?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to extend the shelf life of raw buffalo meat by coating with high solubility bioactive peptides (BPs). In this work, BPs (11S) of pea and red kidney beans were obtained by different isolation techniques. Alcalase-red kidney bean hydrolysate (RBAH) successfully obtained after 60 min enzymatic hydrolysis for red kidney bean protein isolate (RPI) by Alcalase (E/S ratio of 1:100, hydrolysis degree 30%). The 11S pea globulin (11SGP) was isolated from pea total protein by ammonium sulphate. BPs were characterised by SDS-PAGE and used as a shield coated raw buffalo meat because of their basic nature. The isolated peptides have considerable antioxidant and antimicrobial activity. The antioxidant activity was estimated by DPPH assay. The antibacterial activity was evaluated by well diffusion assay; however, antifungal activity was estimated by disc diffusion assay. RBAH and 11SGP (800 µg/mL) were significantly (p ≤ .05) scavenged 90, 92% of DPPH˙, however, 60 µg/mL concentration was significantly (p ≤ .05) reduced 48–89% of gram-positive, and 38–82% of gram-negative bacteria, respectively, and they were inhibited 88% of fungal growth. The BPs (400 µg/g) were significantly reduced (p ≤ .05) the increment of meat pH and myoglobin oxidation to an acceptable percentage of metmyoglobin (MetMb) (40%) for 15–20 d via eliminating 44% of bacterial load and maintained secured storage for two weeks. RBAH was significantly increased (p ≤ .05) a*, and b* values and enhanced the meat redness, but a* value was decreased during storage. RBAH and 11SGP (400 µg/g) (p ≤ .05) was maintained the meat colour and odour by 48–68% and 64–73% after two weeks of cold storage, respectively. The tested peptides could be safely applied in novel foods.
11SGP and RBAH are high solubility bioactive peptides (BPs) with antioxidant and antimicrobial activity.
11SGP and RBAH reduce myoglobin oxidation to an acceptable percentage of met-myoglobin.
RBAH enhances the whiteness and redness of buffalo meat.
11SGP and RBAH extend meat shelf life for a period exceeds 15 d at 4 °C.
Highlights
Graphical Abstract
Introduction
Chemical deterioration and microbial contamination are the most problems that affected the meat, meat products quality and storage period at refrigeration conditions. All these unwanted alternations can be banned by preservatives supplementation that maintains meat quality, extend its lifetime, and keep it safe (Saguy and Peleg Citation2009). Consumers currently demand food with good quality, suitable price and long storage time; therefore, food manufacturers and producers make every effort to find appropriate food additives that meet consumers’ desires. Many chemicals, such as nitrates, butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) have efficiently used as food preservatives to extend the storage time and vitality, reducing microbial and chemical undesirable changes. Despite their biological activity (Seabra et al. Citation2006; Anyasi et al. Citation2017), various damages on health including neural system, digestive system and respiratory system damages because of intensive usage of chemical preservatives besides, they are expensive (Speranza and Corbo Citation2010; Anand and Sati Citation2013; Carocho et al. Citation2014; Bondi et al. Citation2017). Therefore, consumers are progressively concerned about these effects and preferred natural additives. Researchers have strived to produce bioactive natural preservatives for food use (Osman et al. Citation2013; Abd El-Hack et al. Citation2020; Abdelnour, Swelum, et al. Citation2020; Abdelnour, El-Saadony, et al. Citation2020; Ashour et al. Citation2020). Proteins, protein isolates (PIs), protein hydrolysates, peptides and amino acids have significant antioxidant and antimicrobial activity because of amino acids in their structure (Silphaduang and Noga Citation2001). Bioactive peptides (BPs) obtained by enzymatic hydrolysis are small oligo-peptides isolated from plants, insects, amphibians, crustaceans and marine organisms (Bougatef et al. Citation2010; Centenaro et al. Citation2014). The peptides antiradical activity depends on their ability to bind with metal ion and the free radical (Baltić et al. Citation2014; Choi et al. Citation2015; Wang et al. Citation2015). Natural preservatives isolated from animals and plants, i.e. fish and chicken hydrolysates have antioxidant potential. The incorporation of these hydrolysates in minced beef prevented lipid oxidation with rates of 93% and 80% that reported by Centenaro et al. (Citation2014). Also, plant-derived antimicrobials could decrease lipid oxidation and reduce colour loss besides, increase the fresh sausage lifetime. BPs have multiple antioxidant and microbial activities. Biologically, active peptides derived from plants have a role in improving the food quality and technological properties as they play several roles, e.g. sweetener, colour preservatives, acidity regulator, emulsifier, flavour enhancer and thickener. They can also affect water and oil retention capacity, colloidal stability, viscosity and foam formation as the final product to improve food quality (Halim et al. Citation2016; Faustino et al. Citation2019).
Red kidney bean (Phasolus vulgarus L.) and green pea (Pisum sativum) are members of the leguminous family. They are a good protein source (25–44%) and other nutrients (Boye et al. Citation2010; Ahmed et al. Citation2018). Various studies investigated that common beans and pea reduced the risk of many diseases, such as cancer and diabetes (Dueñas et al. Citation2015). The main protein stored in kidney bean and pea was globulin, 8S in bean and 11S in pea (Romero et al. Citation1975). They are oligopeptides contains 2–20 amino acids with a molecular weight ranging from 43 to 55 KD (Los et al. Citation2020). These BPs from legumes can be produced from total proteins by in vitro enzymatic hydrolysis with alcalase, pepsin, trypsin and papain (Chalamaiah et al. Citation2018; Al-Ruwaih et al. Citation2019; Saad et al. Citation2020). Besides BPs exhibit antibacterial activity against various spoilage bacteria (Osman et al. Citation2013; Li et al. Citation2014), so they can incorporate in functional foods (Mamboya Citation2012; Abd El-Hack et al. Citation2020). Alcalase is a serine endopeptidase from Bacillus licheniformis marketed by NOVO, and it more active than trypsin and papain. Alcalase analyses various proteins to small polypeptides (Mamboya Citation2012). Enzymatic hydrolysis decreases the peptide size, making protein hydrolysate the most available amino acid source in human and animal food (Noman et al. Citation2018). Bumrungsart and Duangmal (Citation2019) explored the influence of Flavourzyme® (1–7%) on hydrolysis of gram bean PI for 2 h and the antioxidant potential of hydrolysate investigated, also, Meshginfar et al. (Citation2017) and Saad et al. (Citation2020) incorporated various protein hydrolysates as natural preservatives in meat products. In this work, Red kidney bean (Phaseolus vulgaris L.) protein isolates (RPIs) were hydrolysed by alcalase (Bacillus licheniformis) to obtain RBAH then RPI and RBAH were characterised. Pea PI was prepared, characterised and 11SGP globulin was isolated by precipitation with ammonium sulphate. Biological activities: antioxidant activity of RBAH and 11SGP, and their antimicrobial activity against certain spoilage bacteria and fungi were evaluated. Furthermore, the role of 11SGP and RBAH in raw buffalo meat preservation during cold conditions by coating technique.
