Abstract
This article reviews current knowledge on candidate genes and fatty acids in beef meat. It highlights the general situation of beef cattle in the world, the anatomy, and genetics of cattle, and discusses fatty acid groups in beef and their percentage in musculus longissimus dorsi and the musculus longissimus thoracis. Some selected genes that have been most researched in recent years are discussed in terms of many fatty acids that are associated with as well as sensory attributes of meat and the influence of the fatty acids themselves on sensory evaluation of meat.
Introduction
Beef is an important and nutritionally valuable food in the diet of the world’s population where the level of beef production is largely determined by available feed resources and production technologies. Beef consumption is determined by sociological, religious, cultural, economic, and other determinants. In total there are 1.51 billion cattle in the world, of which 325 million are in meat production, and beef production alone is estimated at 68 million tons. Globally, the average annual beef consumption is 9.1 kg/capita, ranging from 4.72 (Asia) to 36.1 kg North America (FAOSTAT Citation2020). Dairy cattle as well as local breeds, also significantly participate in the production of various categories of beef (excreted cows and male calf meat). At regional and global levels, a significant environmental footprint of beef production due to greenhouse gas emissions is a challenge for the future development of beef cattle production.
Meat played an essential role in the human diet both during evolution and in modern times. Although several studies reported that red meat consumption may increase cardiovascular disease and cancers, leading to a negative perception of the role of meat in health, current literature data do not support the association between high consumption of red meat and the risk of myocardial ischaemia (Soliman Citation2018). Moderate consumption of a variety of foods, including meat, enjoyed by people remains the best dietary recommendation, considering that meat consumption has increased recently and is likely to continue to increase in the future (Razmaite et al. Citation2020). This review attempts to summarise recent advances in scientific research on fatty acids in beef, focussing on the candidate genes most commonly used in scientific research on meat quality. The majority of articles available on Scopus from 2000 to 2020 with the keywords beef, fatty acids, candidate genes and gene polymorphism were considered in this review.
Introduction to cattle anatomy and genetics
The bovine genome was originally selected for sequencing because of the unique biology of ruminants, their importance as a major source of protein for humans, and the evolutionary position of cattle relative to other mammals (Tellam et al. Citation2009). The Bovine Genome Sequencing and Analysis Consortium sequenced the first genome of cattle (Hereford cattle) in 2009, which was the Year of the Ox according to the Chinese zodiac. It was part of an effort to improve livestock production and was one of the largest genomes ever sequenced at the time. Similarly, many single nucleotide polymorphisms (SNPs) were also generated from the partial sequence of six breeds (Holstein, Angus, Jersey, Limousin, Norwegian Red and Brahman). Together with the Hereford sequence, which served as the reference bovine genome, these six represent a valuable resource for marker-assisted selection of genetic traits in commercial breeding programs (Burt Citation2009). We now know that the bovine genome consists of 3 billion base pairs and contains approximately 22,000 genes (Tellam et al. Citation2009).
Beef is a source of protein and fat with saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), all of which contain essential amino acids. It is also a rich source of B group vitamins, as well as vitamins D, E and K, and minerals, such as iron, calcium, magnesium, phosphorus, potassium, sodium, selenium and zinc. Muscle tissue is very low in carbohydrates and contains no fibre (USDA Citation1989). Intramuscular fat (IMF) content and fatty acid composition are the most important factors contributing to nutritional value, along with the biological value of protein, trace minerals and vitamins (Wyness Citation2013). Fat deposits in the muscle fibres soften the meat during cooking and improve flavour through chemical changes induced by heat that allow protein and fat molecules to interact. When fat is cooked with the meat, the meat also appears juicier. The nutritional contribution of fat is mainly calories, as opposed to protein. The higher the fat content, the lower the nutritional contribution of meat (Usman et al. Citation2015).
The fatty acid composition of bovine fat is considered an important factor for several reasons. They reduce saturated fat, which can contribute to elevated serum cholesterol levels and coronary heart disease in humans, contribute fatty acids to the flavour attributes of beef, and give variation in fat softness (Kelly et al. Citation2013).
Skeletal muscles, including the longissimus dorsi muscle, are composed of myofibres (i.e. skeletal muscle fibres), fat cells, connective tissue or extracellular matrix, and various other cell types, including satellite cells, immune cells and blood vessels (Park et al. Citation2018). To better understand IMF deposition, it is important to identify the mechanisms responsible for the initial growth of muscle and fat cells. Their development is dependent on myogenesis (muscle cell formation) and adipogenesis (fat cell formation) (Du et al. Citation2010).
There are four main depots for adipose tissue in animals: abdominal, subcutaneous, intermuscular and intramuscular. Adipogenesis in cattle is first initiated in the abdominal adipocytes around the middle period of gestation (Du et al. Citation2013). Adipogenesis is a broad term that encompasses the conversion of mesenchymal stem cells into preadipocytes, the determination and proliferation of preadipocytes and the differentiation and conversion of preadipocytes into mature adipocytes (Hausman et al. Citation2009). Du et al. (Citation2013) also noted that this adipogenesis phase extends from the neonatal period for abdominal adipocytes to the early weaning phase for subcutaneous and intermuscular adipocytes, indicating that during the late fattening phase of cattle, marbling adipocytes may grow continuously, although the development of abdominal, intermuscular and subcutaneous fat may slow or cease.
Fatty acids in beef meat
Fatty acids are widely distributed in nature, food, and organisms. They belong to the class of lipids and are an important component of the cell membranes. They have many important biological functions; structural and functional and they are an important source of energy. Fatty acids are carboxylic acids with a long aliphatic chain that is either saturated or unsaturated. They are not usually found in organisms in their independent form but exist as three main classes of esters: triglycerides, phospholipids and cholesterol esters (Berg et al. Citation2019).
The fatty acid composition of meat, which is composed of muscle and fat tissue, is important for two reasons. First, it determines the nutritional value, and second, it affects various aspects of meat quality, including shelf life and flavour. Nutritional value is determined in part by the ratio of SFA to PUFAs and by the balance between fatty acids of the n-6 and n-3 series (Prado et al. Citation2009). Intermuscular fat in mature beef muscle is proportionally composed of an average of 0.45–0.48 SFA, 0.35–0.45 MUFA, and up to 0.05 PUFA. The ratio of polyunsaturated to SFAs (P:S) is typically low in beef, about 0.1, except in very lean animals (1% IMF) where the P:S ratio is much higher, about 0.5–0.7 (Vahmani et al. Citation2015).
Berg et al. (Citation2019) showed that one way to classify fatty acids can be length. Fatty acids are composed of a carboxylic acid with a long and unbranched chain of carbon atoms. Straight chain fatty acids are either saturated or unsaturated with one or more double bonds. According to the number of carbons, straight-chain fatty acids can be classified into short-chain fatty acids (SCFA, C2–C7), medium-chain fatty acids (MCFAs, C8–C13), long-chain fatty acids (LCFAs, C14–C19) and very-long-chain fatty acids (VLCFAs, C20+). The one other classification group of fatty acids are the straight-chain and odd-chain fatty acids (OFCAs). Most fatty acids are straight-chain, such as stearic and oleic acids. This means that an even number of carbon atoms compose the fatty acids in higher organisms such as mammals. The vast majority of biological mechanisms for fatty acids synthesis work are by the successive addition of two-carbon units in the form of acetyl-CoA. That’s way fatty acids are uniformly even numbers if they are produced from biological systems simply because the building blocks come in units of two, while OFCAs are biosynthesised and metabolised somewhat differently at the molecular level than their straight-chain relatives. The most common OFCAs are saturated C15 and C17 derivatives and pentadecanoic and heptadecanoic acids, respectively.
