?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study is to investigate the responses of egg quality, sensory attributes and lipid profile of eggs and blood serum to different combinations of dietary oil supplementations and storage conditions. Thus, laying hens aged 25 weeks were assigned to six experimental groups and fed different diets for 14 weeks. The group fed an unsupplemented diet served as control, while the other five treatment groups consumed the same basal diet supplemented with 5% corn oil, 5% linseed oil, 2% corn oil + 3% linseed oil, 3% corn oil + 2% fish oil and 3% linseed oil + 2% fish oil, respectively. After 10 weeks, 36 eggs were collected from each experimental group and divided into three sets of 12 eggs to compare the quality of fresh eggs with those obtained after 21 days of storage at 23 °C and 5 °C. In addition, 20 untrained panellists evaluated the sensory characteristics of fresh eggs. Except for shell thickness, shell percentage and shell weight per unit of surface area (SWUSA), the supplementation of oil did not affect the egg quality parameters. The eggs laid by hens fed 3% corn oil and 2% fish oil had the highest quality shells, while the eggs from the control group had the best albumen colour, flavour and general acceptance. However, only shell percentage and shape index were unaffected by storage conditions, while all other parameters were significantly lower in eggs stored at 23 °C for 21 days. Although dietary oil supplementation did not significantly improve egg quality, feeding 3% corn oil and 2% fish oil improved eggshell quality, which declined with increasing storage temperature/period.
Dietary oils are essential components of poultry diets and animal nutrition.
Sensory attributes are essential for human acceptance of animal products.
Lipid profile may reflect lipid utilisation and efficiency.
Different dietary oil combinations affect the eggshell quality and sensory attributes of eggs.
Linseed oil enhanced the nutritional value of egg lipids and serum lipid profiles.
HIGHLIGHTS
Introduction
Following the COVID-19 crisis, significant advances in the poultry, egg and meat industries have been made because of modern technologies and relevant scientific findings (Hafez and Attia Citation2020). For example, eggs are now routinely fortified with omega-3 fatty acids to improve the sustainability of egg quality and benefit human health (Alagawany et al. Citation2019). This initiative is motivated by the fact that an average human diet tends to provide adequate amounts of omega-6 acids (n-6 PUFAs) due to increased consumption of vegetable oils such as soybeans, sunflower and corn oils (Khan et al. Citation2017), whereas omega-3 (n-3 PUFA) sources such as linseed oil and marine fish oil are rarely consumed (Van Dael Citation2021). An ample body of evidence has shown that high omega-6/omega-3 ratios stimulate inflammation, whereas their lower ratios are suppressed inflammation (Klasing Citation1998; González-Esquerra and Leeson Citation2001; Alagawany et al. Citation2019), thus reducing the risk of many chronic diseases.
Regular hen eggs contain about 1.779 g of omega-6 and 0.205 g of omega-3 (0.099 g of alpha-linolenic acid and 0.105 g of docosahexaenoic fatty acid) per 100 g of fresh weight (Kralik et al. Citation2021). Thus, a more favourable omega-6/omega-3 ratio could be achieved by increasing the quantity of omega-3 fatty acids in eggs. However, existing research has suggested that this would result in the greater production of harmful peroxides during storage (Fraeye et al. Citation2012; Ahmad et al. Citation2013). The nutritional quality of poultry products and their values and sustainability of high-quality poultry products has been linked with dietary intake of lipids sources and/or levels having a correlation with lipid metabolism and recent lifestyle diseases such as hypercholesteremia, cardiovascular (Klasing Citation1998; Khan et al. Citation2017; Alagawany et al. Citation2019), high blood pressure, peripheral arterial disease and coronary heart disease (Simopoulos and Salem Citation1996; González-Esquerra and Leeson Citation2001; Djuricic and Calder Citation2021).
Hence, in the current study, laying hens were fed diets supplemented with various levels and sources of omega-3 fatty acids to evaluate the response of egg quality characteristics and sensory attributes and lipid profiles in eggs and blood serum to different oil supplementations and storage conditions (temperature/period).
