Abstract
In the past, taurine was not considered essential in poultry diet; however, heavy reliance on plants derived feed ingredients and environmental stresses have compelled its application inevitable. Six hundred day old male broilers (Hubbard) were supplementation with taurine at the level of 0 (TAN0), 2.5 (TAN2.5), 5 (TAN5) and 7.5 g/kg (TAN7.5) in drinking water during heat stressed conditions for a period of 35 days. On overall basis, feed intake was not significantly (p>.05) different between the treatments. At the end of the experiment, weight gain was significantly (p<.05) higher in the treatment groups. On the overall basis, FCR was significantly (p<.01) lower in the treatment groups. Abdominal fat weight was significantly (p<.01) lower in TAN5 and TAN7.5 compared to the control group. On the slaughter age (35 days) muscle fibre diameter (MFD), muscle fibre cross sectional area (MFCSA), muscle fascicle diameter (MFASD) and muscle fascicle cross-sectional area were significantly (p<.05) higher in TAN7.5 compared to the control. The results indicated that growth performance was enhanced in taurine supplemented birds irrespective of the inclusion levels during the heat stress. Abdominal fat was significantly decreased in TAN5 and TAN7.5 levels; however, muscle histological features were significantly higher in all treatment groups.
Heat stress (HS) adversely affect the performance of broilers.
Taurine improved the performance of birds during HS.
Taurine reduced the abdominal fat in HS broilers.
Highlights
Introduction
Genetic selection in broiler has resulted in dramatically enhanced growth performance; however, this has made the broiler vulnerable to infectious diseases and environmental challenges such as high ambient temperature (Khan et al. Citation2012). Due to high metabolic rate, lack of sweat glands and abundant feathers, broilers are sensitive to heat stress (Khan et al. Citation2011). Huge economic losses are associated with heat stress via impaired growth and loss of meat quality, thus heat stress has become a serious problem for poultry producers (Attia and Hassan Citation2017; Attia et al. Citation2017, Citation2018; Safiullah et al. Citation2019; Shah et al. Citation2019). In addition, heat stress can negatively affect carcass characteristics, quality and muscle histomorphology (Khan et al. Citation2011; Attia and Hassan Citation2017; Attia et al. Citation2017, Citation2018; Lu et al. Citation2019; Shah et al. Citation2020), which further increase the economic losses of the poultry industry (Rehman et al. Citation2018). A number of feed additives have been recommended to reverse the deleterious effects of heat stress (Laudadio et al. Citation2012; Khan et al. Citation2014).
Taurine (2-aminoethanesulfonic acid) is a sulphur-containing non-proteinaceous compound having several biological functions in the body and considered as semi-essential amino acid (Surai et al. Citation2020), though it has no role in protein synthesis and energy production (Murakami Citation2017). Many stress alleviation related functions such as antioxidant (Grove and Karpowicz Citation2017), membrane stabilisation (Zhang et al. Citation2014), anti-inflammatory (Seidel et al. Citation2019), immunomodulation (Walczewska et al. Citation2015), detoxification (Qvartskhava et al. Citation2019) and osmoregulation (Schaffer and Kim Citation2018) have been reported for taurine. Under normal physiological conditions, it seems likely that internal taurine synthesis is adequate, therefore, several studies did not report improved performance in birds subjected to taurine supplementation (Blair et al. Citation1991; Huang et al. Citation2014; Alzawqari et al. Citation2016). During stress, chicken requirements for various nutrients also change and the major ingredients in poultry diet are deficient of taurine (Surai et al. Citation2020). Interestingly, Tomonaga et al. (Citation2018) reported that during heat-stress, taurine and hypotaurine concentration in chickens is significantly reduced. Recently, He et al. (Citation2019) found that taurine supplementation increased the expression of appetite gene in heat-stressed birds. Belal et al. (Citation2018) also reported significantly higher body weight in response to supplementation of taurine in birds housed in heat-stressed condition. We did not find any study showing the effect of taurine on the muscle histomorphology in broiler during heat stress. Therefore, we hypothesised that improving the microstructures of the muscles during heat stress may result in overall improvement in weight gain of broilers. Therefore, the objective of the present study was to study the effects of different levels of taurine supplementation on performance, carcass characteristics and muscle histology in broiler during heat stress.
Materials and methods
Animals
All the experimental procedures were conducted according to the guideline of the Use and Care of Experimental Animals, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, Pakistan.
