Abstract
The aim of this study was to evaluate how different rearing systems (intensive or semi-extensive) and sexual hormones could affect stress parameters and the development of stress-related intramural coronary arteriosclerosis in Lidia bulls (n = 18) and Piemontese oxen (n = 17). At slaughter, hearts were sampled and submitted to histological investigations in order to evaluate coronary arteriosclerosis. Blood and hair samples were also collected to measure d-ROMS and 20β-dihydrocortisol levels, respectively. No significant differences were recorded for serum levels of d-ROMS between Piemontese oxen and Lidia bulls. On the contrary, Lidia bulls presented higher levels of hair 20β-dihydrocortisol compared to Piemontese oxen (p = .007). Arteriosclerosis was recorded in both the groups. In Lidia bulls, mild to moderate coronary arteriosclerosis was recorded in all the evaluated regions of the heart (right and left papillary muscle; interventricular septum; right and left ventricle free wall; right and left atrium). In Piemontese oxen, mild to severe coronary arteriosclerosis was observed, especially in the left papillary muscle. These results suggest that both cattle breed experienced stress during their rearing cycle. Further studies are needed to clarify the role of housing system and sexual hormones in the development of these subclinical vascular pathology.
Rearing systems can affect animal welfare.
Coronary arteriosclerosis, d-ROMS and hair 20β-dihydrocortisol levels can be affected by stress.
Lidia bulls and Piemontese oxen experienced different levels of stress during their rearing cycle.
Highlights
Introduction
Animal welfare means how an animal is coping with the conditions in which it lives and it is not only related to physical health. In this context, an animal is in a good state of welfare if it is healthy, comfortable, well-nourished, safe, able to express innate behaviours, and if it is not suffering from unpleasant states such as pain, fear, and distress (Broom Citation1988). The basal needs of farm animals should be assured by allowing free access to adequate quantities of feed and fresh water, by providing a suitable rearing environment and by avoiding physical pain or suffering of any kind (Webster Citation2001).
As far as cattle husbandry is concerned, housing system represents an important factor that can impair animal welfare (Odore et al. Citation2011). In particular, beef fattening systems may be divided into two main categories, intensive indoor and extensive or semi-extensive grass-based systems involving winter accommodation (European Commission Citation2000). Intensive indoor systems characterised by insufficient space allowance, concentrate feeding and overstocking are generally associated with poor welfare (Park et al. Citation2020). On the contrary, increased space allowance decrease the incidence of disease and improve growth performance (Keane et al. Citation2017). Moreover, traditional rearing systems are still present all over Europe and even if they ensure the final product quality requested by the consumer, in doing so, animal behaviour and welfare may be compromised, generating stress (Odore et al. Citation2011).
Stress indicates a set of physiological and behavioural changes elicited by noxious or unpleasant exogenous or endogenous stimuli that provoke the activation of the hypothalamic–pituitary–adrenal axis and/or the sympatho-adrenomedullary system (Moberg and Mench Citation2000). Stress of cattle increases disease susceptibility and decreases production, reproduction and growth (Chen et al. Citation2016). As such, it is a priority to find more effective ways to manage it, including optimisation of management practices and selection of animals with greater stress tolerance (Lomillos and Alonso Citation2008).
A series of well-known stress indicators could be used to evaluate either acute stress of cattle, such as the reactive oxygen metabolites (d-ROMs) (Celi Citation2011), and salivary/blood cortisol, or chronic stress, such as hair 20β-dihydrocortisol (Heimbürge et al. Citation2019). On the one hand, d-ROMs test is used to assess the oxidative stress status in cattle and it is considered the ‘gold standard’ for evaluating free radical levels, measuring the metabolites deriving from the reaction of free radicals with macromolecules present in the organism (Celi Citation2011). On the other hand, hair cortisol and 20β-dihydrocortisol have been increasingly used to detect long-term retrospective levels of cortisol in cattle, representing a more reliable method than plasma or salivary cortisol that are subject to major physiological daily fluctuations (Sharma et al. Citation2019; Heimbürge et al. Citation2020). For these reasons, previous studies have used both d-ROMs and hair 20β-dihydrocortisol as animal-based measurements to evaluate beef and dairy cows’ welfare as they are simple, non-invasive and painless procedures (Bernabucci et al. Citation2002; Vesel et al. Citation2020).
Moreover, housing system and prolonged stress-related situations may manifest themselves with subclinical pathological changes affecting the cardiocirculatory system (Ratcliffe et al. Citation1969). Particularly, in human medicine, it has been demonstrated that chronic psychosocial stress is associated with an increased risk of arteriosclerosis (Chou et al. Citation2015). Arteriosclerosis can be defined as a chronic arterial change consisting of hardening, loss of elasticity, and luminal narrowing resulting from proliferative and degenerative changes of tunica intima and media (Biasato et al. Citation2018). Despite the incidence of arteriosclerotic changes being common in older animals (Maxie and Robinson Citation2007), some adverse and stressful socio-environmental factors have already been reported to stimulate the onset of intramural coronary arteriosclerosis also in young animals (Ratcliffe and Snyder Citation1967; Ratcliffe et al. Citation1969; Henry et al. Citation1971). Regarding cattle, only Biasato et al. (Citation2018) reported the presence of intramural coronary arteriosclerosis, but the hypothesis of a potential influence of housing condition on its pathogenesis has not been proved yet.