Materials and methods
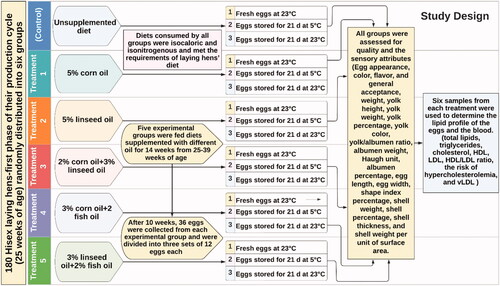
The study sample comprised 180 Hisex laying hens in their first phase of the production cycle (25 weeks of age) randomly distributed into six groups (Figure ), where one group was designated as control and fed a standard unsupplemented diet, as recommended by the Hisex breeder nutrition guide (https://www.tiwebsite.com/exploring/hisex-white-management-guide). The remaining five groups were fed diets supplemented with 5% corn oil, 5% linseed oil, 2% corn oil + 3% linseed oil, 3% corn oil + 2% fish oil and 3% linseed + 2% fish oil, respectively. The levels were chosen based on the available literature (González-Esquerra and Leeson Citation2001; Fraeye et al. Citation2012; Alagawany et al. Citation2019; Kralik et al. Citation2021). Diets consumed by all groups were isocaloric and isonitrogenous and met the requirements of laying hens’ diet (Table ). Throughout the study, the hens were housed in floor pens of 1.5 × 0.6 × 2.0 m, with five birds per pen and six replicates per treatment. Each pen was equipped with a laying feeder and a manual drinker, ensuring that food and water were available ad libitum. All pens were kept in an environmentally controlled room at 22–24 °C with a 16/8 light/dark daily cycle. The experimental was listed for 14 weeks from 25 to 39 weeks of age.
Table 1. Ingredients and chemical composition of diets fed to laying hens.
Eggs were daily collected, and those that were dirty, broken, blood-spotted, or otherwise damaged were discarded. After 10 weeks, 36 eggs per group were collected and divided into three sets of 12 eggs each. The first group was subjected to quality control measurements to examine these attributes in fresh eggs on the day of collection at 23 °C. At the same time, the remaining two groups of eggs were stored for 21 days at 5 °C and 23 °C, respectively, before these measurements were taken. Egg weight, yolk height, yolk diameter, yolk index, yolk weight, yolk percentage, yolk colour, yolk/albumen ratio, albumen weight, Haugh unit, albumen percentage, egg length, egg width, shape index percentage, shell weight, shell percentage, shell thickness and shell weight per unit of surface area (SWUSA) were determined. The measurements were carried out using the methods described by Attia et al. Citation1994; Attia et al. Citation1995 using the EggAnalyzer® (ORKA Food Technology; https://eggtester.com/about-us/).
Twenty untrained panellists with no self-reported egg intolerance or allergy evaluated the sensory attributes of quality attributes, who were instructed not to eat or smoke for at least 2 h before the evaluation. For this purpose, 10 eggs from each group were hard-boiled by placing them in a pot of water in a single layer and cooking them for 10 min (measured when the water reached boiling temperature). After 10 min, the pot was removed from the hotplate, the hot water was discarded and the eggs were then placed in water at 18 °C for 5 min. The eggs were then peeled, cut longitudinally into four equal parts and placed on plastic plates.
The panellists evaluated the eggs on a continuous unstructured line intensity scale (nine-point hedonic scale) ranging from 1 (dislike extremely) to 9 (like extremely) in terms of their appearance, yolk colour, albumen colour, flavour and overall acceptability.
