compositions and in vitro digestibility of mulberry (Morus Alba) leaf silage. Pakistan J Zool. 49:2271–2277.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74(10):3583–3597.
- Wanapat M, Gunun P, Anantasook N, Kang S. 2014. Changes of rumen pH, fermentation and microbial population as influenced by different ratios of roughage (rice straw) to concentrate in dairy steers. J Agric Sci. 152(4):675–685.
- Weinberg ZG, Chen Y, Gamburg M. 2004. The passage of lactic acid bacteria from silage into rumen fluid, in vitro studies. J Dairy Sci. 87(10):3386–3397.
- Weinberg ZG, Shatz O, Chen Y, Yosef E, Nikbahat M, Ben-Ghedalia D, Miron J. 2007. Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages. J Dairy Sci. 90(10):4754–4762.
- Yuan XJ, Guo G, Wen AY, Desta ST, Wang J, Wang Y, Shao T. 2015. The effect of different additives on the fermentation quality, in vitro digestibility and aerobic stability of a total mixed ration silage. Anim Feed Sci Technol. 207:41–50.
- Zhang JG, Kawamoto H, Cai YM. 2010. Relationships between the addition rates of cellulase or glucose and silage fermentation at different temperatures. Anim Science J. 81(3):325–330.
- Zhang Q, Li X, Zhao M, Yu Z. 2016. Lactic acid bacteria strains for enhancing the fermentation quality and aerobic stability of leymus chinensis silage. Grass Forage Sci. 71(3):472–481.
- Zhang Q, Yu Z, Wang XG, Na RS. 2017. Effects of chlorpyrifos and chlorantraniliprole on fermentation quality of alfalfa (Medicago sativa L.) silage inoculated with or without Lactobacillus plantarum LP. Anim Sci J. 88(3):456–462.
- Zheng ML, Niu DZ, Jiang D, Zuo SS, Xu CC. 2017. Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar. J Appl Microbiol. 122(6):1456–1470.