Evidence suggests also that gonadal hormones, especially testosterone can play a role both in the stress response and in the vascular ageing (Kutlikova et al. Citation2020; Moreau et al. Citation2020). In particular, in human, low testosterone is associated with impaired endothelial function and increased arterial stiffness as well as lower stress reactivity due to the activity of the hypothalamic–pituitary–gonadal axis on the hypothalamic–pituitary–adrenal axis (Kutlikova et al. Citation2020; Moreau et al. Citation2020). To the authors’ knowledge, no previous studies have investigated the role of sexual hormones on stress parameters and arteriosclerosis in cattle.
Based on the above-reported background, the aim of this study is to evaluate d-ROMs, hair 20β-dihydrocortisol and arteriosclerosis in two different traditional cattle rearing systems, one with castrated bulls and one with intact bulls.
Materials and methods
Study population
The experimental protocol was designed according to the guidelines of the current European Directive (2010/63/EU) on the care and protection of animals.
Two different traditional cattle breeds were included in the present study: Lidia bulls and Piemontese oxen.
A total of 18 Lidia bulls of 40 months of age on average (39.53 ± 11.82) reared in Andalusia (Spain) were included in the study. In Spain, the Lidia breed is considered the greatest exponent of a semi-extensive traditional breeding system, being the only bovine population selected for a behavioural trait (Lomillos and Alonso Citation2008). All the animals included in the study were reared and fed on the pasture for their first 2–3 years and then underwent to a fattening period for 5–12 months in small fenced areas prior to the bullfighting show. After bullfighting, animals were slaughtered in a commercial abattoir located between 30 and 50 km from the arena. The inclusion criteria were as follows: (1) animals were regularly slaughtered immediately after a bullfight; (2) they were not injured during the bullfighting; and 3) they showed no significant alterations at the routine meat inspection.
A total of 17 Piemontese oxen of 48 months of age on average (47.82 ± 13.33) reared in medium or large herds (over 50 or 100 fattening heads) in Piemonte (Italy) were included in the study. They were housed in free stalls with or without access to external pens, typical of an intensive farming system. The calves were castrated at 3–6 months of age by Burdizzo's pincer (Biagini and Lazzaroni Citation2007). Then, the castrated animals were reared in multiple pens on straw until 3 years of age, when the number of heads per pen was reduced according to the Italian laws on animal welfare (D.lgs 146/01). At 4 years of age with a live weight of 900–1000 kg, they were slaughtered in a commercial abattoir located between 20 and 50 km from the farms. The inclusion criteria were as follows: (1) animals were regularly slaughtered; (2) they showed no significant alterations at the routine meat inspection.
The animals were fed in accordance to the specific breed requirements. In the finisher phase, the diet of Lidia bulls and Piemontese oxen had the following average composition (% as fed): crude protein 16.2 and 14.3%, crude fibre 8.0 and 4.1%, net energy 1.03 and 1.01 UFV (Unité Foragère Viande)/kg, respectively.
Blood reactive oxygen metabolites quantification
Blood samples were collected at slaughterhouse, after shooting, from the jugular vein of 18 Lidia bulls and 17 Piemontese oxen. An aliquot of 6 ml of the blood was placed in a serum-separating tube and then centrifugated at 700× g for 15 min. The obtained sera were immediately frozen at −80 °C and sent to the Department of Veterinary Sciences, University of Turin (Italy). The serum levels of d-ROMs were quantified using the d-ROMs test (Diacron International S.A.S, Grosseto, Italy) following the manufacture’s instruction. In particular, the end-point procedure was used with a double read at 37 °C after 90 min of incubation. Results were expressed in arbitrary units, namely Carratelli units (U.CARR) calculated using the following formula: (sample absorbance/standard absorbance) × standard concentration. The value of 1 U.CARR corresponds to a concentration of 0.08 mg/dl of hydrogen peroxide. This method has a detection limit of 11 U.CARR.