Material and methods
Red kidney bean seeds (Phasolus vulgarus L), pea seeds (Pisum sativum) and buffalo meat sample were purchased from the Zagazig City market, Egypt. Alcalase enzyme, 2,2-diphenyl-1-picrylhydrazyl (DPPH) from Sigma, Muller Hinton agar (MHA) and Nutrient agar media (Oxoid, Thermo scientific, Basingstoke, UK). Electrophoresis reagents were acquired from Bio-Rad laboratories (Hercules, CA). Trichloroacetic acid (TCA), thiobarbituric acid obtained from ALAMIA (10th Ramadan B4, Egypt). All other chemicals used in experiments were of analytical grade. The gram-positive bacteria (Listeria monocytogenes ATCC 15313, Bacillus cereus ATCC 11778 and Streptococcus pyogenes ATCC 19615), and gram-negative bacteria (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Acinetobacter baumannii ATCC 19606), besides, fungi strains: Alternaria alternate, Aspergillus flavus, Fusaruim oxysporum and Monascus purpureus were used in this study.
Protein isolates preparation
Red kidney bean protein isolates (RPI) and red kidney bean alcalase hydrolysate (RBAH) preparation
Red kidney bean seeds were powdered and ten grams of powder was submerged with hexane in Soxhlet apparatus for 7 h for defatting then left to air-dry. RPI was isolated from defatted bean (5 g) according to Johnson and Brekke (Citation1983) then freeze-dried by a lyophiliser. The powdered RPI was mixed with alcalase (1:100, w/w) and dissolved in phosphate buffer pH 6 and incubated at 37 °C at an interval of (0, 15, 30 and 60 min), The enzyme was inhibited in boiling water for 15 min and the hydrolysate was obtained by centrifugation at (6000 xg, 30 min), the supernatant was lyophilised then kept for further analysis.
Pea protein isolate (PI) and 11SGP preparation
Green pea seeds were dried then converted to powder, and the resultant powder was defatted using n-hexane by Soxhlet apparatus for 7 h. Pea PI was separated from the defatted seeds powder using Johnson and Brekke (Citation1983) procedure. The 11S globulin was isolated from the defatted seeds powder of pea as described by Kimura et al. (Citation2008) with some modifications. Defatted pea flour was dissolved in 150 mL buffer (0.03 mol/L Tris–HCl at pH 8.5, 0.4 M NaCl, 10 mM β-mercaptoethanol, 1 mM EDTA, 0.02% (w/v) NaN3). The mixture was stirring for 1 h at 45 °C on a hot plate. 11S globulin was precipitated with ammonium sulphate (50–65%), and then centrifuged at 14,000 xg for 15 min and the precipitate was obtained and dialysed.
Chemical characterisation red kidney bean alcalase hydrolysate (RABH) and 11S pea globulin (11SGP)
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) of RBAH and 11SGP
The RPI with different degrees of hydrolysis (15, 30 and 60 min) and 11SGP fractionated by discontinuous SDS-polyacrylamide slab gel electrophoresis following the method of Laemmli (Citation1970).
Protein pH-solubility curves
The solubility curves were measured in the pH range (2–10) following Chobert et al. (Citation1991) with little modifications. Each peptide (0.125 g) was homogenised in 25 mL of distilled water pH (2–10) was adjusted by either NaOH or HCl (0.5 M) using pH metre (pH 211 HANNA instruments Inc., Woonsocket, RI made in Romania). The slurries were mixed for 1 h at 30 °C, and then cold centrifuged at 2000 xg for 20 min. The supernatant was obtained, and protein content was measured by the Kjeldahl method (AOAC Citation2005). The protein solubility (%) was measured against pH following the equation:
Degree of hydrolysis (DH)
The DH of red kidney total protein after intervals of 15, 30, and 60 min was estimated by Hoyle and Merritt’s (Citation1994) method. One mL of TCA (10%) was homogenised with RBAH supernatant (1:1 v/v), then centrifuged at (10,000 rpm, 10 min and 4 °C) to collect the supernatant in TCA 10%. Total nitrogen in the 10% TCA supernatant and the substrate was measured by the Kjeldahl method (AOAC Citation2005).
Biological activity of red kidney bean alcalase hydrolysate and 11S pea globulin (11SGP)
Antioxidant activity
The radical scavenging activity of RBAH (60 min hydrolysis) and 11SGP concentrations (25, 50, 100, 200, 400 and 800 µg/mL) was evaluated by altering the DPPH˙ purple-coloured solution compared to tertiary butylhydroquinone (TBHQ) as synthetic antioxidant according to Hatano et al. (Citation1988) with some modifications, 100 µL of each concentration was homogenised with 1 mL ethanolic DPPH then incubated in the dark for 30 min (Gülçin et al. Citation2004). The absorbance (Abs) was measured at 517 nm compared to the control colour. DPPH˙ antiradical activity (%) was estimated as the following:
The SC50 value (the lowest concentration that was scavenged 50% DPPH˙) was estimated by Bursal and Gülçin (Citation2011).
Antibacterial activity
Antibacterial activity was estimated by well diffusion assay according to Valgas et al. (Citation2007) with some modifications. For 100 µL of each activated bacterial inoculum (L. monocytogenes, B. cereus, S. pyogenes, E. coli, Ps. aeruginosa and A. baumannii) was spread over MHA plates, then wells (6 mm) were punched in MHA plates by sterilised crok. For 30 μL of each tested peptide concentration (25, 50, 100, 200, 400 and 800 µg/mL) was added to wells, then were incubated at 37 °C for 24 h. A transparent ruler has measured the produced zones of inhibition surrounded the wells (mm). The sterilised distilled water well was a control. The least concentration was inhibited the visible bacterial growth was minimum inhibitory concentration (MIC) and determined as follow 500 μL of each concentration of tested peptides (25, 50, 100, 200, 400 and 800 µg/mL) was added to 9 mL Muller Hinton broth (MHB) inoculated with 500 μL of pathogenic bacteria, the tubes were incubated for 24 h at 37 °C. The turbidity was measured at 600 nm (El-Saadony, Abd El-Hack, et al. Citation2020; El-Saadony, Elsadek, et al. Citation2020; Akl et al. Citation2020). Bacterial growth curve affected by RBAH and 11SGP (60 µg/mL) was conducted at an interval of 0, 6, 18 and 24 h by measuring the turbidity at 600 nm.