The human body can synthesise many of these fatty acids, with the exception of some essential PUFAs, such as linoleic acid (LA) and α-linolenic acid (ALA). LA is the most abundant fatty acid in nature and it is a precursor of other omega-6 fatty acids, while omega-3 fatty acids are synthesised from α-linolenic acid (Nagy and Tiuca Citation2017).
In animals, fatty acids are formed from carbohydrates in the liver, adipose tissue and mammary glands during lactation. Carbohydrates are converted to pyruvate by glycolysis, which is the first step in the conversion of carbohydrates to fatty acids. Pyruvate is then decarboxylated in the mitochondrion to acetyl-CoA. However, the acetyl-CoA must be transported to the cytosol, where acetyl-CoA citrate (formed by the condensation of acetyl-CoA with oxaloacetate) is removed from the citric acid cycle and transported across the inner mitochondrial membrane into the cytosol. There it is cleaved by ATP citrate lyase into acetyl-CoA and oxaloacetate. The oxaloacetate is returned to the mitochondrion as malate. The cytosolic acetyl-CoA is carboxylated by acetyl-CoA carboxylase to malonyl-CoA, the first step in fatty acid synthesis. In the second step, malonyl-CoA is involved in a repetitive series of reactions that lengthens the growing fatty acid chain by two carbons at a time. Almost all-natural fatty acids have an even number of carbon atoms. When synthesis is complete, the free fatty acids are usually combined with glycerol (three fatty acids form a glycerol molecule) to form triglycerides, the main storage form of fatty acids and energy in animals. Fatty acids are also important components of phospholipids, which form the phospholipid layers that make up all cell membranes (Berg et al. Citation2019).
Fatty acid composition has been shown to vary between different breeds of cattle, suggesting that lipid metabolism is under at least some degree of genetic control and that fatty acid profiles could be altered by genetic selection. Genetic variability consists of differences between species, differences between line breeds, differences due to crossbreeding between breeds and differences between animals within breeds (Lemos et al. Citation2017). Fatty acid composition is complex traits and are difficult to measure. But application of genomic selection and candidate genes for fatty acids, it will be valuable in future selection breeding programs (Zhu et al. Citation2017).
Percentage of fatty acids in beef meat
About 80% of the fatty acids in beef consist of SFAs which are predominantly 14:0 (myristic acid), 16:0 (palmitic acid), and 18:0 (stearic acid). The remaining 20% is distributed among 30 different fatty acids (Abbas et al. Citation2009).
Oleic acid (C18:1) is the primary MUFA in beef and accounts for approximately 33% of the fatty acid in beef. Palmitic acid (C16:0) is a SFA that makes up about 27% of the fatty acids in beef. There is very strong evidence that palmitic acid increases serum cholesterol levels, predominantly by increasing bad cholesterol (LDL). This fatty acid is responsible for most of the cholesterol raising effect of beef, increasing the risk of atherosclerosis, cardiovascular disease and stroke (Mozaffarian et al. Citation2005). Stearic acid (18:0) is a SFA that accounts for about 18% of the fatty acid in beef. Several studies have shown that the effect of stearic acid on total cholesterol level is minimal and not harmful to health. Stearic acid is essentially neutral in its effect on total serum cholesterol, like oleic acid.
Beef meat is also a source of two SFA, lauric (C12:0) and myristic (C14:0) acids, which have been associated with human health. Lauric and myristic fatty acids are responsible for increasing bad cholesterol levels in blood serum and are strongly associated with early heart attack. The proportions of lauric (less than 1%) and myristic (2–3%) acids in beef are low.
Fatty acids with an odd number of carbon atoms, such as pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) (less than 1 and 1–2% of fatty acids in beef, respectively), are mainly formed by the accumulation of a 3-carbon fatty acid product resulting from vitamin B12 deficiency. OFCAs can accumulate in the membrane lipids of nervous tissue, leading to altered myelin integrity and demyelination, ultimately resulting in impaired nervous system function (Müller et al. Citation1998).
Elaidic acid (C18:1) (2–5% of the fatty acid in beef), is a trans MUFA that can increase serum bad cholesterol (LDL). Trans fatty acids can act like SFA. Studies have shown that foods enriched with C18:1-trans resulted in higher levels of bad cholesterol (LDL) compared to C18:1-cis (Abbas et al. Citation2009). Although C18:1-trans increased bad cholesterol (LDL) compared to saturated fat, it had no effect on good cholesterol (HDL). Other MUFA include palmitoleic acid (C16:1) (approximately 2–3%), trans-vaccenic acid (TVA, C18:1-trans-11) (3–4%) and vaccenic acid (C18:1-cis-11) (1–2%).
The major influential PUFA in beef are LA (C18:2) (about 3.5%), alpha-linolenic acid (C18:3) (1.5%), arachidonic acid (C20:4) (about 1%), eicosapentaenoic acid (EPA) (C20:5) (<1%), docosanpetaenoic acid (DPA-3) (C22:5) (<1%) and docosahexaenoic acid (DHA) (C22:6) (<1%) (Enser et al. Citation1998).
Dannenberger et al. (Citation2004) reported 10 isomers of conjugated LA (CLA) in beef, with CLA cis-9, trans-11 accounting for approximately 70% of the total CLA isomers. The biological effects of two of these isomers have been extensively studied. The anticarcinogenic and antiatherogenic effects of cis-9, trans-11 and the anti-obesity effects of trans-10, cis-12 are well documented (USDA Citation1989) (Figures ).
Figure 1. Summary of least square means of fatty acid composition of longissimus dorsi muscle in various cattle breeds (*Kelava Ugarković et al. Citation2013; **Papaleo Mazzucco et al. Citation2016; ***Jaborek et al. Citation2019).
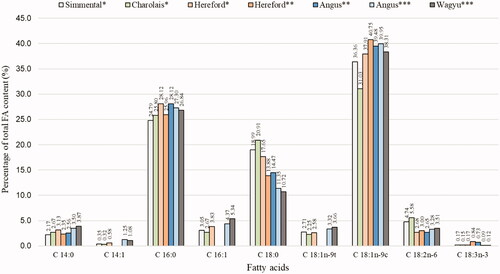
Figure 2. Summary of least-square means of fatty acid composition of longissimus thoracis muscle in various cattle breeds and genotypes (*Bartoň et al. Citation2010; **Matsuhashi et al. Citation2011; ***Oh et al. Citation2012; ****Sasago et al. Citation2017; ‘*’Flowers et al. Citation2018; ‘**’Momot et al. Citation2020).
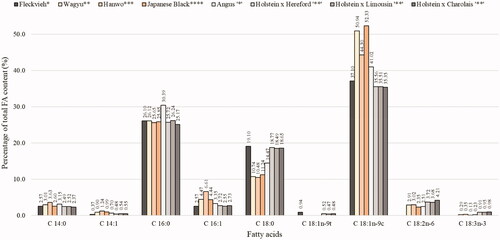
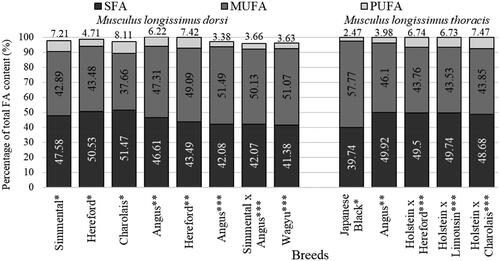
Figure 3. Summary of least-square means of fatty acid composition of longissimus dorsi (*Kelava Ugarković et al. Citation2013; **Papaleo Mazzucco et al. Citation2016; ***Jaborek et al. Citation2019) and longissimus thoracis muscle in various cattle breeds and genotypes (*Sasago et al. Citation2017; **Flowers et al. Citation2018; ***Momot et al. Citation2020).