Hair 20β-dihydrocortisol determination
Hair aliquots of about 200 mg from the base of the tail were sampled at slaughterhouse from 18 Lidia bulls and 17 Piemontese oxen and stored at room temperature. All the samples were sent to the Regional Antidoping and Toxicology centre ‘A. Bertinaria’ (Torino) for 20β-dihydrocortisol determination according to the method previously described by Tarantola et al. (Citation2020). Samples were transferred into 30-mL glass tubes and a decontamination step was performed twice by adding 3.0 mL of dichloromethane. The cleaned hair was dried under a nitrogen stream and then cut into tiny fragments with clean scissors. An aliquot of 100 mg was weighted and the internal standard solution (cortisol d2) was added. Sample extraction was carried out by the addition of 1 mL of methanol followed by incubation for 15 h at 55 °C. The organic phase was collected and evaporated at 25 °C by using a Techne Sample Concentrator (Barloworld Scientific, Burlington, NJ) with a nitrogen blow-dry evaporation system. The residue was dissolved in 100 µL of methanol, transferred into a vial and centrifuged at 4000 g for 10 min. Furthermore, an aliquot of the solution was transferred into a vial for UHPLC-MS/MS analysis and 1 µL was injected into the LC–MS/MS system. The chromatographic separation was performed into a Shimadzu Nexera 30 UHPLC-system (Shimadzu, Duisburg, Germany) interfaced to an AB Sciex API 5500 triple quadrupole mass spectrometer (AB Sciex, Darmstadt, Germany). The analyses were performed in Electro-Spray negative ionisation mode (ESI–). Ion acquisition was operated at unit mass resolution in the multiple reaction monitoring (MRM) mode, using three transitions from the deprotonated molecular ion to specific fragment ions for 20β-dihydrocortisol and one transition for cortisol-d2. An Acquity UPLC BEH C18 column 100 mm × 2.1 mm, 1.7 µm, protected by a VanGuard BEH C18 (5 mm × 2.1 mm) pre-column was used to perform the analytes chromatographic separation. The column oven was maintained at 40 °C and the elution solvents were water/formic acid 5 mM (Solvent A) and acetonitrile/formic acid 5 mM. The mobile phase composition was varied by a linear gradient (A:B; v/v) from 90:10 to 55:45 in 7 min; then the ratio of 10:90 was reached in 0.5 min and an isocratic elution at this conditions was maintained for 0.5 min. The flow rate was 0.4 mL/min and the total run time was 10 min including re-equilibration at the initial conditions. The collision energy (CE), decluttering potential (DP) and the MRM transitions for 20β-dihydrocortisol and cortisol-d2 are reported in Table . This method has a 20β-dihydrocortisol detection limit of 0.5 ng/g.
Table 1. MRM transitions and corresponding potentials for 20β-dihydrocortisol and cortisol-d2 detection.
Heart evaluation
At slaughter, hearts of Lidia bulls and Piemontese oxen were externally examined and sectioned across the median longitudinal plan to evaluate all the cardiac structures. From each heart, seven representative anatomic areas were sampled: (1) papillary muscle of the right ventricle; (2) papillary muscle of the left ventricle; (3) interventricular septum; (4) right ventricle free wall; (5) left ventricle free wall; (6) right atrium; (7) left atrium. Lidia bulls’ samples were fixed in 10% buffered formalin, routinely embedded in paraffin wax blocks at the Department of Veterinary Science, University of Cordoba and then sent to the Department of Veterinary Sciences, University of Turin for the histological evaluation. Piemontese oxen samples were processed at the Department of Veterinary Sciences of University of Turin following the same protocols. Paraffin wax blocks were cut at 5 µm thickness, mounted on glass slides and stained with haematoxylin and eosin. Tissue sections were later examined by light microscopy independently by two observers and the discordant cases were reviewed at a multi-head microscope until a consensus was reached. Pathological intramural coronary arteries with and without lumen narrowing were manually counted in every localisation. At least, five serial sections were obtained from each myocardial localisation and count was performed on the myocardial sections including a total number of 8–12 intramural coronary arteries with a diameter greater than 200 µm (40–60 arteries/localisation/animal) (Biasato et al. Citation2018). The following histopathological parameters were evaluated for each vessel using a semiquantitative scoring system from 0 (normal) to 3 (severe alterations): intimal hyperplasia, degenerative changes of tunica media, and medial hypertrophy/hyperplasia (Table ). Each animal could present lesions of different degrees in each localisation. Moreover, the presence of Anitschkow cells was also recorded. These cells appeared as large mono-nuclear cells in which the nuclear chromatin is present in an undulating wavy ribbon with slender processes radiating from it. Other histopathological findings, such as myocardial replacement fibrosis and myocarditis, were also recorded.
Table 2. Semi-quantitative scoring system used to evaluate intramural coronary arteries.
Statistical analysis
The statistical analysis was performed using GraphPad Prism® software (v 7.0 GraphPad Software, San Diego, CA). The Shapiro–Wilk test was used to test the normality of the data distribution before statistical analyses. Kruskall–Wallis test was performed, followed by Dunn post-hoc test in order to evaluate the distribution and severity of arteriosclerosis in the seven considered districts of the heart within each group. Mann–Whitney U test was then performed to compare the number of pathological arteries, d-ROMs, 20β-dihydrocortisol and the severity of arteriosclerosis between the two groups. Spearman's rank-order correlation was used to measure the strength and di-rection of association between d-ROMs and number of arteriosclerotic arteries in the two groups. p-values <.05 were considered statistically significant. Data were expressed as N and percentage (%) or mean ± standard error of the mean (SE).