Antifungal activity
The antifungal activity of RBAH (60 min hydrolysis) and 11SGP were estimated by disc assay as per Ali‐Shtayeh and Abu Ghdeib (Citation1999) with some modifications. Mycelia disc (6 mm) was picked from fungal cultures and placed in the centre of each potato dextrose agar (PDA) plate, and paper discs (6 mm) were saturated with each peptide concentration (25, 50, 100, 200, 400 and 800 µg/mL) then placed on both sides of PDA plates. The control was paper disc saturated with sterilised distilled water, then the PDA plates were incubated at 28 °C for 5 d, and the reduction in fungal growth was measured (Cm) after 5 d). The least concentration of RBAH and 11SGP, which inhibited the fungal growth, was MIC. The MIC was measured as per Ali‐Shtayeh and Abu Ghdeib (Citation1999) and El-Saadony et al. (Citation2019).
Bio-preservative effect of red kidney bean alcalase hydrolysate (RABH) and 11S pea globulin (11SGP) in raw buffalo meat under cooling conditions
The RBAH with high antioxidant and antimicrobial activity obtained by Alcalase, E/S ratio of 1:100 (hydrolysis time = 60 min and DH = 30%), and 11SGP were considered a bio-preservative agent. The fresh raw buffalo was cut with a sterile knife in two groups. The meat samples were transferred to sterilised polyethylene bags.
Raw buffalo meat preservation experiment
Raw buffalo meat cubes (400 g) were divided into two groups (200 g), and then each group was subdivided into four subgroups (50 g) and placed in a plastic container in room condition. The meat cubes were submerged in RBAH and 11SGP concentrations (100, 250 and 400 µg/g) for 24 h then air-dried under sterile conditions. The treated samples were placed in enclosed plastic bags and stored at 4 °C for two weeks. Physicochemical, microbiological analysis, colour measurement and sensory evaluation of samples have been carried out at different intervals of (0–15 d) cold preservation.
Physicochemical analysis of meat sample
pH estimation
Five grams from each group were ground in a sterilised mincer and homogenised in 50 mL distilled water, then filtrated. Meat filtrate pH was determined following Özyurt et al. (Citation2012) using pH-metre (pH 211 HANNA instruments Inc. Woonsocket, RI made in Romania).
Metmyoglobin (MetMb) analysis
The MetMb contents in raw buffalo meat samples were determined as per Badr (Citation2007) as follow meat samples (5 g) were homogenised in 25 mL 40 mM phosphate buffer pH 6.8 for 10 s undercooling, then was kept for 1 h at 4 °C and centrifuged undercooling at (6000 rpm, 30 min), the supernatant Abs was measured at intervals of (525, 545, 565 and 572 nm) using a spectrophotometer (JENWAY 6405 UV/visible, Staffordshire, UK). MetMb (%) was measured using the following formula according to Krzywicki (Citation1982):
Lipid peroxidation assay
The inhibition of lipid peroxidation (%) was measured using the method of Niehaus and Samuelsson (Citation1968) as follow: meat filtrate acquired from the previous steps was prepared in phosphate buffer (50 mM, pH 7) and centrifuged at 14,000 rpm under cooling for 1 h to obtain the supernatant. Of 100 µL of the supernatant was added to tubes containing 2 mL of TBA–TCA–HCl reagent, boiled for 30 min, then cooled. The sample Abs measured at 535 nm using a spectrophotometer (JENWAY 6405 UV/visible, Staffordshire, UK) against control. Inhibition (%) calculated from the following equation:
Microbial analysis
The microbial load of minced raw buffalo meat supplemented with 100, 250 and 400 µg/g of RBAH and 11SGP was performed during cold storage periods (0–5 d), according to APHA (Citation1992). The minced meat sample was homogenised for 30 min with sterilised saline peptone buffer (1:9 w/v) in screw bottle at room temperature to prepare a suspension; the suspension (1 mL) was used to prepare decimal dilutions to 10−5. One mL of each dilution was placed in sterile one-use Petri-dishes then the medium was added, each dilution was mixed well in the medium (Abdelnour, Swelum, et al. Citation2020; Abdelnour, El-Saadony, et al. Citation2020). The total bacterial count (TBC) was enumerated on plate count agar (PCA) after incubation at 30 °C for 48 h. Also, PCA medium was used for counting psychrophilic bacteria count (PBC) after incubating for 10 d at 7 °C. Microbiological results were converted to logarithms (CFU/g) (Lee Citation2009; Reda et al. Citation2020; Sheiha et al. Citation2020).
Sensory evaluation and colour measurement
Sensory evaluation of raw buffalo meat supplemented with RBAH and 11SGP at different concentrations (100, 250 and 400 µg/g) was performed using 90 members panellists of students and staff of Zagazig University, Egypt. Each panellist was performed four different tests for each sub-sample and control samples at 0, 5, 10 and 15 d of cold storage. Supplemented samples with three random digits codes were presented in individual stands to each panellist for evaluation. Four attributes; colour, appearance, odour and overall acceptability were evaluated using a 9-point hedonic scale (9 = like extremely and 1 = dislike extremely). Samples with scores below five were deemed unacceptable.
Hunter lab colorimeter (ColorFlex EZ's 45°/0°, Virginia, USA) was used to estimate the colour of meat samples supplemented with legumes peptides CIELAB system: L* (lightness-darkness), a* (red-green) and b* (yellow-blueness) (Hunter Citation1975).
Statistical analysis
All experiments were done in five-replicate; the mean of five-replicate data were analysed by two-way ANOVA with a significance level of 5%, followed by LSD test to estimate the differences between means using SPSS version 19 (SPSS Inc., Chicago, IL).