Edible parts of eggs (albumen + yolk; n = 6 per treatment presented each treatment replicates) were carefully separated without albumen for determination of yolk total lipids (Folch et al. Citation1957), triglycerides (Fossati and Prencipe Citation1982), cholesterol (Allain et al. Citation1974), high-density lipoprotein (HDL) (Lopes-Virella et al. Citation1977) and low-density lipoprotein (LDL) (Wieland and Seidel Citation1983) using commercial digenetic kits manufactured by Diamond Diagnostics (23 EL-Montazah St. Heliopolis, Cairo, Egypt, http://www.diamonddiagnostics.com). The HDL/LDL ratio was determined by dividing HDL/LDL. Moreover, the risk of hypercholesterolaemia and very-low-density lipoprotein (vLDL cholesterol) was measured as (mg/dL) equation (Friedewald et al. Citation1972): vLDL = triglycerides/5.
Using the following equation, the risk of hypercholesterolaemia was computed according to Attia et al. (Citation2015):
Blood was collected in nonheparinised tubes at 39 weeks of age (n = 6 per treatment representing all treatment replicates) and centrifuged at 1500 × g for 15 min to obtain the serum. Blood serum was stored at −18 °C until conducting the analyses. As stated previously, serum total lipids, triglycerides, total cholesterol, HDL, LDL, HDL/LDL ratio, the risk of hypercholesterolaemia and vLDL were measured. The blood serum SFA and PUFA concentrations were measured using a fluorometric assay kit and an IL Monarch Automatic Analyser (Instrumentation Laboratories, Ltd., Warrington, UK) (Duncombe Citation1963; Hušek et al. Citation2002) (Item no. 700310). The UFA/SFA ratio was determined by dividing PUFA/SFA.
Data were subjected to a one-way ANOVA using the GLM procedure of SAS (SAS Institute Citation1990). Variables with significant F-test results (p < 0.05) were compared using Student–Newman–Keuls’s test with the pen as the unit of analysis and p < 0.05 indicating statistical significance. p-Values < 0.1 are considered as a tendency.
Results
As shown in Table , the type of oil supplementation did not significantly affect the egg weight change after storage and shape index, but egg weight of the 5% corn diet tended to be higher (p = 0.071) than that of other treatments. However, fresh eggs had the highest weight, followed by those stored at 5 °C and 23 °C for 21 days. The egg weight loss was greater at 23 °C/21 days compared to the other storage conditions. The storage temperature/period did not affect the egg shape index, and no interaction between dietary treatment and storage conditions was observed.
Table 2. Effect of various sources of oils and storage temperature on exterior egg quality characteristics.
The type of oil supplementation did not affect the percentage of dirty, broken and blood-spotted eggs or the total discarded eggs (Table ).
Table 3. Effect of various sources of oils and storage temperature on external egg quality traits.
The results in Table indicate that compared to the control, eggshell quality indicators (such as shell thickness, shell percentage and SWUSA) significantly increased when the hen diet was supplemented with 3% corn oil + 2% fish oil, and with 5% linseed oil and most of eggshell quality traits was decreased when hens fed 5% corn oil diet. Moreover, absolute shell weight exhibited a similar trend.
Table 4. Effect of various sources of oils and storage temperature on eggshell quality characteristics.
Storage temperature greatly affected shell thickness, shell weight, shell percentage and SWUSA, with better values for fresh eggs and eggs stored at 5 °C compared to those kept at 23 °C for 21 days. More specifically, the shell thickness of eggs stored at 5 °C for 21 days was lower than that of fresh eggs but was significantly higher than that for eggs stored at 23 °C. However, there was no significant interaction between dietary oil supplementation and storage conditions on the eggshell quality indicators (Table ).
Dietary oil supplementation did not significantly affect interior egg quality measurements, such as Haugh unit, yolk index, yolk percentage, albumin percentage, yolk/albumin ratio, or egg grade, but yolk colour tended to be higher (p = 0.098) in the control diet than that in the other groups (Table ). However, compared to fresh eggs, interior egg quality (Haugh unit, yolk percentage, yolk/albumin ratio and egg grade) declined significantly due to storage, especially at 23 °C. In contrast, eggs stored at 5 °C had a yolk index and yolk colour like that of fresh eggs. Table shows that, except for the yolk index of eggs stored at 23 °C/21 days (which increased compared to fresh eggs and storage temperature), the interaction between oil supplementation and storage conditions did not significantly affect the interior egg quality except for a tendency in percentage yolk, albumen and yolk:albumen ratio.