Six hundred day old male broilers (Hubbard) were randomly distributed into a control and treatment groups under completely randomised design for a period of 35 days. The treatment groups consisted of control (TAN0), 2.5 (TAN2.5), 5 (TAN5) and 7.5 g/kg (TAN7.5) taurine in drinking water from the second week. Each group had six replicates. The birds were exposed to cyclic heat stress. The weekly temperature and humidity data are shown in Table . Broilers were fed a commercial pelleted diet consisting of starter and finisher phases. Feed in the pellet form and water were supplied during the whole experiment ad libitum (Table ). Mortality was recorded as it occurred.
Table 1. Mean daily temperature and relative humidity during the experimental period.
Table 2. Composition of basal feed and chemical composition.
Performance traits
Body weight and feed intake were measured on replicate basis each week to calculate feed conversion ratio (FCR) and was adjusted with the mortality.
Carcass characteristics
For the determination of carcass characteristics, two male birds per replicate per treatment were slaughtered. Skin and feathers were removed, eviscerated (eviscerated weight) and the feet and head were discarded to find dressing percentage. Abdominal tissue was collected from surrounding of internal organs and muscles of breast and legs to calculate proportion of breast, legs, thighs and wings. Internal organs of each bird such as liver, heart and lungs were also separated and weighed as giblet weight.
Muscle histomorphology
The muscle histomorphology was determined as described by Shah et al. (Citation2019). From already slaughtered birds, thin portions (4 cm) were separated from pectoral muscle and processed. Muscle samples were sectioned (5 µm) with the help of microtome (AEM 450, AMOS Scientific, Clayton South, Australia) and stained with haematoxylin and eosin. Muscle fibre diameter (MFD) and muscle fascicle were determined at ×40. Muscle fibre cross-sectional area (MFCSA) and muscle fascicle cross-sectional area were determined using formula πr2 and muscle fibres/µm2 respectively.
Statistical analysis
Statistical analysis of the data was carried out using general linear model (one-way analysis of variance) with the help of statistical package (SPSS, 21.0 version, Chicago, IL). A pen of birds was considered as an experimental unit. For histological characteristics, single bird was considered as an experimental unit. Means were compared through post hoc Tukey test to find the significant difference between them. Carcass characteristics were measured as percent of the live body weight. p Value less than .05 was considered statistically significant.
Results
The result of feed intake in birds kept under HS conditions is given in Table . The results showed that feed intake was not significantly (p>.05) different between the treatments during the entire period of the experiment including weekly starter and finisher phases.
Table 3. Effect of different levels of dietary inclusion of taurine on feed intake (g) in broilers under cyclic heat stress.
Body weight of birds under HS condition and supplemented with taurine is given in Table . No significant difference was found between the groups during the first three weeks and the starter phase of the experiment. During 4th week, body weight was significantly (p<.01) lower in TAN0 compared to TAN5 and TAN7.5. In the 5th week, body weight of broiler was significantly (p=.02) lower in TAN0 compared to TAN5. At finisher stage, body weight of broiler was significantly (p<.01) lower in TAN0 compared to TAN2.5, TAN5 and TAN7.5. Similar trend was also found in the overall period.
Table 4. Effect of different levels of dietary inclusion of taurine on weight gain (g) in broilers under cyclic heat stress.
The results of FCR in heat stressed broiler in response to taurine treatment are given in Table . The results indicated that during the first three weeks and at the end of starter phase, no significant difference was found between the groups. During the 4th week, FCR was significantly (p<.01) lower in TAN2.5, TAN5 and TAN7.5 compared to TAN0. A similar trend was also recorded during 5th week and finisher phase of the experiment. During the finisher phase and overall basis, FCR was significantly (p<.01) higher in TN0 compared to TAN2.5, TAN5 and TAN7.5.
Table 5. Effect of different levels of dietary inclusion of taurine on feed conversion ratio (FCR) in broiler under cyclic heat stress.
The result of carcass characteristics in heat stressed broiler fed different levels of taurine is given in . The results indicated that dressing percentage, eviscerated weight and giblet weight were not significantly (p>.05) different between the groups. Abdominal fat weight was significantly (p<.01) higher in TAN0 compared to TAN5 and TAN7.5 groups. Other carcass parameters such as breast, leg, thigh and wing weight were not significantly different between the groups. No significant change was found in mortality between the control and taurine treated birds.