Genes involved in fatty acid synthesis
Molecular genetics has made tremendous progress leading to the identification of genes or genetic markers that influence meat quality traits. The candidate gene strategy was proposed by a direct search for quantitative trait loci (QTL). Genetic markers associated with the traits of interest can be searched directly by applying molecular biology techniques. With these techniques, genetic variation at specific loci can be determined and the relationship between genetic variation at QTL and production traits can be analysed. The use of molecular genetics for genetic improvement is based on the ability to genotype individuals for specific genetic loci (Raza et al. Citation2020). QTLs are chromosomal positions delineated by alleles associated with a measurable effect on a quantitative trait. A key step is mapping QTLs to identify genes that contribute to variation in quantitative traits rather than identifying functional candidate genes based on metabolic pathways. Some candidate genes have been found to have DNA polymorphism associated with beef quality traits.
Candidate genes are selected based on a biological understanding of the role of the wild-type product of that gene. A candidate gene is a gene thought to be associated with a particular disease or phenotypic trait whose biological function is inferred either directly from other studies, including animal model studies with other species (using comparative genomic studies), genome-wide association studies (GWAS), classical map-based positional cloning methods and newer next-generation sequencing (NGS) methods (Giri and Mohapatra Citation2017).
A candidate gene approach used in genetic association for a variety of species may be useful to improve our understanding of genes responsible for regulating traits related to the underlying metabolism of muscle growth, as candidate genes possess specific biological actions related to different aspects of a target trait, candidate gene approaches may provide a valuable strategy for detecting the QTL that controls genetic variation in an important trait (Bordbar et al. Citation2019).
Recently, genes responsible for fatty acid composition of beef have been identified, and the number of reports on this topic is increasing. Table provides a summary overview of genes responsible for fatty acid composition over the past 20 years. The following is a description of the genes that are most frequently mentioned in the scientific literature in connexion with the quantitative characteristics of meat quality.
Table 1. Genes associated with fatty acid composition (Hirwa et al. Citation2010; Jeong et al. Citation2012; Berton et al. Citation2016).
Diacylglycerol acyltransferase 1 gene (DGAT1)
Diacylglycerol acyltransferase 1 (DGAT1) is a microsomal enzyme encoded by the DGAT1 gene located on chromosome 14 in cattle that processes diacylglycerol and fatty acid acetyl-CoA as substrates to catalyse the final step of triglyceride synthesis and has been shown to affect IMF in Angus cattle (Anton et al. Citation2011). The deposition of IMF is mainly determined by lipid metabolism, which dictates the balance between fat deposition and breakdown in the longissimus dorsi muscle (Jeong et al. Citation2012). Studies showed that the DGAT1 gene is significantly associated with fat-related traits such as back fat thickness and marbling in beef (Thaller et al. Citation2003; Pannier et al. Citation2010).
Thyroglobulin gene (TG)
Thyroglobulin (TG) is a glycoprotein hormone stored in the thyroid gland that accounts for 75% of total thyroid protein and serves as a substrate for the synthesis of thyroid hormones (Kaczor et al. Citation2017). It is synthesised in the follicular cells of the thyroid gland and is a precursor of triiodothyronine (T3) and thyroxine (T4). It plays an important role in the regulation of metabolism and may influence adipocyte growth and differentiation as well as fat depot homeostasis. A polymorphism in TG has been associated with back fat thickness and marbling in beef cattle (Dubey et al. Citation2014).
Barendse et al. (Citation2006) detected a putative QTL on bovine chromosome 14 by association with the CSSM66 microsatellite locus. Based on the close association with this locus, authors identified the gene encoding TG. TG was also considered as a functional candidate gene because its product is the precursor of hormones that affect lipid metabolism.
The TG gene in cattle is one of the most important genes affecting lipid levels and its metabolism (Yardibi et al. Citation2013) and its DNA genomic region includes 37 exons and covers a genomic region of at least 300,000 bp (Kaczor et al. Citation2017).
Fatty acid-binding proteins gene (FABP4)
Fatty acid-binding proteins (FABPs) are a family of small cytoplasmic proteins that bind long LCFA and other hydrophobic ligands and their main functions are the transport and metabolism of fatty acids. They play a role in beta-oxidation of fatty acids by participating in their transport from the cytoplasmic membrane. The tissue-specific cytoplasmic proteins belonging to this family are known as FABP1-FABP9, and the most common isomorphs are FABP3-FABP5 (Kaczor et al. Citation2017).
Highly expressed in mature tissues, FABP 4 is a carrier protein for fatty acids that is mainly expressed in adipocytes and macrophages and encodes adipocyte FABP, an enzyme involved in the intermuscular transport of fatty acids. FABP4 is involved in the uptake, transport and metabolism of fatty acids and is an important metabolic indicator of an animal's ability to store intermuscular fat (Jurie et al. Citation2007). Blecha et al. (Citation2015) reported that FABP4 is expressed in adipose tissue and plays an important role in lipid metabolism and homeostasis in adipocytes and is associated with lipid metabolism (lipolysis and lipogenesis), marbling and dorsal fat deposition.
Fatty acid composition of adipose tissue in livestock has been recognised as one of the economically important carcase traits affecting meat quality, as higher concentration of MUFA leads to lower fat melting point and affects fat softness and favourable beef flavour (Narukami et al. Citation2011). Fatty acid composition could be controlled by genetic factors, such as lipid synthesis and genes related to fatty acid metabolism, as shown by many authors in their studies (Avilés et al. Citation2013; Urrutia et al. Citation2020). Hoashi et al. (Citation2008) reported that FABP4 is one of the cytoplasmic proteins involved in intracellular transport and metabolism of free fatty acids and binds long-chain fatty acids with high affinity. In their report, Japanese black steers had higher rib thickness and SFA content, while heifers had higher MUFA content.
Berton et al. (Citation2016), in relation to the FABP4 gene, reported that it operates in the FABPs domain, and it was downregulated for omega-3 and omega-6 fatty acids. In general, FABPs are often associated with lipid metabolism by acting as intercellular transport of hydrophobic.
Fatty acid synthase gene (FASN)
Fatty acid synthase (FASN) is a multifunctional enzyme that plays a central role in mammalian lipid metabolism and regulates the de novo biosynthesis of long-chain fatty acids. With seven active sites, FASN catalyses all reaction steps of the conversion of acetyl-CoA and malonyl-CoA to palmitate in the presence of NADPH (Roy et al. Citation2005). In animals, the synthesis of FASN is a regulated process that depends on nutrition and hormones at all life stages, including during neonatal development and differentiation (Roy et al. Citation2005; Morris et al. Citation2007). The bovine FASN gene has been mapped to BTA19 (Zhang Citation2008). Zhang also found that the thioesterase (TE) domain responsible for fatty acid synthesis is located in the four exons (39–42) of the FASN complex. They also concluded that the TE domain determines the product chain length of FASN and it varies in different individuals. In their report, they found that the g.17924A>G SNP, which causes an amino acid change from threonine to alanine, was associated with the C14:0, C16:0, C18:1, SFAs, and MUFAs in the longissimus dorsi muscle of Angus bulls. Yeon et al. (Citation2013) found that the SNP was significantly associated with C16:0, C16:1, C18:1, SFA, and unsaturated fatty acids in Hanwoo steers. Li et al. (Citation2012) found in their study that the g.17924A>G SNP was associated with a wide range of SFAs from 10:0 to 20:0, several PUFAs, and a single long-chain PUFA. On the other hand, the associations of the AA genotype with higher levels of SFA 14:0 and lower levels of 9c-18:1 were consistent with the results reported by Zhang (Citation2008) for the longissimus dorsi muscle of Angus bulls. Bartoň et al. (Citation2016) reported that the mentioned SNP located in exon 39 showed in Holstein cattle that the animals with the AA genotype had higher proportions of C14:0, C16:0, C14:1 n-5 and SFA in muscle fat, while the proportions of C18:1 n-9 and MUFA and the MUFA/SFA ratio were lower than in animals with the GG genotype. Morris et al. (Citation2007) concluded that the listed SNP was associated with the proportion of myristic acid in the adipose tissue of a beef cattle cross between Jersey and Limousin.