Results and discussion
The characterisation of red kidney bean alcalase hydrolysate (RABH) and 11S pea globulin (11SGP)
SDS-PAGE of RBAH and 11SGP
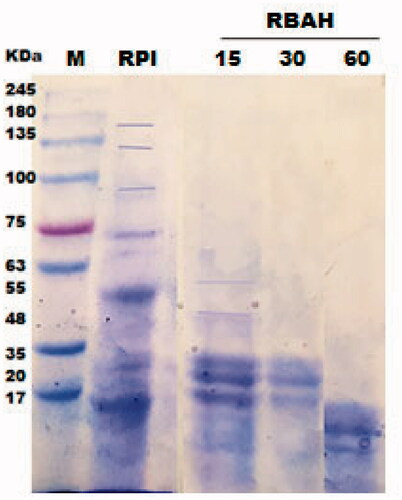
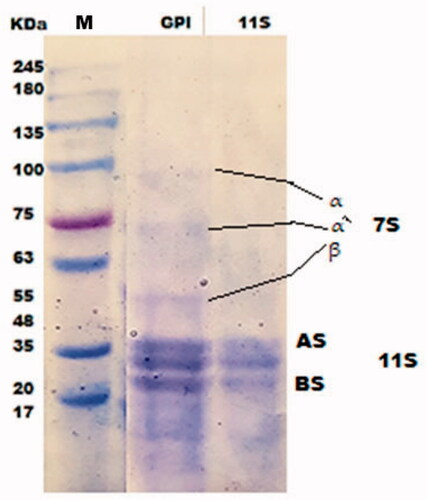
Figure shows SDS-PAGE electropherogram of RPI and RBAH, 12 protein bands with molecular weight ranged from 17 to 150 KD presented in RPI in lane 2, phaseolin or (8S vicilin) was the main storage protein in kidney bean presented in bands (48–55 KD). Similar results obtained by do Evangelho et al. (Citation2017), the 47 and 44 KD bands refer to Phaseolin are seen in SDS-PAGE profiles of black bean PI. Besides, this electrophoretic pattern agree with Montoya et al. (Citation2006) and Los et al. (Citation2020). Carrasco-Castilla et al. (Citation2012) indicated that the phaseolin was the abundant protein of the common bean. Also, Figure clears the profile of RBAH after different alcalase hydrolysis time; 8S vicilin bands remained after 15 min of hydrolysis but disappeared after 30 and 60 min of Alcalase hydrolysis, most RPI bands were disappeared in lane 5. do Evangelho et al. (Citation2017) found that phaseolin bands are seen in black bean PI, and pepsin hydrolysates after 2 h but protein bands higher than 50 KD disappeared after 6 h (Saad et al. Citation2020). Also, Los et al. (Citation2020) found that the presence of carioca bean (Phaseolus vulgaris L.) protein hydrolysates was between 36 and 20 kDa. The SDS-PAGE profile of PI and 11SGP (lanes 2 and 3) are shown in Figure . Pea PIs consisted of two main fractions 11S globulin (The molecular weight of 11S globulin subunits is 40 and 20 KD for the acidic and basic subunits, respectively) and 7S globulin (The molecular weight of 7S globulin subunits is 75, 60 and 52 KD for α/, α and β subunits, respectively). 11S globulin is a hexamer with (330 − 410 KD), each monomer of 60 KD can split into acidic (approximately 40 KDa), and basic (approximately 20 KDa) polypeptides via disulphide bond reduction (O’Kane et al. Citation2004). 11S globulin is originally more basic than 7S globulin (Osman et al. Citation2016).
Protein pH-solubility curves
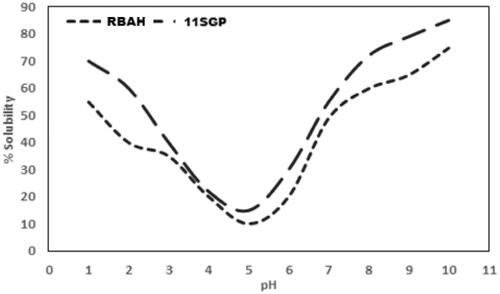
Figure shows the Iso-electric pH (lowest insolubility) of RBAH and 11SGP ranged from 4 to 7, reflecting their basic nature. Generally, protein solubility was significantly reduced (p ≤ .05) with pH increment; high solubility for RBAH and 11SGP was witnessed at pH 10 was 75, and 85%, respectively. Los et al. (Citation2020) found that carioca bean and soybean protein hydrolysate have solubility values of 35.13% at pH 3.0 and 100% at pH 7.0 and 9.0, respectively. Osman et al. (Citation2016) verified that the isoelectric point of 11S globulin isolated from soybean is 6.5. The high solubility of proteins required in many functional applications, particularly food applications, enhances the technological properties of supplemented foods (Faustino et al. Citation2019).
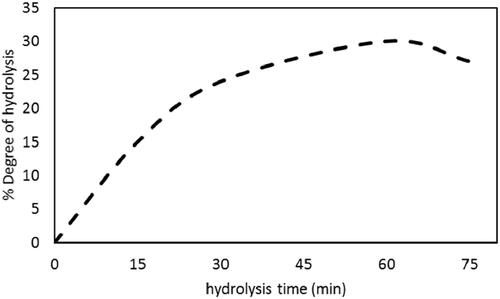
Degree of hydrolysis (DH)
Figure clears that DH of RBAH was significantly increased (p ≤ .05) with hydrolysis time, the highest DH value was accomplished after 60 min Alcalase hydrolysis (30%). Similar results obtained by do Evangelho et al. (2017) showed the highest DH = 27% of pepsin black bean protein hydrolysate achieved after 2 h. The highest DH = 75% when Bumrungsart and Duangmal (Citation2019) used Flavourzyme® (6%) for 6 h to hydrolyse black gram bean PI. DH = 33% when kidney bean PIs were treated with 0.1% pepsin for 6 h (Saad et al. Citation2020). Also, DH = 23 and 27% in buffalo skimmed milk and camel whey protein hydrolysed by Alcalase for 4 h, respectively (Abdel-Hamid et al. Citation2016; Abdel-Hamid et al. Citation2017). The obtained DH in the range of the previously reported values.
The biological activity of red kidney bean alcalase hydrolysate (RABH) and 11S pea globulin (11SGP)
Antioxidant activity
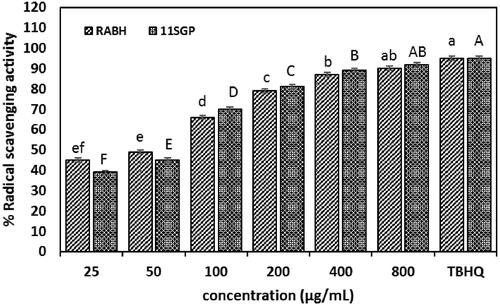
Figure shows that RBAH and 11SGP exhibit scavenging activity against TBHQ where SC50 were 100 and 120 µg/mL low SC50 values indicate strong radical scavenging activity (Zhu et al. Citation2011). RBAH and 11SGP (800 µg/mL) significantly scavenge 90 and 92% of DPPH radical, respectively, compared to 95% for synthetic antioxidant TBHQ. No significant differences in antiradical activity (p ≤ .05) between RBAH and 11SGP. Antioxidant peptides have critical importance in the food industry, where they keep the product quality by preventing the oxidation of proteins, lipids and nucleic acids (Nwachukwu and Aluko Citation2019). The antiradical mechanism of tested peptides depended on aromatic amino acids donating an electron to radicals for stability or hydrogen transfer according to peptides structure; the two mechanisms were acted parallel or dominated. Peptides contain hydrophobic amino acids that are improbably soluble in lipids through hydrophobic side chains. Basic and acidic amino acids were chelated metal ion and donated proton through their NH2 and COOH side chains (Sarmadi and Ismail Citation2010; Esfandi et al. Citation2019).
Figure 5. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of Alcalase-red kidney bean hydrolysate (RBAH) and 11S pea globulin (11SGP) concentration as compared to tertiary butylhydroquinone (TBHQ). Data are presented means ± SE, different lowercase and uppercase letters indicate significant differences p ≤ .05.