Table 6. Effect of dietary inclusion of taurine on carcass characteristics in heat-stressed broiler.
The result of muscular histomorphology in heat stressed broiler fed different levels of taurine is given in Table and Figure . Muscle fibre diameter, MFCSA, muscle fascicle diameter (MFASD) and muscle fascicle cross-sectional area were significantly (p<.05) higher in TAN7.5 compared to the control.
Figure 1. Microstructure of skeletal muscle of heat-stressed (A) and taurine supplementation at the level of 2.5 (B), 5 (C) and 7.5 g/kg (D) in broilers (×40).
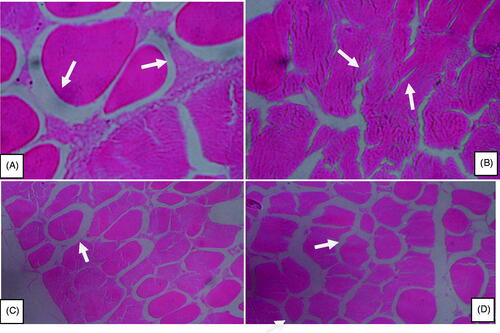
Table 7. Effect of different levels of taurine inclusion on muscle histomorphology in broiler birds under cyclic heat stress.
Discussion
Numerous studies have documented the deleterious impact of heat stress on the performance of broiler (Khan et al. Citation2014; Attia and Hassan Citation2017; Attia et al. Citation2017, Citation2018; Rehman et al. Citation2018; Safiullah et al. Citation2019; Shah et al. Citation2020). Limited studies have been published on the effect of taurine in heat-stressed broiler. In the present study, feed intake was not significantly improved by taurine in heat-stressed birds; however, weight gain was significantly higher and FCR was significantly lower in taurine supplemented birds. Since feed intake was not significantly affected by taurine supplementation, the lower FCR in treatment groups is attributed to the higher weight gain recorded in these groups. In a previous study, taurine supplementation was not effective to improve the feed intake and body weight in heat-stressed birds in comparison with TN zone (Lu et al. Citation2019). In another study, He et al. (Citation2019) reported that taurine supplementation at the rate of 5 g/kg failed to ameliorate the growth performance in heat-stressed broiler. The discrepancy in results may be due to difference in dose, duration and other experimental conditions. In the current study, FCR was improved in taurine supplemented birds under heat stress condition. The reduced feed intake in heat-stressed birds may be an effective surviving strategy at the expense of lower digestibility of nutrients (Khan et al. Citation2012). Higher weight gain in the current study is attributed to the improved nutrient metabolism in response to taurine supplementation in heat-stressed birds (Divakaran Citation2006; Belal et al. Citation2018). In addition, taurine has anti-inflammatory, immune potentiating and antioxidant role which might have played a role in improving the efficiency in heat-stressed birds (Lu et al. Citation2019).
In the present study, abdominal fat was significantly reduced in TAN5 and TAN7.5 groups compared to TAN0 suggesting that heat-stress increased the abdominal fat while taurine supplementation reduced the fat contents during heat stress condition. Heat stress increases fat deposition in broiler which further decreases the carcass quality. Abdominal, subcutaneous and intramuscular fats increase during exposure to high environmental temperature (Geraert et al. Citation1996; Zhang et al. Citation2012; Attia et al. Citation2017, Citation2018). During heat stress, animals are deficient in energy and unable to mobilise the stored fat due to decreased lipolytic enzymes (Torlinska et al. Citation1987). Decreased fat deposition was reported by Lu et al. (Citation2019) in heat-stressed broiler in response to taurine supplementation which may be due to enhanced activity of hormone sensitive lipase enzyme, decreased expression of fatty-acid synthase (FAS) gene and increased expression of muscular isoform of carnitine palmitoyl transferase 1 (M-CPT 1) resulting in overall enhanced lipid aerobic metabolism and lipid mobilisation.