Jeong et al. (Citation2012) reported a correlation between the expression frequency of lipid metabolism genes and IMF content in the longissimus dorsi muscle of Korean cattle and concluded that FASN gene expression had a positive correlation with IMF content (p<.01).
Kaplanová et al. (Citation2013) confirmed no significant effect on SFA, MUFA or PUFA in GG FASN genotype, but they observed a significant difference between AG and GG genotype with the content of myristic acid and palmitic acid in their crossbred cattle. They concluded their study by stating that the A allele has a positive effect on unsaturated fatty acid content, but the A allele is associated with lower content compared to the G allele.
Stearoyl-CoA desaturase gene (SCD)
Stearoyl-CoA desaturase (SCD) is an enzyme encoded by the SCD gene and plays an important role in determining the fatty acid profile of ruminant tissues (Ntambi Citation2013), as it is responsible for the conversion of SFA into MUFA (Hoashi et al. Citation2008). This enzyme, localised in the endoplasmic reticulum, inserts a double bond between carbons 9 and 10 in SFA and affects the fatty acid composition of membrane phospholipids, triglycerides and cholesterol esters, and it is also a key enzyme in the endogenous production of the cis-9, trans-11 isomer of CLA (Ntambi Citation2013). It is responsible for the conversion of SFA to MUFA in mammalian adipocytes (Kay et al. Citation2004) and plays a rate-limiting role in the synthesis of unsaturated fatty acids by insertion of a cis-double bond in the delta9 position of fatty acids, with palmitate (16:0) and stearate (18:0) proposed as preferred substrates to be converted to palmitoleate (9c-16:1) and oleate (9c-18:1), respectively (Ntambi Citation2013). According to Ohsaki et al. (Citation2007), the SCD gene affects the presence of stearic acid and oleic acid in beef while Milanesi et al. (Citation2008) found that the SCD enzyme is also involved in the endogenous synthesis of CLA. Wood et al. (Citation2007) showed that oleic acid (18:1cis-9), an important fatty acid in meat, is formed from stearic acid (18:0) by the enzyme SCD, an important lipogenic enzyme, while Wang et al. (Citation2019) reported that SNPs within the SCD gene showed significant associations with C18:1 cis-13 in Canadian commercial bulls, in Japanese black and in Spanish cattle breeds. Both fatty acids are predominant in the neutral lipid. However, diet has been shown to contribute to the fatty acid profile, while genetic factors remain to be elucidated (Mannen Citation2012). SCD is a candidate gene for genetic variation in fatty acid composition.
The bovine SCD gene is located on chromosome 26 and consists of 6 exons and 5 introns (Alim et al. Citation2012). Jiang et al. (Citation2008) reported that the SCD gene was significantly associated with six traits of fat deposition and fatty acid composition in skeletal muscle but not with depth of subcutaneous fat in a Wagyu × Limousin reference population. Taniguchi et al. (Citation2004) described eight SNPs, including c.878 T>C. This SNP was predicted to cause a substitution of the amino acid valine for alanine at amino acid position 293, and this substitution affects amino acid composition and IMF- melting point (Dujková et al. Citation2015). Mannen (Citation2011) confirmed that alanine, compared to valine, increases the proportion of MUFAs at the expense of SFA and lowers the melting point of IMF.
Taniguchi et al. (Citation2004) identified a SNP in the fifth exon of this gene c.878 T>C that causes an amino acid switch from alanine to valine. They also found that the SNP was associated with the percentage of MUFAs and the melting point of intermuscular fat in Japanese Black steers.
The CC genotype of the SCD c.878 T>C SNP was reported to be associated with a higher amount of MUFA containing 9c-14:1, 9c-16:1 and 9c-18:1 and a lower melting point in the intermuscular fat of Japanese Black steers (Taniguchi et al. Citation2004). Ohsaki et al. (Citation2007) found that the CC genotype of the same SNP was significantly associated with a lower concentration of 18:0 and a higher concentration of 18:1 (apparent 9c-18:1) in both perirenal and intermuscular adipose tissue of Japanese Black cattle, whereas the associations with 16:0 or 16:1 (apparent 9c-16:1) were not significant. Bartoň et al. (Citation2010) found in a population of Simmental bulls that the CC genotype was significantly associated with a lower concentration of 18:0 in both intermuscular and subcutaneous fat and a higher concentration of 9c-18:1 in muscle fat, but associations with 16:0 or 9c-16:1 were not significant.
Li et al. (Citation2012) found in their study that adipose tissue from Canadian Angus and commercial crossbred beef from Charolais cattle had no significant correlations for palmitate (16:0), stearate (18:0), palmitoleate (9c-16:1), or oleate (9c-18:1). They also found a remarkable consistency of the association of SCD c.878 T>C SNP with 9c-14:1 reported previously, indicating a predominant effect of SCD on 14:0 desaturation in cattle, and the results also suggest that the SCD SNP is a causative or closely associated with causative mutations for 14:0 desaturation, while Kaplanová et al. (Citation2013) reported in their study that the influence of CC genotype on lower stearic acid (C18:0) was not confirmed.
Mannen (Citation2012) reported that the polymorphism of SCD gene is associated with fatty acid composition. Since gene expression may be related to this trait, the effect of gene expression was investigated, and the results showed that the level of SCD mRNA expression was significantly higher (p<.05) in Japanese Black cattle than in Holstein Cattle. In addition, Japanese Black cattle consistently had higher (p<.05) levels of MUFAs than Holstein cattle. The authors concluded that these results suggest that differences in SCD gene expression may contribute to the differences in fatty acid composition between Japanese Black and Holstein cattle.
SCD (delta-9 desaturase) mRNA expression level was associated with MUFA content in Holstein Japanese Black cattle and SNP in Japanese Black cattle, which contributed to higher MUFA content and lower melting point in IMF (Taniguchi et al. Citation2004). The relationship between fat content and P:S ratio is noteworthy. Since the content of SFA and MUFA increases faster than the content of PUFA with increasing fat content, the relative proportion of PUFA and the P:S ratio decrease with increasing fat content. A low fat content (1%) explains the advantageously high P:S ratio (0.5–0.6) in the double-muscled Belgian Blue bulls (Dannenberger et al. Citation2004).
Growth hormone gene (GH)
Many studies considered growth factors and other regulatory proteins associated with the somatotropic axis as promising candidate markers for quantitative traits in livestock. Genes encoding growth hormone (GH), GH receptor (GHR), transcription factor PIT-1 (which activates expression of GH and prolactin genes in the anterior pituitary), insulin-like growth factor-I (IGF-1) and perhaps yet unknown genes encoding GH signal transduction pathways could contribute to progress in genetic selection of livestock (Oprządek et al. Citation2005). Bovine GH has been intensively studied as a genetic marker because it has important functions related to animal growth and production. It acts directly by binding to its receptors on progenitor bone, muscle and fat cells and triggers cell proliferation (Ardiyanti et al. Citation2009). The GH gene has been proposed as a candidate gene for genetic variants in meat production traits because of its essential role in physiological mechanisms related to growth in their products. The biological effects of GH involve a variety of tissues and the metabolism of all nutrients: carbohydrates, lipids, proteins and minerals.
Growth hormone is a single-chain polypeptide composed of 191 amino acids that is synthesised and secreted by the anterior pituitary gland under the hypothalamic control of two hormones. The first is GH-releasing hormone (GHRH), which increases GH secretion, and the second is somatotropin-releasing inhibitory factor (SRIF, also called somatotropin), which inhibits its secretion (Silveira et al. Citation2008).
