 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Tenebrio molitor (mealworm) larvae are one of the most intriguing edible insects, and they may be raised on a variety of substrates, including by-products, side-stream products, and former foodstuff. The substrates could affect drastically the productive performances as well as the chemical-nutritional value of the larvae. In this study we tested two main substrate ingredients, brewery spent grains (SG) and bread leftovers (B), without adding any other dry ingredients. In order to correlate the chemical composition of the substrates to the larvae characteristics, five different diets were tested as 100% of a single ingredient (SG100 and B100), 75–25% mixes (SG75B25 and SG25B75) and 50–50% mixes (SG50B50). The effects of the substrate were tested on the development rates, chemical composition, and microbial loads of mealworm larvae. The effects of fasting, washing, and cooking were also tested on the microbiological determinations. Results indicate that all the parameters were affected by the chemical compositions of the substrates. The larvae fed the higher contents of SG showed the best growth performances along with higher nutritional values. The diet with only bread (B100) showed the worst parameters, both on the growth performances and on the nutritive values. Microbial loads were also affected by the diets, with minor effects in relation to the washing and fasting procedures, while cooking drastically reduced all the microbial loads. Taking into account that the two employed ingredients were former foodstuff or by-products it is important to highlight the capacity of mealworms to positively convert side-stream materials into rich nutritional animal products.
Mealworm could be proficiently reared on brewery spent grains and bread leftovers converting them into nutritional animal products.
Mealworm productive performances and nutritional value could be affected by the employed substrate.
Evidence of high mealworms plasticity and potential tailor made of the final outcomes.
Highlights
Introduction
In the last decade, edible insects rapidly increased their importance in animal science research fields and insect farming has grown in popularity throughout the world. As result, a lot of attention has been given by the media and the public opinion, especially when new legislative milestones or scientific results on their potential role in food production have been announced. The high protein content and relative ease in rearing have made yellow mealworms (Tenebrio molitor) one of the most popular edible insects along with black soldier flies (Hermetia illucens) and house crickets (Acheta domesticus). Since 2017, the European Union has listed mealworm larvae for their possible use as processed animal proteins (PAPs) aimed at the feeding of aquaculture animals (Commission Regulation 2017/893). Most recently PAPs derived from the insect species listed in the Commission Regulation were also authorised for feeding poultry and swine (Commission Regulation 2021/1372). Moreover, mealworm larvae were also considered as novel foods following the Regulation (EU) 2015/2283. Two out of six requests as novel food were already positively evaluated, and mealworms can now be used as ingredients by authorised producers in a wide list of products (Mancini et al. Citation2022). In addition, mealworms are capable of growth on organic matrixes derived from different production sectors, reducing the environmental impact in a view of a circular economy (Moruzzo et al. Citation2021). One of the main reasons of this interest in edible insects is based on the growing global population and the consequent increasing demand for proteins in food intake. Edible insects, indeed, showed a lower environmental impact than conventional production animals (mostly due to greenhouse gas production, water use, and arable land use), along with higher reproductive capacity, nutritional quality, and feed conversion efficiency (van Huis et al. Citation2013). Dreyer et al. (Citation2021) reported a comparison between Austrian organic broiler farming to mealworm ones. Authors highlighted that the impacts related to the production of 1 kg of edible mealworm protein were lower than broilers’ (18–72% lower impacts), with the exception of freshwater eutrophication (6% higher impacts, related to a higher heat demand in the mealworm rearing system that leads to increase freshwater eutrophication potential due to phosphate emissions related to fossil energy). The higher feed conversion efficiency (feed conversion ratio, FCR, of 3.91 and 4.91 respectively for mealworm larvae and organic broiler), the higher edible portion (94.5% and 43%, respectively) and the negligible greenhouse gasses emissions (direct and indirect) of mealworm larvae compared to organic broilers farming strengthen the interest in those insects as a sustainable alternative to conventional livestock (Dreyer et al. Citation2021).