In the current study, MFD and MFASD and their corresponding cross-sectional areas were decreased in chronic heat-stressed birds which were restored by the supplementation of taurine. Muscle fascial diameter as well as muscle fascial cross-sectional area was significantly higher in all treatment groups compared to the control. We did not find any published literature where muscle histomorphology has been investigated under supplementation of taurine in heat-stressed broiler. Furthermore, most of the published studies are dealing with meat quality parameters such as cooking loss, shear force, meat colour and drip loss in broiler under supplementation of taurine (Huang et al. Citation2014; Xu et al. Citation2020). Therefore, it is difficult to pinpoint the exact mechanism of recovery of microscopic changes in muscle tissuesaffacted by heat-stress in the current study. Taurine is integral part of the physiological functions of skeletal muscles such as membrane stabilisation, mitochondrial integrity maintenance, calcium homeostasis and osmoregulation (Zhang et al. Citation2014; Schaffer and Kim Citation2018; Jakaria et al. Citation2019; Seidel et al. Citation2019). Zielińska et al. (Citation2010) reported that taurine injection into the chicken embryo increased the number of muscle cells in the breast tissue. Another study reported that taurine supplementation recovered mitochondrial damage in breast muscle induced by heat stress (Lu et al. Citation2019). We speculate that the improved histomorphological features of the pectoral muscle in heat-stressed broiler in the present study could be due to the antioxidant effect of taurine (Xu et al. Citation2020).
Conclusions
The results indicated that growth performance was enhanced in taurine supplemented birds irrespective of the inclusion levels during the heat stress. Abdominal fat was significantly decreased in TAN5 and TAN7.5 levels; however, muscle histological features were significantly higher in all treatment groups.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alzawqari MH, Al-Baadani HH, Alhidary IB, Al-Owaimer AN, Abudabos AM. 2016. Effect of taurine and bile acid supplementation and their interaction on and molecular mechanisms. Redox Biol. 24:101223.
- Attia YA, Mohammed AA-H, Asmaa SE. 2018. Productive, physiological and immunological responses of two broiler strains fed different dietary regimens and exposed to heat stress. Ital J Anim Sci. 17(3):686–697.
- Attia YA, Al-Harthi MA, El-Shafey AS, Rehab YA, Kim WK. 2017. Enhancing tolerance of broiler chickens to heat stress by supplementation with vitamin E, vitamin C and/or probiotics. Ann Anim Sci. 17(4):1–15.
- Attia YA, Hassan SS. 2017. Broiler tolerance to heat stress at various dietary protein/energy levels. Eur Poult Sci. 81:1–15.
- Belal SA, Kang DR, Cho ESR, Park GH, Shim KS. 2018. Taurine reduces heat stress by regulating the expression of heat shock proteins in broilers exposed to chronic heat. Rev Bras Cienc Avic. 20:479–486.
- Blair R, Jacob JP, Gardiner EE. 1991. Lack of an effect of taurine supplementation on the incidence of sudden death syndrome in male broiler chicks. Poult Sci. 70:554–560.
- Divakaran S. 2006. Taurine: an amino acid rich in fish meal. Advance en Nutricion Acuicola. VIII Simposium Internacional de Nutricion Acuicola. p. 15–17.
- Geraert PA, Padilha JFC, Guillaumin S. 1996. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: biological and endocrinological variables. Br J Nutr. 75:205–216.
- Grove RQ, Karpowicz SJ. 2017. Reaction of hypotaurine or taurine with superoxide produces the organic peroxysulfonic acid peroxytaurine. Free Radic Biol Med. 108:575–584.
- He X, Lu Z, Ma B, Zhang L, Li J, Jiang Y, Zhou G, Gao F. 2019. Effects of dietary taurine supplementation on growth performance, jejunal morphology, appetite-related hormones, and genes expression in broilers subjected to chronic heat stress. Poult Sci. 98(7):2719–2728.
- Huang CX, Wang B, Min Z, Yuan J. 2014. Dietary inclusion level and time effects of taurine on broiler performance, meat quality, oxidative status and muscle taurine content. Br Poult Sci. 55(5):598–604.
- Jakaria M, Azam S, Haque ME, Jo SH, Uddin MS, Kim IS, Choi DK. 2019. Taurine and its analogs in neurological disorders: focus on therapeutic potential and molecular mechanisms. Redox Biol. 24:101223.
- Khan RU, Naz S, Dhama K. 2014. Chromium: pharmacological applications in heat stressed poultry. Int J Pharmacol. 10(4):213–317.
- Khan RU, Naz S, Nikousefat Z, Selvaggi M, Laudadio V, Tufarelli V. 2012. Effect of ascorbic acid in heat-stressed poultry. World’s Poult Sci J. 68(3):477–490.