Table 5. Effect of various sources of oils and storage temperature on interior egg quality characteristics.
As shown in Table , the type of oil supplementation affected the sensory attributes of eggs (e.g. albumen colour, flavour and general acceptance). When compared to the control, eggs produced by hens fed linseed oil, with or without corn oil showed a lighter albumen colour. The flavour of eggs of hens provided corn oil was comparable to the control group. General acceptance: only when diets were supplemented with linseed oil, eggs showed a lower grade. Hens fed the control diet showed the highest appearance (p = 0.066) and yolk colour (p = 0.076).
Table 6. Effect of various sources of oils and storage temperature on egg sensory attributes.
Table illustrates the effect of dietary oil supplementation on serum lipid profiles. Except for serum HDL, LDL and HDL/HDL ratio, different oil sources and/or combinations did not significantly affect most serum lipid profiles. Moreover, compared to the unsupplemented diet (control), corn oil and linseed oil significantly increased HDL levels. The combination of different oils did not further increase serum HDL levels, which were intermediate.
Table 7. Effect of various sources of oils on blood serum lipid profiles.
The group fed 3% corn oil + 2% fish oil had the lowest LDL cholesterol, while the control group and hens fed a combination of corn oil + linseed oil had the highest LDL cholesterol level. Other groups fell in the middle. The HDL/LDL ratio reflects changes in beneficial and harmful cholesterols and indicates that corn oil, linseed oil and the combination of corn oil and fish oil increased the HDL/LDL ratio compared to other groups, except for the linseed oil + fish oil group where the ratio was intermediate. The corn oil + linseed oil had lower HDL/LDL than other combinations but equal to control and linseed + fish oil groups.
Table displays the effect of dietary oil supplementation on the edible egg (albumen + yolk) lipid profile.
Table 8. Effect of various sources of oils on whole egg (edible) lipid profiles.
According to the findings, different oil sources and/or combinations did not significantly affect egg total lipid, total cholesterol, the risk of hypercholesterolaemia, HDL-cholesterol, LDL cholesterol, or the HDL/LDL ratio. On the other hand, oil sources had a significant effect on egg triglycerides and vLDL. The results indicate that hens fed a combination of linseed alone or in combination with corn oil or fish oil had lower levels of egg triglycerides and vLDL than those fed corn oil alone (p < 0.05).
Discussion
Eggs’ flavour and appearance are the two main aspects that consumers are most interested in and place a high value on (Hayat et al. Citation2005; Fraeye et al. Citation2012; Ahmad et al. Citation2013). Even though feeding laying hens different concentrations of linseed and fish oils, to enhance ALA, EPA and DHA fatty acids, did not harmfully affect the external egg quality in this study. Moreover, supplementing 5% linseed oil and 3% corn oil + 2% fish oil resulted in shell percentage and SWUSA values comparable to those of control, showing sustainability of good egg quality. This finding suggests that the used dietary oil sources do not compromise the quality of eggshells and, consequently, the calcium metabolism or the amount of calcium available for the eggshells’ formation (Chen et al. Citation2014). These results are in accordance with the findings reported by Alshaikhi et al. (Citation2021) observing that dietary oil supplementation had no effect on the egg interior quality attributes. In this regard, Panaite et al. (Citation2021) found that eggshell percent and eggshell breaking strength were not affected by linseed meal at 6% in laying hens’ diets, but eggshell thickness was increased significantly. These results indicate that linseed meal had no negative effect on calcium available for eggshell formation.
Except for corn oil, the yolk colour tended to reduce due to the inclusion of the different oils in the hen’s diet. This finding is in accordance with Luquetti et al. (Citation2020), who found that the use of vegetable oils, although it does not compromise the egg quality and production parameters, was insufficient to achieve the same egg yolk colour levels as chicken eggs under corn diets. The differences in egg yolk colour may be due to various pigments in oils and the antioxidant capacity of the omega-3 of each oil source.