Several research studies highlighted the capacity of mealworm larvae to growth on former foodstuff, by-products, and side-stream products (Melis et al. Citation2019; Zhang et al. Citation2019; Mancini et al. Citation2019b; Bordiean et al. Citation2020; Rumbos et al. Citation2020; Mattioli et al. Citation2021; Naser El Deen and Lamaj Citation2021; Montalbán et al. Citation2022). Food loss occurs across the food chain, resulting in a variety of economic, environmental, and social problems. Such losses are frequently created by the end customer, particularly in industrialised nations; it is estimated that approximately 89 Mt of food waste is generated in the EU27 annually. These estimated losses do not take into account the outputs from agricultural production and the side-streams and by-products. Brewers’ spent grain (SG) is the major by-product of the brewing industry, representing around 85% of the total by-products generated. The chemical composition of SG varies depending on the barley variety, harvesting season, malting and mashing conditions, as well as the quality and the kind of adjuncts used in the brewing process. SG is considered a lignocellulosic material rich in protein and fibre (around 20 and 70% of its composition, respectively) (Mussatto et al. Citation2006). Baked goods that are no longer considered sufficiently fresh but are still fit for consumption are no longer sold in shops and supermarkets. These leftover baked goods, mainly bread, are estimated to account for 8–10% of the total output of all bakeries. Bread leftovers, as a former foodstuff, could be used in animal nutrition as defined in the Commission Regulation (EU) No 68/2013 on the catalogue of feed materials.
The majority of the research studies revealed mealworms’ flexibility in response to the substrate, with changes in development periods and nutritional values of the larvae. For instance, differences in growth performances and feed conversion ratios (ranging between 1.57 and 2.08) were reported by Bordiean et al. (Citation2020) in mealworm larvae fed rapeseed meal, wheat bran, willowleaf sunflower, and chicken feed. Similarly, Montalbán et al. (Citation2022) revealed changes in growth performances and FCRs in a mealworm-fed diet with different starch: protein ratios (FCR ranged between 1.39 and 1.67). In quite all the research studied conduced changing in nutritional values of the larvae were reported in relation to the chemical composition of the substrates, affecting both in the proximate composition as well as in fatty acids profile, amino acid and mineral compositions (Melis et al. Citation2019; Zhang et al. Citation2019; Rumbos et al. Citation2020; Mattioli et al. Citation2021; Montalbán et al. Citation2022). However, because the major objective of the experiments was to evaluate the effects of combinations of high/low levels of proteins, lipids, or carbohydrates, researchers used diet derived from mixtures of several ingredients. One of the main challenges of animal production is to relocate the productions near to the feed suppliers and/or the consumers, reaching the goal to reduce pollution caused by feed/food transport. Indeed, because insect farming does not require special geographical or natural environmental qualities (as it is done in indoor controlled environment), it is plausible to assume that new farms will be built near substrate suppliers, with minimal feed modification to keep prices down. The aim of this research was to test two different food chain derived materials in mealworm rearing, i.e. brewery spent grains and bread leftovers, focussing on the growth performances, nutritional values, and microbiological loads of larvae. In addition, the two main ingredients were also tested in mixed diets in order to correlate the performances of the larvae to the feed characteristics.
Material and methods
Diet composition
Five different diets were formulated based on brewery spent grains (SG) and bread (B). Brewery spent grains were directly collected by a local brewery (Birrificio del Forte, Pietrasanta, Lucca, Italy) as a by-product and stored at −20 °C. Before the use, spent grains were thawed at 4 °C for 18 hours and completely dried at 90° C (for approximately 4 days). Bread leftovers were collected from different local market shops at the daily closure and immediately dried at 90 °C to remove excessive humidity. Both breweries spent grains and bread were then finely grinded, bread leftovers were all mixed together after being dried and grinded. Diets were formulated as only brewery spent grains (SG100), only bread (B100) and three mixes of these ingredients as % 75–25, 50–50 and 25–75 (SG75B25, SG50B50, SG25B75). The proximate composition of SG, B, and mixed diets is reported in Table .
Table 1. Proximate composition of brewery spent grains (SG100), bread (B100) and the three mix diets SG75B25, SG50B50, and SG25B75.
Insect rearing and growth performances
Mealworms were reared in plastic containers (39 × 28 × 14 cm) at the Department of Veterinary Sciences (University of Pisa, Pisa, Italy) under a laboratory scale production. The temperature was maintained at 25 ± 1 °C with 50%–60% of relative humidity. About one hundred adults (one-two weeks old) were placed in the rearing boxes (500 g substrate) for ten days to lay eggs (3 boxes per diet, 15 boxes in total, about 1500 adults). Beetles were then removed, and larvae were left to grow in each box. In order to homogenise the number of larvae per box, at week 9 larvae were harvested by sieving and about 300 larvae per box were employed for the second step of the trial. Nine weeks old larvae were placed in new boxes maintaining the same diet of the previous step (150 g of diet per box, 3 boxes per diet, 15 boxes in total). Boxes were visually evaluated three times per week to check larvae health and to remove the dead ones; feed was added weekly if needed (ad libitum, weighed before adding), and potato slices were deposited once a week to provide moisture (weighted fresh and as leftovers). Every week, representative samples of larvae (100 per box) were weighed to quantify the growth performance. Finally, larvae were harvested again when the first pupa was observed.