- Khan RU, Naz S, Nikousefat Z, Tufarelli V, Javdani M, Rana N, Laudadio V. 2011. Effect of vitamin E in heat-stressed poultry. World’s Poult Sci J. 67(3):469–478.
- Laudadio V, Dambrosio A, Normanno G, Khan RU, Naz S, Rowghani E, Tufarelli V. 2012. Effect of reducing dietary protein level on performance responses and some microbiological aspects of broiler chickens under summer environmental conditions. Avian Biol Res. 5(2):88–92.
- Lu Z, He X, Ma B, Zhang L, Li J, Jiang Y, Zhou G, Gao F. 2019. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J Sci Food Agric. 99(3):1066–1072.
- Murakami S. 2017. The physiological and pathophysiological roles of taurine in adipose tissue in relation to obesity. Life Sci. 186:80–86.
- Qvartskhava N, Jin CJ, Buschmann T, Albrecht U, Bode JG, Monhasery N, Oenarto J, Bidmon HJ, Görg B, Häussinger D. 2019. Taurine transporter (TauT) deficiency impairs ammonia detoxification in mouse liver. Proc Natl Acad Sci U S A. 116:6313–6318.
- Rehman ZU, Chand N, Khan RU, Khan S, Qureshi MS. 2018. An assessment of the growth and profitability potential of meat-type broiler strains under high ambient temperature. Pak J Zool. 50(1):429–432.
- Safiullah , Chand N, Khan RU, Naz S, Ahmad M, Gul S. 2019. Effect of ginger (Zingiber officinale Roscoe) and organic selenium on growth dynamics, blood melanodialdehyde and paraoxonase in broilers exposed to heat stress. J Appl Anim Res. 47:212–216.
- Schaffer S, Kim HW. 2018. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (Seoul). 26:225–241.
- Seidel U, Huebbe P, Rimbach G. 2019. Taurine: a regulator of cellular redox-homeostasis and skeletal muscle function. Mol Nutr Food Res. 63(16):e1800569.
- Shah M, Zaneb H, Masood S, Khan RU, Din S, Shakirullah S, Khan I, Tariq A, Rehman HU. 2019. Ameliorative effect of zinc and multistrain probiotic on muscle and bone characteristics in broiler reared under cyclic heat stress. Pak J Zool. 51(3):1041–1046.
- Shah M, Zaneb H, Masood S, Khan RU, Mobashar M, Khan I, Din S, Khan MS, Rehman H, Tinelli A. 2020. Single or combined applications of zinc and multi-strains probiotic on intestinal histomorphology of broilers under cyclic heat stress. Probiotics Antimicrob Proteins. 12(2):473–480.
- Surai PF, Kochish II, Kidd MT. 2020. Taurine in poultry nutrition. Anim Feed Sci Technol. 260:114339.
- Tomonaga S, Okuyama H, Tachibana T, Makino R. 2018. Effects of high ambient temperature on plasma metabolomic profiles in chicks. Anim Sci J. 89:448–455.
- Torlinska T, Banach R, Paluszak J, Gryczka-Dziadecka A. 1987. Hyperthermia effect on lipolytic processes in rat blood and adipose tissue. Acta Physiol Pol. 38:361–366.
- Walczewska M, Ciszek-Lenda M, Surmiak M, Kozlowska A, Jozefowski S, Marcinkiewicz J. 2015. Impact of taurine on innate and adaptive immunity as the result of HOCl neutralization. Adv Exp Med Biol. 803:109–120.
- Xu SW, Lu Z, Ma BB, Xing T, Li JL, Zhang L, Jiang Y, Gao F. 2020. Dietary taurine supplementation enhances antioxidative capacity and improves breast meat quality of broiler chickens. Br Poult Sci. 61(2):140–145.
- Zhang ZY, Jia GQ, Zuo JJ, Zhang Y, Lei J, Ren L, Feng DY. 2012. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult Sci. 91(11):2931–2937.
- Zhang Z, Liu D, Yi B, Liao Z, Tang L, Yin D, He M. 2014. Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol Med Rep. 10:2255–2262.
- Zielińska MK, Sawosz E, Chwalibog A, Ostaszewska T, Kamaszewski M, Grodzik M, Skomiał J. 2010. Nano-nutrition of chicken embryos. Effect of gold and taurine nanoparticles on muscle development. J Anim Feed Sci. 19(2):277–285.