Few studies have investigated the effects of this gene on growth performance and carcase traits in cattle, such as the study by Ardiyanti et al. (Citation2009). While a SNP in bovine GH was detected in Holstein cattle by a nucleotide substitution of CTG/GTG at codon 127 (Sørensen et al. Citation2002), a polymorphic substitution of ACG/ATG at codon 172 was observed by Ardiyanti et al. (Citation2009) in Japanese Black and Brown cattle. Silveira et al. (Citation2008) reported significant effects of GH polymorphism at codon 127 on somatic measurements and body weight, as well as on breeding values for meat traits and growth hormone concentration. The presence of the AluI restriction site corresponds to the presence of the amino acid leucine (L) at position 127 in the bovine GH polypeptide chain, whereas the absence of this site indicates the presence of valine (V) at the same position. Schlee et al. (Citation1994) in their study on the GH gene showed a significant effect of the L/V genotype on the beef breeding values of Simmental bulls, with the heterozygous LV -genotype being superior to the homozygotes LL and VV in both carcase gain and beef value. In their study, di Stasio et al. (Citation2002) found that the GH locus showed greater effects on body weight in Piedmontese cattle. Ardiyanti et al. (Citation2009) in their study on Japanese Black cattle investigated the polymorphism of GH codon at 127 and 172 and found that one group of cattle had higher proportion of C18:1, MUFA, USFA and MUFA/USFA and USFA/SFA in contrast to the other group which had lower proportion of C16:0 and C18:0. Maharani et al. (Citation2012) in their study on Hanwoo cattle found that nucleotide substitution at codons 127 and 172 was not associated with fatty acid trait. A more recent study by Bordonaro et al. (Citation2020) conducted on Modicana cattle in southern Italy showed that the LL genotype of the GH gene had higher levels of total unsaturated and MUFAs in codon region 127.
Sensory attributes of beef meat
According to Ressurreccion (Citation2004), the main reasons that the average consumer considers when choosing meat and meat products are hygiene, safety, freshness, nutritional value, clear declaration, ingredients, price, packaging, brand reputation, fitness for use, shelf-life characteristics, suitability for specific occasions, origin, ethical aspects, environmental aspects, appearance and sensory characteristics (taste, colour, smell, etc.).
Sensory analysis is a scientific discipline concerned with the measurement, analysis and interpretation of the responses of those food characteristics that we perceive through the senses of sight, smell, taste, hearing, and touch. The decision to purchase beef is guided by the perception of a variety of sensory characteristics including organoleptic properties such as colour, tenderness, juiciness and flavour. These characteristics are used by consumers as the basis for determining meat quality. Taste is an important meat quality characteristic related to consumer perceived satisfaction with the food, followed by appearance and tenderness (López-Pedrouso et al. Citation2020).
Sensory acceptability is an important factor that addresses consumers' needs and influences their purchasing decisions, although unfortunately the sensory profile of meat products often has a high and uncontrollable variability due to production and technology-related factors and intrinsic meat characteristics. The main sensory meat attributes relate to visual appearance and oral perception in terms of texture and taste (López-Pedrouso et al. Citation2020). Texture is a sensory attribute with several parameters (tenderness, juiciness, suppleness, fibrousness and coarseness), of which tenderness and juiciness are the most positively evaluated. Moreover, meat flavour is produced after a thermal process that releases volatile compounds mainly due to lipid degradation and Maillard reactions. Sensory characteristics are influenced by the age of the animal, breed, feeding, pre-slaughter treatment and maturation.
Differences in the taste and flavour of certain meats are related to the amount, composition and nature of lipid breakdown during processing. The action of lipases and phospholipases of muscle and adipose tissue on triacylglycerols (triglycerides) and phospholipids leads to the accumulation of free fatty acids, whose autoxidation (or enzymatic oxidation) produces volatile components with specific aromas and flavours associated with aroma and taste (Timón et al. Citation2001). Free fatty acids depend on the raw material (type and category of meat, composition of breeding, feeding, etc.) and on the processing technology (for meat cuts: curing, method and duration of drying and maturing). The taste and aroma of fatty acids depend to a large extent on their melting point. Free fatty acids are believed to act as precursors of chemical compounds or flavour and aroma components in the final product by reacting or partially oxidising with protein degradation products (peptides and amino acids) to form various volatile substances that directly influence the flavour of the product.
The characteristic flavour and aroma of certain meats depend largely on the amount and composition of fat, and the flavour and aroma are also associated with carbonyls and other polar compounds (Sink Citation1973). The most common carbonyl compounds are ketones and all amino acids have an active carbonyl group at physiological or neutral pH. Volatile components of cooked meat are formed by lipid oxidation and Maillard reactions. The compounds formed by lipid oxidation, most commonly aldehydes, ketones, hydrocarbons, alcohols and alkylfurans, while Maillard reactions most commonly form heterocyclic nitrogen and sulphur compounds (pyrazines, thiophenes, thiazoles, furans, furfurol, etc.) and various ethyl ethers (Elmore et al. Citation2000).
The most studied muscular traits related to organoleptic traits, especially tenderness, are fibre types (I, IIA, IIX + B) and intermuscular connective tissue (IMCT) (Listrat et al. Citation2020). It is known that IMF content, specifically its percentage of total lipids (TLips), has an influence on muscle tenderness, juiciness and flavour of beef (Listrat et al. Citation2020). However, a thorough knowledge of the relationship between muscle components and organoleptic properties is essential to understand and control the biological basis of meat quality.
SFAs, trans fatty acids and cholesterol have become major health concerns for consumers. Conjugated LAs are a group of fatty acids that have beneficial effects on human health. Conjugated LAs and n-3 fatty acids are higher in cattle fed a high pasture diet than in cattle fed a high grain diet (Daley et al. Citation2010). Bjorklund et al. (Citation2014) hypothesised that meat from conventional steers would have better meat quality and higher consumer acceptance than meat from organic steers, in contrast to meat from organic steers, which would have higher levels of beneficial fatty acids than meat from conventional steers. Their results showed that the fat from organically fed steers had higher (p<.05) mean content of palmitic acid (C16:0), stearic acid (C18:0), linolenic acid (C18:3) and henicosanoic acid (C21:0) than the fat from organically and conventionally fed steers.
Older studies reported that animals fed grain-based diets had muscles with higher concentrations of n-6 PUFA and a different flavour profile, while animals fed grass had muscles with elevated n-3 PUFA. Grain-fed ruminants have high C18:2/C18:3 ratios compared to grass-fed animals (Joo et al. Citation2017). Saturated fats, trans fats and cholesterol have become major health concerns for consumers. Conjugated LAs are a group of FA that have beneficial effects on human health and conjugated LAs and n-3 FA are higher in cattle fed a high pasture diet than in cattle fed a high grain diet (Daley et al. Citation2010). Avilés et al. (Citation2015) showed in their results that in three different commercial beef breeds (Charolais, Limousin and Retinta), animals with CT genotype in SCD g.878 T>C had higher scores for acceptability of tenderness of their meat. Same authors also suggested that CAST, LEP and SCD1 genes have a potential effect on the different measurements of sensory meat quality. Sevane et al. (Citation2014) reported that the T allele of the TG SNP g.1696 C < T was associated with marbling as well as the C allele increases meat toughness at 48h of maturation.