After the harvesting larvae were stored at −20 °C until analysed (for microbiology quantification live larvae were frozen for 1 hour following the processes described below). The development time was calculated between the first day of the experiment and the day of harvesting. The feed conversion ratio (FCR), efficiency of conversion of ingested food (ECI) and the nitrogen conversion efficiency (N-ECI) were calculated assuming that all the provided feed was consumed as reported by Oonincx et al. (Citation2015). The growth performance of the larvae was also determined as mg weight gain per day as:
where 63 stands for week 9 as beginning of the trial.
Proximate composition
Proximate compositions were determined in triplicate on each experimental unit. Frozen larvae were grounded before being analysed. The dry matter, ether extract, crude proteins and ash were determined following the AOAC (Citation1995) methods. The crude protein content of larvae was determined by the nitrogen-to-protein conversion factor of 4.76 as suggested by Janssen et al. (Citation2017), while factors of 5.83 and 5.70 were used respectively for brewery spent grains and bread (Merrill and Watt Citation1973; AOAC Citation2012). The carbohydrates were calculated by difference (100%-moisture%-crude protein%-ether extract%-ash%).
Microbiological analyses
Effects of starving, washing, and cooking, along with the effect of the diet, were tested on the microbiological quantifications following the scheme reported by Mancini et al. (Citation2019b). Larvae were starved for 48 h in sterile plastic containers with a plastic net placed on the base in order to separate faeces and to avoid faecal contact. Larvae (unfasted-fasted) were washed in distilled water inside a sterile stomacher bag (1:9 w/v). A cooking step was performed on the larvae after all the mix effects (unfasted-fasted, unwashed-washed) in a pre-heated oven at 150 °C for 10 min. Samples were diluted in sterile stomacher bags in a sterile saline solution thorough homogenisation, then ten-fold serial dilution series were performed and samples plated on different media. Total viable aerobic count and aerobic bacterial endospores (after heating the 10:1 dilution at 80 °C for 10 min) were counted on plate count agar (PCA) after incubation at 30 °C for 72 h; Enterobacteriaceae were enumerated on violet red bile glucose agar (VRBGA) incubated at 37 °C for 24 h; Escherichia coli was quantified on Tryptone Bile X-Glucuronide medium (TBX) incubated at 42 °C for 24 h; enumeration of staphylococci, both presumptive coagulase-positive and negative, was performed on Mannitol Salt Agar (MSA) incubated at 37 °C for 24–48 h; Enterococcus spp. was counted on kanamycin aesculin azide agar base (K) incubated at 42 °C for 24 h; yeast and mould counts were performed on yeast extract, dextrose, chloramphenicol medium (YEDC) incubated at 25 °C for 120 h; Pseudomonas spp. enumeration was performed on Pseudomonas Penicillin Pimaricin Agar (PPA) incubated at 30 °C for 24–48 h; lactic acid bacteria was enumerated on de Man–Rogosa–Sharpe agar (MRS) incubated at 37 °C for 48 h in anaerobic conditions. Presumptive Bacillus cereus was detected on B. cereus MYP agar incubated at 37 °C for 24 h. Absence of Listeria monocytogenes and Salmonella spp. in 25 g was assessed according to ISO 11290 and ISO 6579, respectively. All culture media and supplements were purchased from ThermoFisher Scientific (Waltham, United States) except for B. cereus MYP agar, which was purchased from Biolife (Milan, Italy). Microbiological counts were expressed as log colony forming unit per g (log cfu/g).
Statistical analysis
Linear regression and second-order polynomial quadratic equation were performed to evaluate the effect of each diet on the growth performance. The data obtained from the proximate composition were statistically analysed using a one-way ANOVA with regards to the different diets (SG100, SG75B25, SG50B50, SG25B75 and B100). The data of microbial determinations in relation to the diet (D), the effect of fasting (F, un-fasted and fasted), the effect of washing (W, un-washed and washed) and the effect of cooking (C, un-cooked and cooked) were tested with a multi-factor analysis of variance (4-way ANOVA). Statistical analysis taken into consideration the effects of the D, F, W and C their interactions (D × F, D × W, D × C, F × W, F × C, W × C, D × F × W, D × F × C, D × W × C, F × W × C, and D × F × W × C). Statistical significance was set at 0.05 and differences were assessed using Tukey’s test. In order to assess the effects of feed on larval development and chemical composition, a principal components analysis (PCA) was performed on proximate compositions of the substrates, proximate composition of larvae and growth performance (mg weight gain per day); all the data were mean centred and scaled to a unit standard deviation before analysis. R free statistical software was used (R Core Team Citation2015).