Antibacterial activity
Table and Figure show the diameter of inhibition zones (DIZ) of RBAH and 11SGP. Gram-positive bacteria were more susceptible than gram-negative bacteria. The DIZ was in the range of 19–43 mm for B. cereus, S. pyogenes and L. monocytogenes. In the range (24–32 mm) for gram-negative bacteria E. coli, A. baumannii and P. aeruginosa. 11SGP had significant p ≤ .05 higher DIZ than RBAH. However, RBAH was more pronounced against gram-positive bacteria than 11SGP. The MIC of RBAH was in range (80–90 µg/mL) and 11SGP was (120–160 µg/mL) against gram-positive bacteria, respectively, and (250–290 µg/mL), (145–190 µg/mL) against gram-negative bacteria, respectively. The lower DIZ observed in the gram-negative bacteria compared to gram-positive bacteria because the lipopolysaccharide layer in gram-negative bacteria membrane acts as a block banning the antibacterial agents penetration, besides enzymes in periplasmic space that annealing the foreign molecules (Holetz et al. Citation2002; Breijyeh et al. Citation2020).
Figure 6. Diameter of inhibition zones (DIZ) in tested G + and G − bacteria, i.e. 1) Bacillus cereus; 2) Staphylococcus pyogenes; 3) Escherichia coli; 4) Acinetobacter baumannii affected by Alcalase-red kidney bean hydrolysate (RBAH) and 11S pea globulin (11SGP), A-F, Alcalase-red kidney bean hydrolysate (RBAH) and 11S pea globulin (11SGP) concentration (25, 50, 100, 200, 400 and 800 µg/mL).
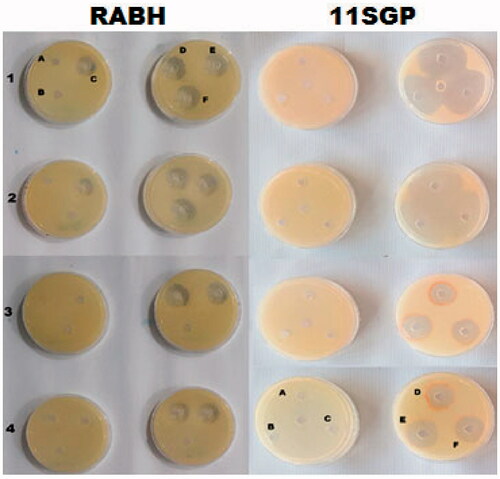
Table 1. Antimicrobial activity of red kidney bean alcalase hydrolysate (RABH) and 11S pea globulin (11SGP) (n = 5).
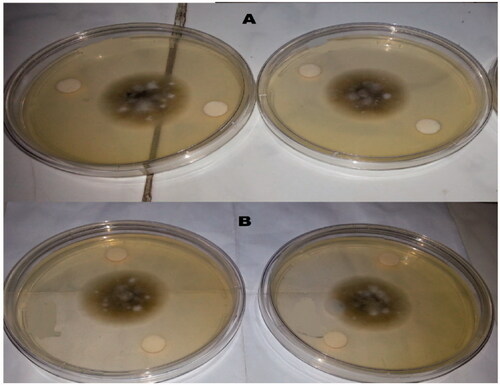
Antifungal activity
Table and Figure clear the reduction in fungal radial growth (Cm) after 5 d by RBAH and 11SGP, where the MIC was in the range of 55–80 µg/mL for all tested fungi. No significant difference between 11SGP and RBAH in fungal reduction. The antifungal activity of peptides cleared by Lei et al. (Citation2019) who stated that the function of antimicrobial peptides (AMPs) is to inhibit against a wide range of pathogens, including bacteria, fungi, and viruses as well as eukaryotic parasites. The activity of these peptides depends on their molecular structural characteristics, i.e. peptide size, amino acid composition, charge, hydrophobicity and the secondary structure. The antifungal peptides with molecular mass of 10 kDa isolated from mung bean (Phaseolus mungo) seeds (Wang et al. Citation2006). It exerted a potent inhibitory action towards a variety of fungal species including Physalospora piricola, Mycosphaerella arachidicola, Botrytis cinerea, Pythium aphanidermatum, Sclerotium rolfsii and Fusarium oxysporum.
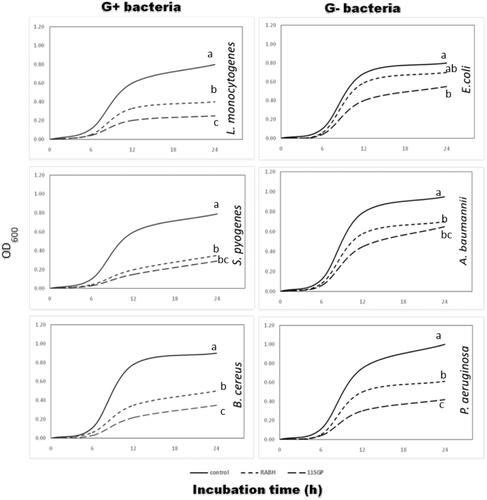
Bacterial growth curve
Figure shows that 11GSP (60 µg/mL) was significantly p ≤ .05 reduced gram-positive bacteria by 90%, and gram-negative bacteria by 60% compared to RBAH (60 µg/mL) that reduced the growth of gram-positive bacteria with 85% and 56% against gram-negative bacteria. Similar results obtained by Saad et al. (Citation2020) who stated that pepsin kidney bean protein hydrolysate after 6 h was inhibited the growth of gram-positive bacteria with 70–75% and gram-negative bacteria with 68–70%. The antimicrobial action of obtained peptides depends on their positive charges that electrostatically bound to compounds with negative charges on the bacterial cell wall and finally were demolished (Gobbetti et al. Citation2004; Jenssen et al. Citation2006; Cheruiyot et al. Citation2009). The hydrophobic nature of peptide plays a vital role in disquieting the cell wall and membrane of bacteria. The acting mechanism of AMPs is thought to be dependent on their capacity to form channels or pores within the microbial membranes which reduce the anabolic processes (Toldrá et al. Citation2018).