Conclusion
This review has attempted to summarise recent studies on genetic markers of fatty acid synthesis in beef cattle. Fatty acids in meat will continue to be an important area of research for animal and meat scientists because they play a central role in animal growth, product quality and nutritional value and are recognised as an important characteristic of beef cattle. Advances in molecular genetics have led to the identification of genes and markers associated with genes affecting important traits in beef cattle. Most traits considered in animal genetic improvement programmes are quantitative traits controlled by many genes along with environmental factors. The development of molecular markers has been greeted with great enthusiasm for their role in classical genetic improvement programmes. Fat content varies greatly depending on the cut and the degree of trimming. Fat marbling is important for meat quality and is closely related to juiciness, flavour and tenderness and is of greatest interest to human health. However, the focus may be expanded as more information becomes available, as further analysis will provide new insights into the contribution of the beef industry around the world.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The articles used in this review are available on request from the corresponding author, A.I., or can be found in the Scopus base.
Additional information
Funding
References
- Abbas KA, Mohamed A, Jamilah B. 2009. Fatty acids in fish and beef and their nutritional values: a review. J Food Agric Environ. 7(3–4):37–42.
- Alim MA, Fan YP, Wu XP, Xie Y, Zhang Y, Zhang SL, Sun DX, Zhang Y, Zhang Q, Liu L, et al. 2012. Genetic effects of stearoyl-coenzyme A desaturase (SCD) polymorphism on milk production traits in the Chinese dairy population. Mol Biol Rep. 39(9):8733–8740.
- Anton I, Kovács K, Holló G, Farkas V, Lehel L, Hajda Z, Zsolnai A. 2011. Effect of leptin, DGAT1 and TG gene polymorphisms on the intramuscular fat of Angus cattle in Hungary. Livest Sci. 135(2–3):300–303.
- Ardiyanti A, Oki Y, Suda Y, Suzuki K, Chikuni K, Obara Y, Katoh K. 2009. Effects of GH gene polymorphism and sex on carcass traits and fatty acid compositions in Japanese Black cattle. Anim Sci J. 80(1):62–69.
- Avilés C, Polvillo O, Peña F, Juárez M, Martínez AL, Molina A. 2013. Associations between DGAT1, FABP4, LEP, RORC, and SCD1 gene polymorphisms and fat deposition in Spanish commercial beef. J Anim Sci. 91(10):4571–4577.
- Avilés C, Peña F, Polvillo O, Barahona M, Campo MM, Sañudo C, Juárez M, Horcada A, Alcalde MJ, Molina A. 2015. Association between functional candidate genes and organoleptic meat traits in intensively-fed beef. Meat Sci. 107:33–38.
- Barendse W, Bunch RJ, Harrison BE, Thomas MB. 2006. The growth hormone 1 GH1:c.457C>G mutation is associated with intramuscular and rump fat distribution in a large sample of Australian feedlot cattle. Anim Genet. 37(3):211–214.
- Bartoň L, Kott T, Bureš D, Řehák D, Zahrádková R, Kottová B. 2010. The polymorphisms of stearoyl-CoA desaturase (SCD1) and sterol regulatory element binding protein-1 (SREBP-1) genes and their association with the fatty acid profile of muscle and subcutaneous fat in Fleckvieh bulls. Meat Sci. 85(1):15–20.
- Bartoň L, Bureš D, Kott T, Řehák D. 2016. Associations of polymorphisms in bovine DGAT1, FABP4, FASN, and PPARGC1A genes with intramuscular fat content and the fatty acid composition of muscle and subcutaneous fat in Fleckvieh bulls. Meat Sci. 114:18–23.
- Berg J, Streyer L, Tymoczko J, Gatto G. 2019. Biochemistry. 9th ed. New York (NY): W.H. Freeman and Company.
- Berton MP, Fonseca LFS, Gimenez DFJ, Utembergue BL, Cesar ASM, Coutinho LL, de Lemos MVA, Aboujaoude C, Pereira ASC, Silva RDO, et al. 2016. Gene expression profile of intramuscular muscle in Nellore cattle with extreme values of fatty acid. BMC Genomics. 17(1):1–16.
- Bjorklund EA, Heins BJ, DiCostanzo A, Chester-Jones H. 2014. Fatty acid profiles, meat quality, and sensory attributes of organic versus conventional dairy beef steers. J Dairy Sci. 97(3):1828–1834.
- Blecha IMZ, Siqueira F, Ferreira ABR, Feijó GLD, Torres RAA, Medeiros SR, Sousa II, Santiago GG, Ferraz ALJ. 2015. Identification and evaluation of polymorphisms in FABP3 and FABP4 in beef cattle. Genet Mol Res. 14(4):16353–16363.
- Bordbar F, Jensen J, Zhu B, Wang Z, Xu L, Chang T, Xu L, Du M, Zhang L, Gao H, et al. 2019. Identification of muscle-specific candidate genes in Simmental beef cattle using imputed next generation sequencing. PLoS One. 14(10):e0223671–17.
- Bordonaro S, Tumino S, Marletta D, De Angelis A, Di Paola F, Avondo M, Valenti B. 2020. Effect of GH p.L127V polymorphism and feeding systems on milk production traits and fatty acid composition in modicana cows. Animals. 10(9):1651–1610.
- Burt DW. 2009. The cattle genome reveals its secrets. J Biol. 8(4):36.
- Daley CA, Abbott A, Doyle PS, Nader GA, Larson S. 2010. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr J. 9(1):10.
- Dannenberger D, Nuernberg G, Scollan N, Schabbel W, Steinhart H, Ender K, Nuernberg K. 2004. Effect of diet on the deposition of n-3 fatty acids, conjugated linoleic and C18:1 trans fatty acid isomers in muscle lipids of German Holstein bulls. J Agric Food Chem. 52(21):6607–6615.
- Di Stasio L, Sartore S, Albera A. 2002. Lack of association of GH1 and POU1F1 gene variants with meat production traits in Piemontese cattle. Anim Genet. 33(1):61–64.
- Du M, Tong J, Zhao J, Underwood KR, Zhu M, Ford SP, Nathanielsz PW. 2010. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. 88(13):51–60.
- Du M, Huang Y, Das AK, Yang Q, Duarte MS, Dodson MV, Zhu MJ. 2013. Meat science and muscle biology symposium: manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J Anim Sci. 91(3):1419–1427.
- Dubey PK, Goyal S, Yadav AK, Sahoo BR, Kumari N, Mishra SK, Niranjan SK, Arora R, Mukesh M, Kataria RS. 2014. Genetic diversity analysis of the thyroglobulin gene promoter in buffalo and other bovines. Livest Sci. 167(1):65–72.
- Dujková R, Ranganathan Y, Dufek A, Macák J, Bezdíček J. 2015. Polymorphic effects of FABP4 and SCD genes on intramuscular fatty acid profiles in longissimus muscle from two cattle breeds. Acta Vet Brno. 84(4):327–336.
- Elmore JS, Mottram DS, Enser M, Wood JD. 2000. The effects of diet and breed on the volatile compounds of cooked lamb. Meat Sci. 55(2):149–159.
- Enser M, Hallett KG, Hewett B, Fursey GAJ, Wood JD, Harrington G. 1998. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. 49(3):329–341.
- FAOSTAT. 2020. Food and Agriculture Organization of the United Nations, Statistical Database; Rome. [accessed 2021 Aug 8]. http://www.fao.org/faostat/en/#home.
- Flowers S, Hamblen H, Leal-Gutiérrez JD, Elzo MA, Johnson DD, Mateescu RG. 2018. Fatty acid profile, mineral content, and palatability of beef from a multibreed Angus-Brahman population1. J Anim Sci. 96(10):4264–4275.
- Giri P, Mohapatra B. 2017. Encyclopedia of animal cognition and behavior. Cham, Switzerland: Springer International Publishing AG; p. 1–4.
- Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, Jiang Z, Poulos SP, Sainz RD, Smith S, et al. 2009. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 87(4):1218–1246.
- Hirwa C. d`A, Wallace P, Shen X, Nie Q, Yang G, Zhang X. 2010. Genes related to economically important traits in beef cattle. Asian J Anim Sci. 5(1):34–45.