Results
Blood reactive oxygen metabolites quantification
The serum level of d-ROMs in Lidia bulls was (76.94 ± 17.91 U.CARR), while in Piemontese oxen, it was (27.99 ± 4.15 U.CARR). However, no significant differences were recorded between the two groups (p = .166).
Hair 20β-dihydrocortisol determination
A significant difference was recorded for the hair 20β-dihydrocortisol between the two groups (p = .007). In particular, Lidia bulls showed a mean of 5.86 ± 2.76 ng/g of 20β-dihydrocortisol in the hair, while the concentration in all the Piemontese oxen was below the detection limit (0.5 ng/g).
Heart evaluation
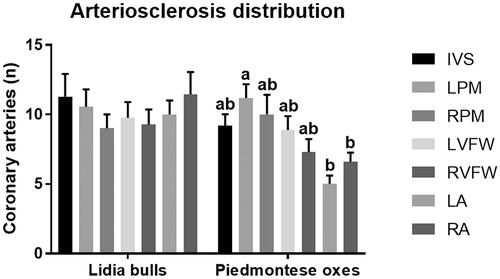
At gross examination, in both groups, all the hearts showed no alterations. In Lidia bulls, the seven sampled areas of the heart were equally affected by arteriosclerotic changes (p = .956). On the contrary, in the left papillary muscle of Piemontese oxen, a higher number of considered arteries showed arteriosclerosis when compared to the left and the right atrium free wall (p = .002, Figure ).
Figure 1. Arterioscelrosis distribution in the seven sampled area of the heart in Piemontese oxen and Lidia bulls. Non-statistically significant differences were recorded in Lidia bulls, while in Piemontese oxen the left papillary muscle was the most affected area compared to left and right atrium. The columns with different superscript letters (a,b) differ significantly (p < .05). IVS: interventricular septum; LPM: left papillary muscle; RPM: right papillary muscle; LVFW: left ventricle free wall; RVFW: right ventricle free wall; LA: left atrium; RA: right atrium.
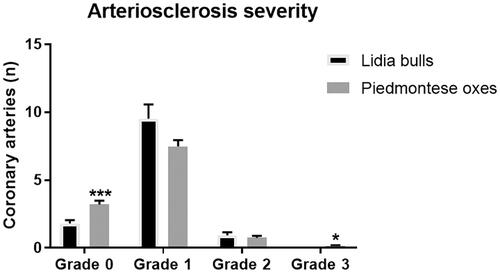
Comparing the two groups, no statistically significant differences were observed concerning the number of affected vessels (p = .192). However, in both groups, most of the vessels showed slight degenerative changes of the tunica media (grade 1). In particular, lesions of grades 0 and 1 were recorded in all the examined Lidia bulls’ and Piemontese oxen’ s hearts (Figure ); grade 2 lesions were observed in the interventricular septum, left papillary muscle and right atrium free wall of 14 out of 18 Lidia bulls (77.7%) (Figure ), as well as in the interventricular septum and right/left papillary muscles of 16 out of 17 Piemontese oxen (94.1%). On the contrary, no grade 3 arteriosclerotic arteries were observed in Lidia bulls, whereas 5 out of 17 oxen (29.4%) showed stenotic coronary arteries in the left papillary muscle (Figure ). Furthermore, a higher number of the considered arteries was normal or showed grade 3 arteriosclerotic changes in Piemontese oxen than in Lidia bulls (p = .007 and p = .019, respectively) (Figure ).
Figure 2. Main arteriosclerotic changes observed in Piemontese oxen (left column) and Lidia bulls (right column). (a) Normal intramural coronary artery, haematoxylin & eosin, 200×. (b) Normal intramural coronary artery, haematoxylin & eosin, 200×. (c) Grade 1 arteriosclerotic intramural coronary artery with degeneration of tunica media (black line), H–e, 200×; (d) grade 1 arteriosclerotic intramural coronary artery with degeneration of tunica media (black line), H–e, 200×; (e) grade 2 arteriosclerotic intramural coronary artery with fragmentation of the elastic membrane (asterisk) and hyperplasia of the tunica intima, H–e, 200×; (f) grade 2 arteriosclerotic intramural coronary artery with fragmentation of the elastic membrane (asterisk) and hyperplasia of the tunica intima; (g) grade 3 arteriosclerotic intramural coronary artery with severe narrowing of the lumen, due to hyperplasia and hypertrophy of tunica intima and media (black arrow), H–e, 200×; (h) Anitschkow cells (black arrows) in the coronary artery wall, H–e, 400×.