In contrast, hens supplemented with linseed oil at a concentration of 5%, or with 3% linseed oil combined with 2% fish oil produced eggs with a less robust flavour. Even though the former treatments led to eggs with a lower general acceptance score, these findings were consistent with those reported by Ayerza and Coates (Citation2001) and Berkhoff et al. (Citation2020). In this regard, Caston et al. (Citation1994) and Leeson et al. (Citation1998) reported that a high concentration (>10%) of flaxseed in the diet of laying hens decreased the egg acceptability, as measured by flavour and aroma. Although long-chain PUFAs are beneficial to human health, their use causes an increase in the production of peroxide during storage, which has an adverse impact on the quality of eggs as well as their nutritional value (González-Esquerra and Leeson Citation2001; Kralik et al. Citation2021), negatively influencing the consumer preference. The lack of significant effect of different oil sources tested herein in egg quality criteria is in general agreement with those reported by Attia et al. (Citation2018) who found that a 6% linseed meal diet did not affect eggs’ albumen percent and Haugh unit score. Previous studies showed that adding a single type of oil or fat to feed is difficult to meet the poultry needs of fatty acids due to the low variety of fatty acids and especially of essential fatty acids. Therefore, different mixing of oils and/or fat, in different proportions, have been studied to identify the best combination (Ceylan et al. Citation2011). However, due to the interaction of different types of ingredients in mixed oils, its effect becomes difficult to control and it is difficult to determine and explain its effect. Gao et al. (Citation2021) demonstrated that mixed oils produced evident damage to the structure of egg yolk and reduced egg production in comparison to the use of single oils or fats.
Fresh eggs outperformed those stored for 21 days, as expected; however, eggs stored at 5 °C performed better than those at 23 °C. This is because of high temperatures increase water evaporation and migration from albumen to yolk, which reduces the nutrient content (Alshaikhi et al. Citation2021). High PUFA and lower levels of antioxidants can accelerate the process of oxidative stress (Caston et al. Citation1994; Leeson et al. Citation1998), which is resulted from damage to the egg yolk membrane (Ayerza and Coates Citation2001; Ceylan et al. Citation2011). However, supplementation with antioxidants can improve egg quality and delay oxidative stress, thereby extending the shelf life of eggs (Meluzzi et al. Citation2000; Kirunda et al. Citation2001).
According to the obtained findings, linseed oil was more effective than corn oil and fish oil in reducing the levels of vLDL and triglycerides. The fact that adding fish oil did not make the effect of linseed oil on its own any more potent demonstrates that linseed oil alone would be sufficient. Similarly, Guenter et al. (Citation1971) found that increasing the amount of linoleic acid (n-6) in the diets of laying hens led to an increase in lipids when compared to egg yolks from laying hens fed a diet with lower levels of linoleic acid. Furthermore, Van Elswyk (Citation1997) suggested that flaxseed lowers plasma triglycerides in laying hens, which may reduce the availability of lipids to form yolk (Sanz et al. Citation1999). This could be the result of a low rate of lipid synthesis in combination with a high rate of lipid oxidation (Van Elswyk Citation1997), resulting from decreasing lipid digestibility and low ME of the diet due to flaxseed mucilage content (Leeson et al. Citation1998).
The association between the changes in egg lipid profile and the change in serum HDL/LDL ratio (Table ) suggests that the lipid source altered lipid metabolism in laying hens (Oliveira et al. Citation2011). However, this effect seems to be affected by ALA source and concentration as Panaite et al. (Citation2021) observed that the 6% linseed meal fed to laying hens did not significantly influence yolk lipid but reduced yolk total cholesterol. On the other hand, the lipid source and/or 5% marine byproducts seemed not to have any noticeable impact on the egg’s total cholesterol (Carrillo-Dominguez et al. Citation2005). Moreover, Ayerza and Coates (Citation2001) found that yolk lipid was affected by ALA source and lipid was decreased as ALA concentration increased, but yolk cholesterol was not affected. The present result agrees with the finding observed by (Yalçyn et al. Citation2007), who found that there was no significant difference in the total cholesterol found in the yolk as a result of the 24.5, 2.7, 1.8, 1.2 and a 1:1 − 6/−3 PUFA ratio. It is concluded that egg lipid and cholesterol contents were affected by lipid sources, levels and concentration and source of `n-3 fatty acid.