- Hoashi S, Hinenoya T, Tanaka A, Ohsaki H, Sasazaki S, Taniguchi M, Oyama K, Mukai F, Mannen H. 2008. Association between fatty acid compositions and genotypes of FABP4 and LXR-alpha in Japanese Black cattle. BMC Genet. 9(1):3–9.
- Jaborek JR, Zerby HN, Moeller SJ, Fluharty FL, Relling AE. 2019. Evaluation of feedlot performance, carcass characteristics, carcass retail cut distribution, Warner-Bratzler shear force, and fatty acid composition of purebred Jersey and crossbred Jersey steers. Transl Anim Sci. 3(4):1475–1491.
- Jeong J, Kwon EG, Im SK, Seo KS, Baik M. 2012. Expression of fat deposition and fat removal genes is associated with intramuscular fat content in longissimus dorsi muscle of Korean cattle steers. J Anim Sci. 90(6):2044–2053.
- Jiang Z, Michal JJ, Tobey DJ, Daniels TF, Rule DC, MacNeil MD. 2008. Significant associations of stearoyl-CoA desaturase (SCD1) gene with fat deposition and composition in skeletal muscle. Int J Biol Sci. 4(6):345–351.
- Joo ST, Joo SH, Hwang YH. 2017. The relationships between muscle fiber characteristics, intramuscular fat content, and fatty acid compositions in M. longissimus lumborum of Hanwoo steers. Korean J Food Sci Anim Resour. 37(5):780–786.
- Jurie C, Cassar-Malek I, Bonnet M, Leroux C, Bauchart D, Boulesteix P, Pethick DW, Hocquette JF. 2007. Adipocyte fatty acid-binding protein and mitochondrial enzyme activities in muscles as relevant indicators of marbling in cattle. J Anim Sci. 85(10):2660–2669.
- Kaczor U, Famielec M, Dudziak P, Kaczor A, Kucharski M, Mandecki A. 2017. Fatty acid binding protein 4 (FABP4) and thyreoglobulin (TG) polymorphisms in relation to milk performance traits in the Holstein-Friesian cattle. Acta Sci Pol Zootech. 16(4):11–16.
- Kaplanová K, Dufek A, Dračková E, Simeonovová J, Šubrt J, Vrtková I, Dvořák J. 2013. The association of CAPN1, CAST, SCD, and FASN polymorphisms with beef quality traits in commercial crossbred cattle in the Czech Republic. Czech J Anim Sci. 58(11):489–496.
- Kay JK, Mackle TR, Auldist MJ, Thomson NA, Bauman DE. 2004. Endogenous synthesis of cis-9, trans-11 conjugated linoleic acid in dairy cows fed fresh pasture. J Dairy Sci. 87(2):369–378.
- Kelava Ugarković N, Ivanković A, Konjačić M. 2013. Effect of breed and age on beef carcass quality, fatness and fatty acid composition. Arch Anim Breed. 56(1):958–970.
- Kelly MJ, Tume RK, Newman S, Thompson JM. 2013. Genetic variation in fatty acid composition of subcutaneous fat in cattle. Anim Prod Sci. 53(2):129–133.
- Lemos M. d, Pereira ASC, Regatieri IC, Feitosa FLB, Baldi F. 2017. Genetic factors that determine the meat fatty acids composition. In: Catala A, editor. Fattty acids. London: Intech Open Science; p. 221–237.
- Li C, Aldai N, Vinsky M, Dugan MER, McAllister TA. 2012. Association analyses of single nucleotide polymorphisms in bovine stearoyl-CoA desaturase and fatty acid synthase genes with fatty acid composition in commercial cross-bred beef steers. Anim Genet. 43(1):93–97.
- Listrat A, Gagaoua M, Andueza D, Gruffat D, Normand J, Mairesse G, Picard B, Hocquette JF. 2020. What are the drivers of beef sensory quality using metadata of intramuscular connective tissue, fatty acids and muscle fiber characteristics? Livest Sci. 240(4):104209.
- López-Pedrouso M, Rodríguez-Vázquez R, Purriños L, Oliván M, García-Torres S, Sentandreu MÁ, Lorenzo JM, Zapata C, Franco D. 2020. Sensory and physicochemical analysis of meat from bovine breeds in different livestock production systems, pre-slaughter handling conditions, and ageing time. Foods. 9(2):176–117.
- Maharani D, Jung Y, Jung WY, Jo C, Ryoo SH, Lee SH, Yeon SH, Lee JH. 2012. Association of five candidate genes with fatty acid composition in Korean cattle. Mol Biol Rep. 39(5):6113–6121.
- Mannen H. 2011. Identification and utilization of genes associated with beef qualities. Anim Sci J. 82(1):1–7.
- Mannen H. 2012. Genes associated with fatty acid composition of beef. FSTR. 18(1):1–6.
- Matsuhashi T, Maruyama S, Uemoto Y, Kobayashi N, Mannen H, Abe T, Sakaguchi S, Kobayashi E. 2011. Effect of bovine fatty acid synthase, stearoyl-coenzyme A desaturase, sterol regulatory element-binding protein 1, and growth hormone gene polymorphisms on fatty acid composition and carcass traits in Japanese Black cattle. J Anim Sci. 89(1):12–22.
- Milanesi E, Nicoloso L, Crepaldi P. 2008. Stearoyl CoA desaturase (SCD) gene polymorphisms in Italian cattle breeds. J Anim Breed Genet. 125(1):63–67.
- Momot M, Nogalski Z, Pogorzelska‐Przybyłek P, Sobczuk‐Szul M. 2020. Influence of genotype and slaughter age on the content of selected minerals and fatty acids in the longissimus thoracis muscle of crossbred bulls. Animals. 10(11):2004–2012.
- Morris CA, Cullen NG, Glass BC, Hyndman DL, Manley TR, Hickey SM, McEwan JC, Pitchford WS, Bottema CDK, Lee MAH. 2007. Fatty acid synthase effects on bovine adipose fat and milk fat. Mamm Genome. 18(1):64–74.
- Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. 2005. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 111(2):157–164.
- Müller H, Jordal O, Seljeflot I, Kierulf P, Kirkhus B, Ledsaak O, Pedersen JI. 1998. Effect on plasma lipids and lipoproteins of replacing partially hydrogenated fish oil with vegetable fat in margarine. Br J Nutr. 80(3):243–251.
- Nagy K, Tiuca ID. 2017. Importance of fatty acids in physiopathology of human vody. In: Catala A, editor. Fatty acids. London: Intech Open Science; p. 3–22.
- Narukami T, Sasazaki S, Oyama K, Nogi T, Taniguchi M, Mannen H. 2011. Effect of DNA polymorphisms related to fatty acid composition in adipose tissue of Holstein cattle. Anim Sci J. 82(3):406–411.
- Ntambi JM. 2013. Stearoyl-CoA desaturase-1Is a biological regulator of energy homeostasis. In: Ntambi JM, editor. Stearoyl-CoA desaturase genes in lipid metabolism. Medison (WI): Springer-Verlag New York; p. 27–37.
- Oh D, Lee Y, La B, Yeo J, Chung E, Kim Y, Lee C. 2012. Fatty acid composition of beef is associated with exonic nucleotide variants of the gene encoding FASN. Mol Biol Rep. 39(4):4083–4090.
- Ohsaki H, Sawa T, Sasazaki S, Kano K, Taniguchi M, Mukai F, Mannen H. 2007. Stearoyl-CoA desaturase mRNA expression during bovine adipocyte differentiation in primary culture derived from Japanese Black and Holstein cattle. Comp Biochem Physiol A Mol Integr Physiol. 148(3):629–634.