Bio-preservation of raw buffalo meat under cooling with RBAH and 11SGP
Physicochemical analysis
Table presents the changes in physicochemical properties of meat supplemented with RBAH and 11SGP during cold preservation; these changes affect the meat quality. The pH values significantly p ≤ .05 increased during the storage period from pH 6 to 7.2 in control samples, this increment reduced with a relative decrease of about 6–14% in meat samples supplemented with RBAH and 11SGP concentrations (100, 250 and 400 µg/g). Saad et al. (Citation2020) observed that minced beef supplementation with pepsin kidney bean hydrolysate (100 and 200 µg/g) reduced the pH increase by 10%. The pH was increased during storage because microorganisms were hydrolysed the proteins and lipids that produced NH3 (Karabagias et al. Citation2011). Nevertheless, the addition of BPs inhibited microbial growth. The met-myoglobin percentage on the meat surface was increased during cold storage because of myoglobin oxidation to metmyoglobin, and undesirable meat colour obtained (Chaijan Citation2008). The acceptable met-myoglobin percentage of consumers in meat products was 40%. This percentage significantly accomplished after 10 d in RBAH and 11SGP (100 µg/g). Still, the 400 µg/g concentration significantly maintained the acceptable rate for 15–20 d (Table ), similar results obtained by Hashemi Gahruie et al. (Citation2017). Lipid oxidation is the main factor affecting the meat lifetime during storage. The formation of hydroperoxides and aldehydes are indicators of lipid oxidation (Citta et al. Citation2017). Table shows the inhibition of lipid oxidation in RBAH and 11SGP-supplemented meat samples as compared to control, non-supplemented raw buffalo meat samples showed much significant p ≤ .05 decrease in lipid oxidation inhibition (10%) compared to RBAH and 11SGP-supplemented buffalo meat samples (15–27%) after 15 d of cold storage, similar results obtained by Hashemi Gahruie et al. (Citation2017).
Table 2. Physicochemical changes during raw buffalo meat supplemented with red kidney bean alcalase hydrolysate (RABH and 11SGP) during cold preservation for 15 d (n = 5).
Microbial changes during cold preservation of raw buffalo meat
Table shows a significant p ≤ .05 increases in microbial load during raw buffalo meat under cold preservation. Total count bacteria (mesophilic) and psychrophilic bacterial count significantly p ≤ .05 reduced by 44 and 37.5%, respectively, after 15 d of secured storage by the potential activity of RBAH and 11SGP (400 µg/g) as compared to control. Therefore, the meat quality extended for more than 15 d under refrigeration conditions. Saad et al. (Citation2020) used pepsin kidney bean protein hydrolysate in maintaining minced beef quality by reducing microbial load with 22%. The acceptable TBC in raw buffalo meat (<1 × 106 CFU/g) based on Egyptian Standards (E.S.) No. 4334/2004 (Egyptian Standard 2004) that set by the International Commission on Microbiological Specification for Food (ICMSF 1982).
Table 3. Total bacterial count (TBC) and psychrophilic bacterial count (PBC) of raw buffalo meat during storage for 15 d at 4 °C (n = 5).
Colour measurement and sensory properties during raw meat cold storage
Table displays raw meat colour parameters. Generally, a* and b* values significantly increased during storage. The a* and b* values were increased with increasing RBAH concentration but significantly decreased during the storage period because of the increment of % met-myoglobin (Hashemi Gahruie et al. Citation2017). The RBAH significantly increased p ≤ .05 the redness of meat than 11SGP because of the greenish colour of 11SGP, therefore, RBAH slightly enhanced the whiteness of meat colour. El-Saadony, Elsadek, et al. (Citation2020) revealed that kidney bean hydrolysate increased the whiteness of cucumber juice. Natural extracts have antioxidant activities can be potentially used to develop natural colour stabilisers, particularly for controlling L∗ and b∗ values in frozen beef burgers (Hashemi Gahruie et al. Citation2017).
Table 4. The fluctuations in colour parameters during RBAH and 11SGP-supplemented meat during storage for 15 d at 4 °C. (n = 5).
The sensory traits of RBAH and 11SGP-supplemented raw buffalo meat shown in Table . A reverse relation between all sensorial properties deterioration and storage time. Colour has significantly deteriorated in the control samples during storage and reached the minimum value after two weeks. This level is the lowest among all samples, representing 18% of the zero-point value. The colour deterioration of RBAH and 11SGP-supplemented raw buffalo meat samples was significantly delayed in a concentration-dependent manner. The (400 µg/g) concentration could maintain colour trait after two weeks of cold storage and equalled 48 and 68% of the zero time, respectively. The supplementation with RBAH and 11SGP significantly p ≤ .05 reduced the deterioration of odour corresponding to the concentration. The supplemented meat samples with RBAH and 11SGP (400 µg/g) significantly p ≤ .05 maintained (64 and 73%) of odour quality after two weeks of cold storage. The appearance and overall acceptability of stored raw buffalo meat followed the same trend in response to supplementation with RBAH and 11SGP. Generally, from the obtained results, it can be concluded, the supplementation of raw meat with RBAH and 11SGP lead to improve the colour, odour, appearance and overall acceptability during the storage periods (0–15 d), same results were obtained by Saad et al. (Citation2020).
Table 5. Sensory properties of RBAH and 11SGP-supplemented buffalo meat during the storage period (n = 5).
Conclusion
BPs RBAH and 11SGP exhibited potent antioxidant and antimicrobial activities because of their basic nature. The functionality of RBAH and 11SGP, i.e. solubility, was significantly improved as compared to total protein. Peptides coating of raw buffalo meat significantly increased the secure storage period by about ten days compared to control because of high antioxidant and antimicrobial activity of tested peptides. The obtained high solubility functional peptides might be integrated into functional foods.
Author contributions
M.T.E.-S., M.M.N. and B.A.A. designed the study plan, collected literature. M.T.E-S., M.M.N. and B.A.A. drafted the manuscript. M.E.A.E.-H., A.S., G.M.S., H.A.B.-A., E.O.S.H., W.R.E. and A.E.T. provided technical help in writing the manuscript and data analysis. All the authors read and approved the final manuscript.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1438-066).
Disclosure statement
All authors declare that they do not have any conflicts of interest that could inappropriately influence this manuscript.
Additional information
Funding
References
- Abd El-Hack ME, El-Saadony MT, Shafi ME, Zabermawi NM, Arif M, Batiha GE, Khafaga AF, Abd El-Hakim YM, Al-Sagheer AA. 2020. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int J Biol Macromol. 164:2726–2744.
- Abdel-Hamid M, Goda HA, De Gobba C, Jenssen H, Osman A. 2016. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int Dairy J. 61:91–98.
- Abdel-Hamid M, Otte J, De Gobba C, Osman A, Hamad E. 2017. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int Dairy J. 66:91–98.
- Abdelnour SA, El-Saadony MT, Saghir SAM, Abd El-Hack ME, Al-Shargi OYA, Al-Gabri N, Salama A. 2020. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest Sci. 240:104220.
- Abdelnour SA, Swelum AA, Salama A, Al-Ghadi MQ, Qattan SY, Abd El-Hack ME, Khafaga AF, Alhimaidi AR, Almutairi BO, Ammari AA, et al. 2020. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital J Anim Sci. 19(1):1046–1056.
- Ahmed J, Al-Ruwaih N, Mulla M, Rahman MH. 2018. Effect of high pressure treatment on functional, rheological and structural properties of kidney bean protein isolate. LWT Food Sci Technol. 91:191–197.