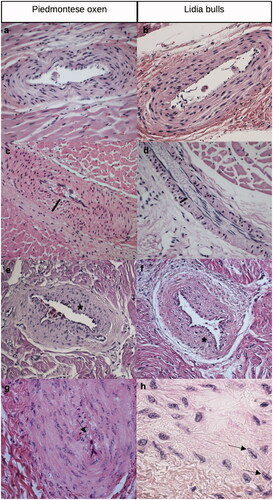
Figure 3. Arteriosclerosis severity in Piemontese oxen and Lidia bulls. Non-statistically significant differences were recorded for grades 1 and 2 between the two groups. Piemontese oxen showed more grade 0 and grade 3 arteries compared to Lidia bulls. *p < .05; ***p < .001.
Anitschkow cells were detected both in Lidia bulls (60%) and Piemontese oxen (100%), being concentrated in left papillary muscle’s and in right atrium free wall’s coronary arteries (Figure ).
No statistically significant correlation was observed between d-ROMs and number of affected intramural coronary arteries in both groups (p = .126 [bulls] and p = .766 [oxen], respectively).
Mild, non-suppurative (lymphoplasmacytic) myocarditis was recorded in 11/18 bulls (61.1%) and in 12/17 oxen (70.5%); 9/17 oxen (52.9%) showed areas of fibrosis in the left papillary muscle, and 11/18 bulls (61.1%) showed mild pericarditis.
Discussion
The present study aimed to evaluate d-ROMs, hair 20β-dihydrocortisol and stress-related arteriosclerosis in two traditional cattle breeds: Lidia bulls and Piemontese oxen. Over the last two decades, an increasing number of consumers and citizens demanded ethical production systems and claimed to refuse to buy products that did not meet their animal welfare concerns (Alonso et al. Citation2020). Due to the need of optimising the production processes and meet the consumers’ expectations, in recent years, also researchers’ interest on animal welfare has increased. Among farm animals, beef cattle may frequently suffer from poor welfare due to their intensive rearing systems adopted in many EU Countries (Tarantola et al. Citation2020).
The best scientific method to assess animal welfare is to measure the response of animals on the basis of functional indicators classified into four criteria groups: behavioural, physiological, pathological and performance (Smidt Citation1983). Particularly, among physiological measures, d-ROMs and neuro-endocrine hormones such as cortisol and their metabolites are commonly used as their alterations are related to depression of the immune response and to negative endocrine, metabolic and productive changes (Lykkesfeldt and Svendsen Citation2007). Therefore, d-ROMs and hair 20β-dihydrocortisol levels were evaluated in the present study. Lidia bulls and Piemontese oxen presented d-ROMs levels within the physiological ranges (<90 UCARR, d-ROMs test – VET, Diacron International S.A.S, Grosseto, Italia). However, Lidia bulls showed higher d-ROMs levels that could be attributed to the stress due to bullfighting immediately before slaughtering. Also, Lidia bulls showed significantly higher levels of hair 20β-dihydrocortisol when compared to Piemontese oxen (p = .007).
The increasing levels of hair 20β-dihydrocortisol observed in Lidia bulls should not be influenced by acute stress generating by bullfighting but seem to suggest a higher level of chronic stress in this group. It is well known that inadequate rearing conditions can produce a stress response that occurs when animals experience changes in the environment that stimulate body responses to restore homeostasis (Odore et al. Citation2011). Indeed, in the fattening period, the Lidia bull’s environment changed from a pasture based-rearing system with no human contact to a tie-stall or single pen rearing system with restricted space allowance and increased human contact, which are known to be psychological stressors for cattle (Ingvartsen and Refsgaard Andersen Citation1993). In fact, restricted space allowance, inadequate type of floor, inadequate space at the manger, lack of specific moving and handling facilities as well as microclimatic conditions and feeding plan are the main critical factors that can cause stress and impar cattle welfare (Cozzi et al. Citation2009). The obtained results are in accordance with Gupta et al. (Citation2007), who reported that bulls housed in a restricted space allowance were stressed and there was an activation of their adrenal glands with increased plasma cortisol concentrations. As a partial confirmation, Tarantola et al. (Citation2020) recently observed that Piemontese cattle reared in a tie-stall system displayed higher d-ROMs and hair 20β-dihydrocortisol levels than Blonde d’Aquitaine cattle housed in pen groups. Moreover, another study also demonstrated that Piemontese cattle housed in a tie-stall system showed higher levels of salivary cortisol compared to those housed in a loose housing system with free stalls, confirming that changing from pasture to pens negatively affect stress-response. However, it is always fundamental to underline that stress response appears to be conditioned by individual predisposition, type of breeding, and composition of the feed (Tarantola et al. Citation2020). For example, Illera del Portal et al. (Citation2007) reported that Lidia bulls are characterised by a higher adaptability to acute stress as a consequence of their genetic selection, showing lower acute stress-related response when compared to other breeds. However, no information about chronic stress response is available in Lidia bulls. Similarly, no results about stress response are available for Piemontese oxen, in which the lack of sexual hormones could influence their stress response. Calves’ castration influences several anatomical, physiological and behavioural characteristics by modifying the expression of some primary and secondary sexual characters, influencing steroids hormones levels (e.g. androsterone and testosterone) and animal organic functions (Heimbürge et al. Citation2019). In fact, it has been reported that after castration the animals are generally quieter thanks to the lowering of testosterone levels (Biagini and Lazzaroni Citation2007). In a recent study, Bolado-Sarabia et al. (Citation2018) reported that immunocastrated Holstein bulls showed less sexual and aggressive behaviours, reducing head butts, mounting, threats, flehmen sign and sniffing compared to intact males. However, Bolado-Sarabia et al. (Citation2018) did not record significant differences for seric cortisol between immunocastrated and intact males at slaughter.