According to the findings related to blood serum biochemistry, both corn oil and linseed oil had a beneficial effect on the process by which lipoprotein cholesterol is metabolised, and they contribute to the preservation of a healthy HDL/LDL. Similarly, flax oil and sunflower oil supplementation increased serum HDL and reduced total cholesterol, LDL, vLDL and triglyceride, showing a beneficial effect on serum lipoprotein (Celebi and Utlu Citation2004). In addition, Van Elswyk (Citation1997) observed that flaxseed decreased plasma triglycerides, which may have been the result of an increase in lipid oxidation and a decrease in lipid synthesis. It was found that changes in dietary lipid intake were correlated with changes in serum and egg lipid profiles (Sim and Bragg Citation1977; Dedousi et al. Citation2022).
Conclusions
Supplementing the laying hens’ diets with various oil sources and combinations had no negative effect on egg quality. Specifically, diets including 3% corn oil + 2% fish oil improved eggshell quality; however, increasing storage temperature/period diminished egg quality. Linseed oil improved the nutritional value of egg lipids and the HDL/LDL ratio of serum. In addition to the beneficial effect of linseed oil on the sustainability of egg quality, it is expected that it can enhance egg nutritive value and produce omega-3 enriched eggs for human health benefits.
Ethical approval
This research work was approved by the Deanship of Scientific Research, King Abdulaziz University, Saudi Arabia, under protocol no. RG-1-155-43 H. The protocol recommends general humane treatment of animals that did not cause animal(s) pain, suffering, distress, or lasting harm, according to the Royal Decree no. M59 in 14/9/1431H and institutional approval code ACUC-22-1-2.
Author contributions
Y.A.A, M.A.A. and A.A.A. conducted the experimental design and first draft of the manuscript; M.J.O., A.A.A., E.O.H., R. A. A. and G. M. S carried out the data collection and contribution to the experimental set-up and laboratory work; Y. A. A. and N. M. A. carried out the statistical analyses and revision; Y.A.A, M.A.A., A.A.A., E.O.H., R.A.A., G. M. S and N.M.A. proofread the manuscript. All authors have final read, approved and agreed to the published version of the manuscript.
Acknowledgements
The authors acknowledge the administrative, technical and financial support by DSR, King Abdulaziz University, Jeddah, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data are available upon official request from the principal investigator upon the permission of the funding agent.
Additional information
Funding
References
- Ahmad DS, Haq A, Yousaf M, Kamran Z, Rehman A-U, Sohail MU, Abdus Samad H. 2013. Effect of canola oil and vitamin a on egg characteristics and egg cholesterol in laying hens during hot summer months. Pak Vet J. 33:346–349.
- Alagawany M, Elnesr SS, Farag MR, Abd El-Hack ME, Khafaga AF, Taha AE, Tiwari R, MohdI Y, Bhatt P, Khurana SK, et al. 2019. Omega-3 and omega-6 fatty acids in poultry nutrition: effect on production performance and health. Animals. 9(8):573.
- Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. 1974. Enzymatic determination of total serum cholesterol. Clin Chem. 20(4):470–475.
- Alshaikhi AM, Abdullatif AA, Badwi MA, Alsobayel AA. 2021. Effects of storage period, marketing channels and season on internal and external quality of commercial table eggs marketed in Riyadh City (Saudi Arabia). Braz J Poult Sci. 23 (1):1–10
- Attia YA, Al-Harthi MA, Korish MM, Shiboob MM. 2015. Fatty acid and cholesterol profiles and hypocholesterolemic, atherogenic, and thrombogenic indices of table eggs in the retail market. Lipids Health Dis. 14:136.