- Akl B, Nader MM, El-Saadony MT. 2020. Biosynthesis of Silver Nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J Agric Chem Biotechnol. 11(1):1–8.
- Ali‐Shtayeh M, Abu Ghdeib SI. 1999. Antifungal activity of plant extracts against dermatophytes. mycoses. 42(11–12):665–672.
- Al-Ruwaih N, Ahmed J, Mulla MF, Arfat YA. 2019. High-pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. LWT Food Sci Technol. 100:231–236.
- Anand S, Sati N. 2013. Artificial preservatives and their harmful effects: looking toward nature for safer alternatives. Int J Pharm Sci Res. 4(7):2496.
- Anyasi TA, Jideani AI, Mchau GR. 2017. Effects of organic acid pretreatment on microstructure, functional and thermal properties of unripe banana flour. Food Measure. 11(1):99–110.
- AOAC. 2005. Official methods of analysis 18th ed. Gaithersburg (MD): Pub AOAC International.
- APHA. 1992. American public health association. Compendium of methods for the microbiological examination of foods. 3rd ed. Washington, DC: APHA.
- Ashour EA, Abd El-Hack ME, Shafi ME, Alghamdi WY, Taha AE, Swelum AA, Tufarelli V, Mulla ZS, El-Ghareeb WR, El-Saadony MT. 2020. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 10(10):457. 2020,
- Badr HM. 2007. Antioxidative activity of carnosine in gamma irradiated ground beef and beef patties. Food Chem. 104 (2):665–679.
- Baltić MŽ, Bošković M, Ivanović J, Janjić J, Dokmanović M, Marković R, Baltić T. 2014. Bioactive peptides from meat and their influence on human health. Tehnol Mesa. 55(1):8–21.
- Bondi M, Lauková A, de Niederhausern S, Messi P, Papadopoulou C. 2017. Natural preservatives to improve food quality and safety. J Food Qual. 2017 (4):1–3.
- Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M. 2010. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 118(3):559–565.
- Boye J, Zare F, Pletch A. 2010. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res Int. 43(2):414–431.
- Breijyeh Z, Jubeh B, Karaman R. 2020. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 25(6):1340.
- Bumrungsart N, Duangmal K. 2019. Optimization of enzymatic hydrolysis condition for producing black gram bean (Vigna mungo) hydrolysate with high antioxidant activity. Food Appli Biosci J. 7(3):105–117.
- Bursal E, Gülçin İ. 2011. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int. 44(5):1482–1489.
- Carocho M, Barreiro MF, Morales P, Ferreira IC. 2014. Adding molecules to food, pros and cons: a review on synthetic and natural food additives. Compr Rev Food Sci Food Saf. 13(4):377–399.
- Carrasco-Castilla J, Hernández-Álvarez AJ, Jiménez-Martínez C, Jacinto-Hernández C, Alaiz M, Girón-Calle J, Vioque J, Dávila-Ortiz G. 2012. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 131(4):1157–1164.
- Centenaro GS, Salas-Mellado M, Pires C, Batista I, Nunes ML, Prentice C. 2014. Fractionation of protein hydrolysates of fish and chicken using membrane ultrafiltration: investigation of antioxidant activity. Appl Biochem Biotechnol. 172(6):2877–2893.
- Chaijan M. 2008. Review: Lipid and myoglobin oxidations in muscle foods. Songklanakarin J Sci Technol. 30(1):47–53.
- Chalamaiah M, Yu W, Wu J. 2018. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: a review. Food Chem. 245:205–222.
- Cheruiyot K, Olila D, Kateregga J. 2009. In vitro antibacterial activity of selected medicinal plants from Longisa region of Bomet district, Kenya. Afr Health Sci. 9(1):S42–S46.
- Chobert JM, Touati A, Bertrand-Harb C, Dalgalarrondo M, Nicolas MG, Haertle T. 1991. In vitro proteolysis and functional properties of reductively alkylated β-casein derivatives. J Dairy Res. 58(3):285–298.
- Choi JH, Kim KT, Kim SM. 2015. Biofunctional properties of enzymatic squid meat hydrolysate. Prev Nutr Food Sci. 20(1):67–72.
- Citta A, Folda A, Scalcon V, Scutari G, Bindoli A, Bellamio M, Feller E, Rigobello MP. 2017. Oxidative changes in lipids, proteins, and antioxidants in yogurt during the shelf life. Food Sci Nutr. 5(6):1079–1087.
- do Evangelho JA, Vanier NL, Pinto VZ, De Berrios JJ, Dias ARG, da Rosa Zavareze E. 2017. Black bean (Phaseolus vulgaris L.) protein hydrolysates: physicochemical and functional properties. Food Chem. 214:460–467.
- Dueñas M, Martínez-Villaluenga C, Limón RI, Peñas E, Frias J. 2015. Effect of germination and elicitation on phenolic composition and bioactivity of kidney beans. Food Res Int. 70:55–63.
- Egyptian Standard Specification “ESS” (No.4334/2004). 2004. Fresh meat. Egyptian Organization for Standardization and Quality Control.
- El-Saadony MT, Abd El-Hack ME, Taha AE, Fouda MMG, Ajarem JSN, Maodaa S, Allam AA, Elshaer N. 2020. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 10(3):587.
- El-Saadony MT, Elsadek MF, Mohamed AS, Taha AE, Ahmed BM, Saad AM. 2020. Effects of chemical and natural additives on cucumber Juice’s quality, shelf life, and safety. Foods. 9(5):639.
- El-Saadony MT, El-Wafai N, El-Fattah H, Mahgoub S. 2019. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv Anim Vet Sci. 7(4):238–249.
- Esfandi R, Walters ME, Tsopmo A. 2019. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon. 5(4):e01538.
- Faustino M, Veiga M, Sousa P, Costa EM, Silva S, Pintado M. 2019. Agro-food byproducts as a new source of natural food additives. Molecules. 24(6):1023–1056.
- Gobbetti M, Minervini F, Rizzello CG. 2004. Angiotensin I‐converting‐enzyme‐inhibitory and antimicrobial bioactive peptides. Int J Dairy Technol. 57(2–3):173–188.
- Gülçin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME. 2004. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 90(2–3):205–215.
- Halim NRA, Yusof HM, Sarbon NM. 2016. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci Technol. 51:24–33.
- Hashemi Gahruie H, Hosseini SMH, Taghavifard MH, Eskandari MH, Golmakani M-T, Shad E. 2017. Lipid oxidation, color changes, and microbiological quality of frozen beef burgers incorporated with Shirazi thyme, cinnamon, and rosemary extracts. J Food Qual. 2017:1–9.
- Hatano T, Kagawa H, Yasuhara T, Okuda T. 1988. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo). 36(6):2090–2097.