Considering heart evaluation, arteriosclerosis is generally reported as an age-related chronic incidental or clinically relevant pathology (Detweiler and Patterson Citation1965; Tsujino et al. Citation2005). Nevertheless, adverse socio‐environmental factors have been reported to be a major stimulus to the development of intramural coronary arteriosclerosis in young swine (Ratcliffe et al. Citation1969), chicken (Ratcliffe and Snyder Citation1967), mice (Henry et al. Citation1971), elephants (Sikes Citation1968) and monkey (Ratcliffe Citation1974). Furthermore, Biasato et al. (Citation2018) recently characterised the prevalence and the histopathological features of intramural coronary arteriosclerosis in beef cattle, suggesting a potential influence of the intensive farming on this disease development.
In the present study, arteriosclerotic coronary arteries were equally distributed among the seven sampled areas in Lidia bulls, while the most affected myocardial localisation in Piemontese oxen was the left papillary muscle. The prevalence of these arteriosclerotic lesions in the left papillary muscle confirms the observations of Biasato et al. (Citation2018) in veal calves and beef cattle, also partially agreeing with previous studies conducted in dogs ( Detweiler and Patterson Citation1965; Detweiler Citation1989; Falk et al. Citation2006) and swine (Kammermann et al. Citation1976). In human medicine, papillary left muscle is more prone to develop ischaemic lesions, which represent a risk factor for developing degenerative vascular pathologies (Nappi et al. Citation2016). Even though no study on coronary vascular distribution in the papillary muscle is available in cattle, a similar anatomical predisposition to develop degenerative lesions in this heart region cannot be excluded.
Histologically, intimal fibromuscular hyperplasia, medial dysplasia/degeneration and medial hypertrophy/hyperplasia were identified in the intramural coronary arteries’ wall, being in accordance with Biasato et al. (Citation2018) and reflecting the temporal evolution of arteriosclerotic lesions. Arteriosclerosis pathogenesis could be related to myocardial infarcts and consequent fibrosis or myxomatous mitral valve disease (Falk and Jönsson Citation2000; Falk et al. Citation2006). Interestingly, in this study, the presence of foci of fibrosis was observed in the left papillary muscle of Piemontese oxen and they could be related to microinfarcts, thus explaining the more severe arteriosclerotic changes recorded in this localisation.
Moreover, non-significant correlation was found between d-ROMS levels and severity of arteriosclerosis. In human medicine, it has been pointed out that oxidative stress can be considered a negative prognostic factor for cardiovascular diseases (Vassalle et al. Citation2012). However, in the present study, both groups presented low d-ROMs levels, within physiological limits, and this can explain the lack of a significant correlation. Moreover, arteriosclerosis is a chronic arterial change and it is unlikely that bullfighting and the acute stress-related response measured by d-ROMS could play a role in the onset of this pathology. The presence of mild to moderate arteriosclerosis in both Lidia bulls and Piemontese oxen could be related to the final rearing stage, in which the animals were exposed to chronic stress due to lower space allowance, even this evidence has not yet been confirmed. Moreover, the presence of grade 3 arteriosclerotic lesions recorded in Piemontese oxen could be related to the lack of sexual hormones. In human medicine, in fact, it is well known that testosterone has a cardio-protective role and lower levels of this hormone are generally associated to higher risk of coronary artery disease (Oskui et al. Citation2013).
Finally, Anitschkow cells were detected in left papillary muscle’s and in right atrium free wall’s coronary arteries in 60% of Lidia bulls and in all the Piemontese oxen. Also, Biasato et al. (Citation2018) recorded the presence of Anitschkow cells in veal calves and beef cattle housed in intensive farming conditions. Anitschkow cells may be of either muscular or inflammatory origin from the macrophage-histiocyte population and their exclusive detection in the pathological coronary walls allows hypothesising a potential involvement of these cells in the pathogenesis of the arteriosclerotic changes (Colombino et al. Citation2019).
Nevertheless, this study presents several potential limitations that should be acknowledged. In particular, the sample size was considerably small and it could possibly have led to a bias in the results.