Results and discussion
Growth performances
The growth performances of larvae fed the five different diets are reported in Figure . Small variations in the growth performances were highlighted when SG were included into the diet, indeed only larvae fed B100 showed a different trend. Following the slow growth performance of larvae of groups B100 the trial was ended at week 21 as all the other diets presented pupae in the boxes. The larvae fed the diets SG100, SG75B25, SG50B50, and SG25B75 reached respectively the final weight of 136.15, 144.65, 134.79 and 123.59 mg, while the larvae fed only bread (B100) were ten time lighter (final weight 14.67 mg). Indeed, mg weight gain per day per larvae for B100 diet was 0.14 mg/d, while the other diets showed ten-fold gains, 1.51, 1.61, 1.50 and 1.37 mg/d (SG100, SG75B25, SG50B50, and SG25B75 respectively). Coefficient of determinations indicate that the models revealed a high percentage of the variability, with R2 between 0.90 and 0.94 for linear regression and between 0.96 and 0.99 for second order polynomial quadratic equation. The growth performances were in line with those reported in a similar trial performed with spent grains, bread, and cookies (Mancini et al. Citation2019b).
Figure 1. Tenebrio molitor larval growth in relation to the diet (brewery spent grains, SG100, bread, B100 and their mixtures, SG75B25, SG50B50, SG25B75).
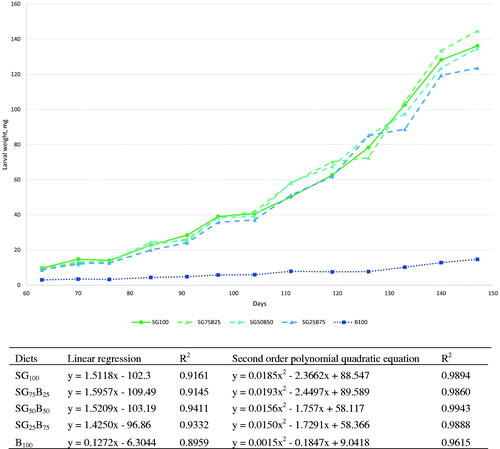
The substrate to body conversion indexes (FCR, ECI and N-ECI) are reported in Table . The Feed Conversion Ratio (FCR, calculated on fresh basis) confirmed the high performance of mealworms in convert substrate into body mass. The FCR data are in line with those of other studies that reported an index of about 2/2.5 kg of substrate to obtain 1 kg of insect (as fresh weight) with maximum peaks (in relation to the diet and to the slightly differences in FCR calculation, i.e. the real total utilisation of all the feed offered) about 3.5 points (Oonincx and de Boer Citation2012; Thévenot et al. Citation2018; Mancini et al. Citation2019b; Bordiean et al. Citation2020; Dreyer et al. Citation2021). In this paper we also calculated the FCR on dry matter (DM) as a better index to show mealworm efficiency in relation to the diet, indeed substrates employed in this type of insect rearing are mostly dry and the FCR calculated on fresh basis numerically does not separate consistently the different diets themselves. Mathematically, the ratio between FCR on fresh basis and FCR on DM is equal to the ratio between the DM of the larvae and the DM of the provided feed. Therefore, calculating the FCR on the DM maintained the concept of the feed conversion ratio but highlight the capacity of mealworm larvae to stock DM in their body without be affected of feed-larvae moistures. Basing on these statements, the data highlight a wide tendency to a worse feed conversion in relation to bread percentage present into the diet (P = 0.04). This trend was also comparable to ECI and N-ECI of larvae, which showed decreased values in relation to the amount of bread provided (P = 0.018 and P = 0.014, respectively). Bordiean et al. (Citation2020) reported a mean ECI value of 20.1 for mealworm fed industrial residues (rapeseed meal, wheat bran and willow leaf sunflower biomass), while a lower ECI, similar to the one obtained in this research, were shown by Oonincx et al. (Citation2015) and Mancini et al. (Citation2019b) ranged between 7 and 21.
Table 2. Feed conversion ratio (FCR), efficiency of conversion of ingested food (ECI) and the nitrogen conversion efficiency (N-ECI) of larvae fed the different diets.