- Attia YA, Burke WH, Yamani KA. 1994. Response of broiler breeder hens to forced molting by hormonal and dietary manipulations. Poult Sci. 73(2):245–258.
- Attia YA, Burke WH, Yamani KA, Jensen LS. 1995. Daily energy allotments and performance of broiler breeders.: 2. Females. Poult Sci. 74(2):261–270.
- Attia YA, El-Hamid AE, Abdallah AA, Berikaa MA, El-Gandy MF, Sahin K, Abou-Shehema BM. 2018. Effect of betaine, vitamin C, and vitamin E on egg quality, hatchability, and markers of liver and renal functions in dual-purpose breeding hens exposed to chronic heat stress. Europ Poult Sci. 82:1-12.
- Ayerza R, Coates W. 2001. Omega-3 enriched eggs: the influence of dietary α-linolenic fatty acid source on egg production and composition. Can J Anim Sci. 81(3):355–362.
- Berkhoff J, Alvarado-Gilis C, Keim JP, Alcalde JA, Vargas-Bello-Pérez E, Gandarillas M. 2020. Consumer preferences and sensory characteristics of eggs from family farms. Poult Sci. 99(11):6239–6246.
- Carrillo-Dominguez S, Carranco-Jauregui ME, Castillo-Dominguez RM, Castro-Gonzalez MI, Avila-Gonzalez E, Perez-Gil F. 2005. Cholesterol and n-3 and n-6 fatty acid content in eggs from laying hens fed with red crab meal (Pleuroncodes planipes). Poult Sci. 84(1):167–172.
- Caston LJ, Squires EJ, Leeson S. 1994. Hen performance, egg quality, and the sensory evaluation of eggs from SCWL hens fed dietary flax. Can J Anim Sci. 74(2):347–353.
- Celebi S, Utlu N. 2004. Laying performance, serum lipoproteins, cholesterol and triglyceride of hens as influenced by dietary fat sources. J Appl Anim Res. 25(2):121–124.
- Ceylan N, Ciftçi I, Mızrak C, Kahraman Z, Efil H. 2011. Influence of different dietary oil sources on performance and fatty acid profile of egg yolk in laying hens. J Anim Feed Sci. 20(1):71–83.
- Chen W, Jiang YY, Wang JP, Huang YQ, Wang ZX. 2014. Effects of dietary flaxseed meal on production performance, egg quality, and hatchability of Huoyan geese and fatty acids profile in egg yolk and thigh meat from their offspring. Livestock Sci. 164:102–108.
- Dedousi A, Kritsa M-Z, Đukić Stojčić M, Sfetsas T, Sentas A, Sossidou E. 2022. Production performance, egg quality characteristics, fatty acid profile and health lipid indices of produced eggs, blood biochemical parameters and welfare indicators of laying hens fed dried olive pulp. Sustainability. 14(6):3157.
- Djuricic I, Calder PC. 2021. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients. 13(7):2421.
- Duncombe WG. 1963. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 88(1):7–10.
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226(1):497–509.
- Fossati P, Prencipe L. 1982. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 28(10):2077–2080.
- Fraeye I, Bruneel C, Lemahieu C, Buyse J, Muylaert K, Foubert I. 2012. Dietary enrichment of eggs with omega-3 fatty acids: a review. Food Res Int. 48(2):961–969.
- Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18(6):499–502.
- Gao Z, Zhang J, Li F, Zheng J, Xu G. 2021. Effect of oils in feed on the production performance and egg quality of laying hens. Animals. 11(12):3482.
- González-Esquerra R, Leeson S. 2001. Alternatives for enrichment of eggs and chicken meat with omega-3 fatty acids. Can J Anim Sci. 81(3):295–305.
- Guenter W, Bragg DB, Kondra PA. 1971. Effect of dietary linoleic acid on fatty acid composition of egg yolk, liver and adipose tissue. Poult Sci. 50(3):845–850.
- Hafez HM, Attia YA. 2020. Challenges to the poultry industry: current perspectives and strategic future after the COVID-19 outbreak. Front Vet Sci. 7:516.