- Oprządek J, Fliskowski K, Zwierzchowski L, Juszczuk-Kubiak E, Rosochacki S, Dymnicki E. 2005. Associations between polymorphism of some candidate genes and growth rates, feed intake and utilisation, slaughter indicators and meet quality in cattle. Arch Tierz. 48:81–87.
- Pannier L, Mullen AM, Hamill RM, Stapleton PC, Sweeney T. 2010. Association analysis of single nucleotide polymorphisms in DGAT1, TG and FABP4 genes and intramuscular fat in crossbred Bos taurus cattle. Meat Sci. 85(3):515–518.
- Papaleo Mazzucco J, Goszczynski DE, Ripoli MV, Melucci LM, Pardo AM, Colatto E, Rogberg-Muñoz A, Mezzadra CA, Depetris GJ, Giovambattista G, Villarreal EL. 2016. Growth, carcass and meat quality traits in beef from Angus, Hereford and cross-breed grazing steers, and their association with SNPs in genes related to fat deposition metabolism. Meat Sci. 114:121–129.
- Park SJ, Beak SH, Jung DJS, Kim SY, Jeong IH, Piao MY, Kang HJ, Fassah DM, Na SW, Yoo SP, Baik M. 2018. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle - A review. Asian-Australas J Anim Sci. 31(7):1043–1061.
- Prado JM, Prado IN, Visentainer JV, Rotta PP, Perotto D, Moletta JL, Prado IM, Ducatti T. 2009. The effect of breed on the chemical composition and fatty acid profile of the Longissimus dorsi muscle of Brazilian beef cattle. J Anim Feed Sci. 18(2):231–240.
- Raza SHA, Khan S, Amjadi M, Abdelnour SA, Ohran H, Alanazi KM, Abd El-Hack ME, Taha AE, Khan R, Gong C, Schreurs NM, et al. 2020. Genome-wide association studies reveal novel loci associated with carcass and body measures in beef cattle. Arch Biochem Biophys. 694:108543.
- Razmaite V, Šiukščius A, Šveistiene R, Bliznikas S, Jatkauskiene V. 2020. Relationships between fat and cholesterol contents and fatty acid composition in different meat-producing animal species. Acta Vet Br. 70(3):1–12.
- Ressurreccion AVA. 2004. Sensory aspects of consumer choices for meat and meat products. Meat Sci. 66:11–20.
- Roy R, Taourit S, Zaragoza P, Eggen A, Rodellar C. 2005. Genomic structure and alternative transcript of bovine fatty acid synthase gene (FASN): comparative analysis of the FASN gene between monogastric and ruminant species. Cytogenet Genome Res. 111(1):65–73.
- Sasago N, Abe T, Sakuma H, Kojima T, Uemoto Y. 2017. Genome-wide association study for carcass traits, fatty acid composition, chemical composition, sugar, and the effects of related candidate genes in Japanese Black cattle. Anim Sci J. 88(1):33–44.
- Schlee P, Graml R, Rottmann O, Pirchner F. 1994. Influence of growth‐hormone genotypes on breeding values of Simmental bulls. J Anim Breed Genet. 111(1–6):253–256.
- Sevane N, Armstrong E, Wiener P, Pong Wong R, Dunner S, GemQual Consortium. 2014. Polymorphisms in twelve candidate genes are associated with growth, muscle lipid profile and meat quality traits in eleven European cattle breeds. Mol Biol Rep. 41(7):4721–4731.
- Silveira LGG, Furlan LR, Curi RA, Ferraz ALJ, de Alencar MM, Regitano LCA, Martins CL, de Beni Arrigoni M, Suguisawa L, Silveira AC, et al. 2008. Growth hormone 1 gene (GH1) polymorphisms as possible markers of the production potential of beef cattle using the Brazilian Canchim breed as a model. Genet Mol Biol. 31(4):874–879.
- Sink JD. 1973. Lipid-soluble components of meat flavors/odors and their biochemical origin. J Am Oil Chem Soc. 50(11):470–474.
- Soliman GA. 2018. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients. 10(6):780.
- Sørensen P, Grochowska R, Holm L, Henryon M, Løvendahl P. 2002. Polymorphism in the bovine growth hormone gene affects endocrine release in dairy calves. J Dairy Sci. 85(7):1887–1893.
- Taniguchi M, Utsugi T, Oyama K, Mannen H, Kobayashi M, Tanabe Y, Ogino A, Tsuji S. 2004. Genotype of stearoyl-CoA desaturase is associated with fatty acid composition in Japanese Black cattle. Mamm Genome. 15(2):142–148.
- Tellam RL, Lemay DG, Van Tassell CP, Lewin HA, Worley KC, Elsik CG. 2009. Unlocking the bovine genome. BMC Genomics. 10:193.
- Thaller G, Kühn C, Winter A, Ewald G, Bellmann O, Wegner J, Zühlke H, Fries R. 2003. DGAT1, a new positional and functional candidate gene for intramuscular fat deposition in cattle. Anim Genet. 34(5):354–357.
- Timón ML, Ventanas J, Carrapiso AI, Jurado A, García C. 2001. Subcutaneous and intermuscular fat characterisation of dry-cured Iberian hams. Meat Sci. 58(1):85–91.
- Urrutia O, Mendizabal JA, Alfonso L, Soret B, Insausti K, Arana A. 2020. Adipose tissue modification through feeding strategies and their implication on adipogenesis and adipose tissue metabolism in ruminants. Int J Mol Sci. 21(9):3183.
- Usman MT, Tanko AS, Alhassan AJ. 2015. Effects of water soaking on the nutritional compositions of beef in Nigeria: a review. Int J Chem Biomol Sci. 1(3):129–133.
- [USDA] U.S. Department of Agriculture (US). 1989. FoodData central, (US).
- Vahmani P, Mapiye C, Prieto N, Rolland DC, McAllister TA, Aalhus JL, Dugan MER. 2015. The scope for manipulating the polyunsaturated fatty acid content of beef: a review. J Anim Sci Biotechnol. 6(1):1–13.
- Wang Z, Zhu B, Niu H, Zhang W, Xu L, Xu L, Chen Y, Zhang L, Gao X, Gao H, et al. 2019. Genome wide association study identifies SNPs associated with fatty acid composition in Chinese Wagyu cattle. J Anim Sci Biotechnol. 10(1):1–13.
- Wyness L. 2013. Nutritional aspects of red meat in the diet. In: Wood JD, Rowlings C, editors. Nutrition and climate change: major issues confronting the meat industry. Leicestershire: Nottingham University Press; p. 1–22.
- Wood JD, Enser M, Richardson RI, Whittington FM. 2007. Fatty acids in meat and meat products. In: Chow CK, editor. Fatty acids in foods and their health implications. 3rd ed. Boca Raton (FL): CRP Press; p. 87–107.
- Yardibi H, Gürsel FE, Ates A, Akıs I, Hosturk GT. 2013. BTN1A1, FABP3 and TG genes polymorphism in East Anatolian red cattle breed and South Anatolian red cattle breed. African J Biotechnol. 12(20):2802–2807.
- Yeon SH, Lee SH, Choi BH, Lee HJ, Jang GW, Lee KT, Kim KH, Lee JH, Chung HY. 2013. Genetic variation of FASN is associated with fatty acid composition of Hanwoo. Meat Sci. 94(1):133–138.
- Zhang S. 2008. Genetic regulation of the healthfulness of beef fatty acid composition [dissertation]. Ames (IA): Iowa State University.
- Zhu B, Niu H, Zhang W, Wang Z, Liang Y, Guan L, Guo P, Chen Y, Zhang L, Guo Y, et al. 2017. Genome wide association study and genomic prediction for fatty acid composition in Chinese Simmental beef cattle using high density SNP array. BMC Genomics. 18(1):1–15.