Conclusion
In conclusion, this is the first study evaluating d-ROMs, hair 20β-dihydrocortisol and intramural coronary arteriosclerosis in two traditional cattle rearing systems: Lidia bulls and Piemontese oxen. Non-significant levels of d-ROMs and hair 20β-dihydrocortisol were detected in Piemontese oxen, while Lidia bulls showed higher levels of hair 20β-dihydrocortisol. Arteriosclerosis was recorded in both groups, being more severe in the left papillary muscle of Piemontese oxen. These results suggest that both cattle breed experienced stress during their rearing cycle. Further studies are needed to clarify the role of housing system in the development of these subclinical pathological arteries changes and to better explore the role of sexual hormones in stress response.
Disclosure statement
The authors declare no conflict of interest
Data availability statement
The datasets analysed in the present study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Alonso ME, González-Montaña JR, Lomillos JM. 2020. Consumers’ concerns and perceptions of farm animal welfare. Animals. 10(3):385.
- Bernabucci U, Ronchi B, Lacetera N, Nardone A. 2002. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. Int J Dairy Sci. 85(9):2173–2179.
- Biagini D, Lazzaroni C. 2007. Effect of pre- and post-pubertal castration on Piemontese male calves: I. Live and slaughtering performances. Livest Sci. 110(1–2):181–186.
- Biasato I, Biasibetti E, Biagini D, Bruatto G, Cenacchi G, Guarda F, Capucchio MT. 2018. Spontaneously occurring intramural coronary arteriosclerosis in regularly slaughtered veal calves and beef cattle: a screening study about prevalence and histopathological features. J Vet Cardiol. 20(1):55–63.
- Bolado-Sarabia JL, Pérez-Linares C, Figueroa-Saavedra F, Tamayo-Sosa AR, Barreras-Serrano A, Sánchez-López E, García-Reynoso IC, Ríos-Rincón FG, Rodríguez-Poché MY, García-Vega LA, et al. 2018. Effect of immunocastration on behaviour and blood parameters (cortisol and testosterone) of Holstein bulls. Austral J Vet Sci. 50(2):77–81.
- Broom DM. 1988. The scientific assessment of animal welfare. Appl Anim Behav Sci. 20(1–2):5–19.
- Celi P. 2011. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol Immunotoxicol. 33(2):233–240.
- Chen Y, Stookey J, Arsenault R, Scruten E, Griebel P, Napper S. 2016. Investigation of the physiological, behavioral, and biochemical responses of cattle to restraint stress. J Anim Sci. 94(8):3240–3254.
- Chou LP, Li CY, Hu SC. 2015. Work-related psychosocial hazards and arteriosclerosis. Int Heart J. 56(6):644–650.
- Colombino E, Biasato I, Biasibetti E, Sereno A, Chiappino L, Evangelista R, Cenacchi G, Guarda F, Capucchio MT. 2019. Potential role of Anitschkow cells in cardiovascular disease in human and veterinary medicine: a review of the literature. Anat Histol Embryol. 48(3):201–206.
- Cozzi G, Brscic M, Gottardo F. 2009. Main critical factors affecting the welfare of beef cattle and veal calves raised under intensive rearing systems in Italy: a review. Ital J Anim Sci. 8(Sup1):67–80.
- Detweiler DK. 1989. Spontaneous and induced arterial disease in the dog: pathology and pathogenesis. Toxicol Pathol. 17(1 Pt 2):94–108.
- Detweiler DK, Patterson DF. 1965. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci. 127(1):481–516.
- European Commission. 2000. “The Welfare of Cattle Kept for Beef Production.”
- Falk T, Jönsson L. 2000. Ischaemic heart disease in the dog: a review of 65 cases. J Small Anim Pract. 41(3):97–103.
- Falk T, Jönsson L, Olsen LH, Pedersen HD. 2006. Arteriosclerotic changes in the myocardium, lung, and kidney in dogs with chronic congestive heart failure and myxomatous mitral valve disease. Cardiovasc Pathol. 15(4):185–193.
- Gupta S, Earley B, Crowe MA. 2007. Pituitary, adrenal, immune and performance responses of mature Holstein × Friesian bulls housed on slatted floors at various space allowances. Vet J. 173(3):594–604.
- Heimbürge S, Kanitz E, Otten W. 2019. The use of hair cortisol for the assessment of stress in animals. Gen Comp Endocrinol. 270:10–17.
- Heimbürge S, Kanitz E, Tuchscherer A, Otten W. 2020. Within a hair’s breadth – factors influencing hair cortisol levels in pigs and cattle. Gen Comp Endocrinol. 288:113359.
- Henry JP, Ely DL, Stephens PM, Ratcliffe HL, Santisteban GA, Shapiro AP. 1971. The role of psychosocial factors in the development of arteriosclerosis in CBA mice. Observations on the heart, kidney and aorta. Atherosclerosis. 14(2):203–218.
- Portal Id, Gil F, Silván Granado G. 2007. Regulación Neuroendocrina Del Estrés y Dolor En El Toro de Lidia (Bos Taurus L.): Estudio Preliminar. Rev complut. cienc Vet. 1(2)1-6–6.