- Hayat S, Fariduddin Q, Ali B, Ahmad A. 2005. Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agronomica Hungarica. 53(4):433–437.
- Hušek P, Šimek P, Tvrzická E. 2002. Simple and rapid procedure for the determination of individual free fatty acids in serum. Anal Chim Acta. 465(1–2):433–439.
- Khan SA, Khan A, Khan SA, Beg MA, Ali A, Damanhouri G. 2017. Comparative study of fatty-acid composition of table eggs from the Jeddah food market and effect of value addition in omega-3 bio-fortified eggs. Saudi J Biol Sci. 24(4):929–935.
- Kirunda DF, Scheideler SE, McKee SR. 2001. The efficacy of vitamin E (DL-alpha-tocopheryl acetate) supplementation in hen diets to alleviate egg quality deterioration associated with high temperature exposure. Poult Sci. 80(9):1378–1383.
- Klasing K. 1998. Comparative avian nutrition. Cambridge (UK): CAB International.
- Kralik G, Kralik Z, Grčević M, Galović O, Hanžek D, Biazik E. 2021. Fatty acid profile of eggs produced by laying hens fed diets containing different shares of fish oil. Poult Sci. 100(10):101379.
- Leeson S, Caston L, MacLaurin T. 1998. Organoleptic evaluation of eggs produced by laying hens fed diets containing graded levels of flaxseed and vitamin E. Poult Sci. 77(9):1436–1440.
- Lopes-Virella MF, Stone P, Ellis S, Colwell JA. 1977. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 23(5):882–884.
- Luquetti BC, Santos Sd, Litz FH, Crosara FSG, Limão VA, Andrade PL, Fernandes EdA 2020. Inclusion of crude corn oil with high acidity in the feed of laying hens: analysis of egs production and quality. Biosci J. 36(3):949–955.
- Meluzzi A, Sirri F, Manfreda G, Tallarico N, Franchini A. 2000. Effects of dietary vitamin E on the quality of table eggs enriched with n-3 long-chain fatty acids. Poult Sci. 79(4):539–545.
- Oliveira Dd, Baião NC, Cançado SdV, Oliveira Bd, Lana ÂMQ, Figueiredo Td 2011. Effects of the use of soybean oil and animal fat in the diet of laying hens on production performance and egg quality. Ciênc Agrotec. 35(5):995–1001.
- Panaite TD, Nour V, Saracila M, Turcu RP, Untea AE, Vlaicu PA. 2021. Effects of linseed meal and carotenoids from different sources on egg characteristics, yolk fatty acid and carotenoid profile and lipid peroxidation. Foods. 10(6):1246.
- Sanz M, Flores A, Lopez-Bote CJ. 1999. Effect of fatty acid saturation in broiler diets on abdominal fat and breast muscle fatty acid composition and susceptibility to lipid oxidation. Poult Sci. 78(3):378–382.
- SAS Institute. 1990. User’s guide. Version 6.06. Cary (NC): SAS Institute.
- Sim JS, Bragg DB. 1977. Effect of dietary factors on serum and egg yolk cholesterol levels of laying hens. Poult Sci. 56(5):1616–1621.
- Simopoulos AP, Salem N. 1996. Fatty acids and lipids from cell biology to human disease. Lipids. 31(1):S1.
- Van Dael P. 2021. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: review of recent studies and recommendations. Nutr Res Pract. 15(2):137–159.
- Van Elswyk ME. 1997. Nutritional and physiological effects of flax seed in diets for laying fowl. World’s Poult Sci J. 53(3):253–264.
- Wieland H, Seidel D. 1983. A simple specific method for precipitation of low density lipoproteins. J Lipid Res. 24(7):904–909.
- Yalçyn H, Ünal MK, Basmacyoolu H. 2007. The fatty acid and cholesterol composition of enriched egg yolk lipids obtained by modifying hens’ diets with fish oil and flaxseed. Grasas Aceites. 58(4):372–378.