- Holetz FB, Pessini GL, Sanches NR, Cortez DAG, Nakamura CV, Dias Filho BP. 2002. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz. 97(7):1027–1031.
- Hoyle NT, Merritt JH. 1994. Quality of fish protein hydrolysates from herring (Clupea harengus). J Food Sci. 59(1):76–79.
- Hunter R. 1975. Scales for the measurements of color difference. The measurement of appearance. New York (NY): John Willy & Sons; p. 133–140.
- Jenssen H, Hamill P, Hancock RE. 2006. Peptide antimicrobial agents. Clin Microbiol Rev. 19(3):491–511.
- Johnson EA, Brekke C. 1983. Functional properties of acylated pea protein isolates. J Food Sci. 48(3):722–725.
- Karabagias I, Badeka A, Kontominas M. 2011. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 88(1):109–116.
- Kimura A, Fukuda T, Zhang M, Motoyama S, Maruyama N, Utsumi S. 2008. Comparison of physicochemical properties of 7S and 11S globulins from pea, fava bean, cowpea, and french bean with those of soybean french bean 7S globulin exhibits excellent properties. J Agric Food Chem. 56(21):10273–10279.
- Krzywicki K. 1982. The determination of haem pigments in meat. Meat Sci. 7(1):29–36.
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227(5259):680–685.
- Lee PS. 2009. Quantitation of microorganisms. Practical handbook of microbiology. Vol. 3. Boca Raton (FL): CRC Press; p. 19–38.
- Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q. 2019. The antimicrobial peptides and their potential clinical applications. Am J Trans Res. 11(7):3919–3931.
- Li YQ, Han Q, Feng JL, Tian WL, Mo HZ. 2014. Antibacterial characteristics and mechanisms of ɛ-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control. 43:22–27.
- Los FGB, Demiate IM, Prestes-Dornelles RC, Lamsal B. 2020. Enzymatic hydrolysis of Carioca bean (Phaseolus vulgaris L.) protein as an alternative to commercially rejected grains. LWT Food Sci Technol. 125:109191.
- Mamboya EAF. 2012. Papain, a plant enzyme of biological importance: a review. Am J Biochem Biotechnol. 8(2):99–104.
- Meshginfar N, Sadeghi Mahoonak A, Ghorbani M, Aalami M. 2017. Effects of protein hydrolysate from sheep visceral on oxidative stability of soybean oil and chicken sausage. J Food Process Preserv. 41(2):e12875.
- Montoya CA, Lallès JP, Beebe S, Montagne L, Souffrant WB, Leterme P. 2006. Influence of the Phaseolus vulgaris phaseolin level of incorporation, type and thermal treatment on gut characteristics in rats. Br J Nutr. 95(1):116–123.
- Niehaus W, Jr, Samuelsson B. 1968. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 6(1):126–130.
- Noman A, Xu Y, Al-Bukhaiti WQ, Abed SM, Ali AH, Ramadhan AH, Xia W. 2018. Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem. 67:19–28.
- Nwachukwu ID, Aluko RE. 2019. Structural and functional properties of food proteinderived antioxidant peptides. J Food Biochem. 43(1):e12761–13.
- O’Kane FE, Happe RP, Vereijken JM, Gruppen H, van Boekel MA. 2004. Heat-induced gelation of pea legumin: Comparison with soybean glycinin. J Agric Food Chem. 52(16):5071–5078.
- Osman A, Daidamony G, Sitohy M, Khalifa M, Enan G. 2016. Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int J Appl Res Nat Prod. 9:17–26.
- Osman AO, Mahgoub SA, Sitohy MZ. 2013. Preservative action of 11S (glycinin) and 7S (β-conglycinin) soy globulin on bovine raw milk stored either at 4 or 25 C. J Dairy Res. 80(2):174–183.
- Özyurt G, Kuley E, Balikçi E, Kaçar Ç, Gökdogan S, Etyemez M, Özogul F. 2012. Effect of the icing with rosemary extract on the oxidative stability and biogenic amine formation in sardine (Sardinella aurita) during chilled storage. Food Bioprocess Technol. 5(7):2777–2786.
- Reda FM, El-Saadony MT, Elnesr SS, Alagawany M, Tufarelli V. 2020. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 10(5):754.
- Romero J, Sun S-MM, McLeester RC, Bliss FA, Hall TC. 1975. Heritable variation in a polypeptide subunit of the major storage protein of the bean, Phaseolus vulgaris L. Plant Physiol. 56(6):776–779.
- Saad AM, Osman AOM, Mohamed AS, Ramadan MF. 2020. Enzymatic hydrolysis of phaseolus vulgaris protein isolate: characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int J Pept Res Ther. 26(1):567–577.
- Saguy, I.S. and Peleg, M. 2009. Accelerated and parallel storage in shelf-life studies. In: “An Integrated Approach to New Food Product Development” (Moskowitz, H.R., Saguy, I.S. and Straus, T., Eds.). Chapter 25 (pp. 429–455). CRC Press (Taylor and Francis), Boca Raton, FL.
- Sarmadi BH, Ismail A. 2010. Antioxidative peptides from food proteins: a review. Peptides. 31(10):1949–1956.
- Seabra RM, Andrade PB, Valentao P, Fernandes E, Carvalho F, Bastos M. 2006. Anti-oxidant compounds extracted from several plant materials. Biomaterials from aquatic and terrestrial organisms. Boca Raton (FL): CRC Press; p. 115–174.
- Sheiha AM, Abdelnour SA, El-Hack AME, Khafaga AF, Metwally KA, Ajarem JS, Maodaa SN, Allam AA, El-Saadony MT. 2020. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 10(3):430.
- Silphaduang U, Noga EJ. 2001. Peptide antibiotics in mast cells of fish. Nature. 414(6861):268–269.
- Speranza B, Corbo MR. 2010. Essential oils for preserving perishable foods: possibilities and limitations. In: Application of alternative food-preservation technologies to enhance food safety and stability. Vol. 23. Sharjah, UAE: Bentham Science Publishers; p. 35–37.
- Toldrá F, Reig M, Aristoy MC, Mora L. 2018. Generation of bioactive peptides during food processing. Food Chem. 267:395–404.
- Valgas C, Souza SMd, Smânia EF, Smânia A. Jr. 2007. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 38(2):369–380.
- Wang LS, Huang JC, Chen YL, Huang M, Zhou GH. 2015. Identification and characterization of antioxidant peptides from enzymatic hydrolysates of duck meat. J Agric Food Chem. 63(13):3437–3444.
- Wang S, Lin J, Ye M, Ng TB, Rao P, Ye X. 2006. Isolation and characterization of a novel mung bean protease inhibitor with antipathogenic and anti-proliferative activities. Peptides. 27(12):3129–3136.
- Zhu KX, Lian CX, Guo XN, Peng W, Zhou HM. 2011. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 126(3):1122–1126.