- Ingvartsen KL, Refsgaard Andersen H. 1993. Space allowance and type of housing for growing cattle: a review of performance and possible relation to neuroendocrine function. Acta Agric Scand A: Anim Sci. 43(2):65–80.
- Kammermann KL, Luginbühl H, Ratcliffe HL. 1976. Intramural coronary arteriosclerosis of normal and dwarfed swine. Vet Pathol. 13(2):104–109.
- Keane MP, McGee M, O’Riordan EG, Kelly AK, Earley B. 2017. Effect of space allowance and floor type on performance, welfare and physiological measurements of finishing beef heifers. Animal. 11(12):2285–2294.
- Kutlikova H, Durdiakova J, Wagner B, Vlček M, Eisenegger C, Lamm C, Riečanský I. 2020. The effects of testosterone on the physiological response to social and somatic stressors. Psychoneuroendocrinology. 117(4):104693.
- Lomillos JM, Alonso ME. 2008. The Lidia breed: management and medicine. In Animal Reproduction in Veterinary Medicine. Vol. 32. London: IntechOpen; p. 137–144. doi:https://doi.org/http://dx.doi.org/10.5772/intechopen.92008.
- Lykkesfeldt J, Svendsen O. 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 173(3):502–511.
- Maxie MG, Robinson WF. 2007. Cardiovascular system. In: KVF Jubb, PC Kennedy, NC Palmer, editors. Pathology of domestic animals. 5th ed. Philadelphia: Elsevier Saunders; p. 141–575.
- Moberg GP, Mench JA. 2000. The biology of animal stress: basic principles and implications for animal. Wallingford: CABI Publishing.
- Moreau KL, Babcock MC, Hildreth KL. 2020. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 11(1):18–18.
- Nappi F, Cristiano S, Nenna A, Chello M. 2016. Ischemic mitral valve prolapse. J Thorac Dis. 8(12):3752–3761.
- Odore R, Badino P, Re G, Barbero R, Cuniberti B, D'Angelo A, Girardi C, Fraccaro E, Tarantola M. 2011. Effects of housing and short-term transportation on hormone and lymphocyte receptor concentrations in beef cattle. Res Vet Sci. 90(2):341–345.
- Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. 2013. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. JAHA. 2(6):e000272.
- Park RM, Foster M, Daigle CL. 2020. A scoping review: the impact of housing systems and environmental features on beef cattle welfare. Animals. 10(4):565.
- Ratcliffe HL. 1974. Arteriosclerosis of nonhominid primates maintained on a controlled diet. Aktuel Probl Angiol. 23:1–92.
- Ratcliffe HL, Luginbuhl H, Schnarr WR, Chacko K. 1969. Coronary arteriosclerosis in swine: evidence of a relation to behavior. J Comp Physiol Psychol. 68(3):385–392.
- Ratcliffe H, Snyder RL. 1967. Arteriosclerotic stenosis of the intramural coronary arteries of chickens: further evidence of a relation to social factors. Br J Exp Pathol. 48(3):357–365.
- Sharma A, Umapathy G, Kumar V, Phillips CJC. 2019. Hair cortisol in sheltered cows and its association with other welfare indicators. Animals. 9(5):248.
- Sikes SK. 1968. Controlling mechanisms in natural populations in relation to human and animal medicine: the disturbed habitat and its effect on the health of animal populations, with special reference to cardiovascular disease in elephants. J R Soc Med. 61(2):160–161.
- Smidt D. 1983. Indicators relevant to farm animal welfare. In: Martinus Nijhoff, editor. Indicators relevant to farm animal welfare. The Hague, The Netherlands: Springer Netherlands.
- Tarantola M, Biasato I, Biasibetti E, Biagini D, Capra P, Guarda F, Leporati M, Malfatto V, Cavallarin L, Miniscalco B, et al. 2020. Beef cattle welfare assessment: use of resource and animal-based indicators, blood parameters and hair 20β-dihydrocortisol. Ital J Anim Sci. 19(1):341–350.
- Tsujino K, Hikasa Y, Minami S, Okamoto Y, Morita T, Shimada A. 2005. Chronic myocardial infarction due to arteriosclerosis of coronary arteries followed by acute thromboembolism of caudal abdominal aorta in a cat. J Vet Med Sci. 67(6):631–634.
- Vassalle C, Bianchi S, Battaglia D, Landi P, Bianchi F, Carpeggiani C. 2012. Elevated levels of oxidative stress as a prognostic predictor of major adverse cardiovascular events in patients with coronary artery disease. J Atheroscler Thromb. 19(8):712–717.
- Vesel U, Pavič T, Ježek J, Snoj T, Starič J. 2020. Welfare assessment in dairy cows using hair cortisol as a part of monitoring protocols. J Dairy Res. 87(S1):72–78.
- Webster AJF. 2001. Farm animal welfare: the five freedoms and the free market. Vet J. 161(3):229–237.