Proximate compositions
Proximate compositions of larvae are reported in Table . No statistically differences were showed by larvae fed the five diets in terms of dry matter and ash compositions (P > 0.05). Interestingly, the diet induced large variability of the nutritional values of the larvae, indeed larvae fed the different mixes showed ranges that differ about 3.6–4% in the ether extract and protein contents (on wet basis, P < 0.001). Variations in terms of single macronutrient are more noticeable if looked closely, as ether extract increased by 28% between B100 and SG50B50 and crude protein increased by 42% between B100 and SG100. The mean proximate compositions of the larvae are in line with those reported in literature also in relation to the diet effects (Oonincx et al. Citation2015; van Broekhoven et al. Citation2015; Janssen et al. Citation2017; Mancini et al. Citation2019b). Ether extracts were higher in larvae fed mixed diets than in larvae fed single ingredient diets. This trend did not follow the diets ether extracts contents, as it showed to decrease in relation to an increased bread percentage (see Table ). Probably, the inclusion of bread in mixed diets, and the consequent increase of carbohydrates, induced an increasing of energy that was stored as lipids into the larvae body; when bread alone was used a lack of protein might have caused a restriction of energy. Indeed, protein content of the diets was negatively affected by the bread percentages. Larvae protein content followed this trend, showing a decreasing content in SG25B75 and B100.
Table 3. Proximate compositions of larvae fed brewery spent grains (SG100), bread (B100) and the three mix diets SG75B25, SG50B50, and SG25B75.
Microbial load
P-values of the statistical evaluation of the microbiological determinations are reported in Table , while the microbiological counts are reported in Table . E. coli, Pseudomonas spp., presumptive B. cereus, yeast and moulds were not detected in the samples, as well as the absence of L. monocytogenes and Salmonella spp. in 25 g was determined. The absence of food borne pathogens in edible insects reared in controlled environments is in line with the majority of the research studies conducted (Grabowski and Klein Citation2017; Vandeweyer et al. Citation2017; Mancini et al. Citation2019a, Citation2019b). Anyhow, some of these bacterial species could be detected in relation to the rearing conditions (such as the technique, the temperature, the humidity or the employed substrates). For instance, the presence of Pseudomonas spp. was detected in mealworm larvae derived from industrial rearing companies (Vandeweyer et al. Citation2017; Wynants et al. Citation2017) as well as via real-time PCR by Osimani et al. (Citation2018) in frass of mealworm larvae fed organic wheatmeal. Similarly, yeasts and moulds were detected in mealworm larvae with different ranges (Vandeweyer et al. Citation2017; Wynants et al. Citation2017; Mancini et al. Citation2019a, Citation2019b). These variations in microbial flora highlight the importance of the application of good manufacturing practices during mealworm rearing and processing to assure their safety when used as food or feed. The average of microbial counts of the unfasted and unwashed raw larvae were in line with those of previous research studies reported above, meanwhile some differences were detected in relation to the tested factors. The diet affected only the staphylococci counts as the larvae fed SG100 showed the lowest values while B100 the highest, the mixes of SG and B showed intermediate values. This trend is in accordance with Mancini et al. (Citation2019b) confirming that B as substrate is more prone to the growth of this type of bacteria than SG. It is important to highlight that B and SG used in the two trials (the present one and the previous one) are completely different as they were collected just before the start of the experiments and in the meantime two years have passed. Fasting decreased the number of staphylococci of about 0.2 log, it also decreased positively the total viable counts. These effects were not related to the diet fed to the larvae as also reported by the not significant P-value of the interaction D × F. Leaving the larvae for at least 24 h or 48–72 h without contact allow the larvae to discard their bowel content reducing the bacterial counts (Wynants et al. Citation2017, Citation2019; Mancini et al. Citation2019b, Citation2019c). Moreover, during starvation time larvae continue moving inside crates, inducing also a positive “grooming” that allowed the reduction of external pollution deposited on the larvae surfaces. This procedure also allowed the decreases of other risks as the content of gluten or mycotoxins derived from the substrates (Mancini et al., Citation2020; Niermans et al. Citation2019), indeed also the positive opinions of EFSA on mealworm as novel food reported a fasting step by the producers (Turck et al. Citation2021a, Citation2021b). Washing the larvae decreased the numbers of Enterobacteriaceae; other research studies reported the lack of effect of the washing step on the microbiological loads of the larvae, though it could remove pollution derived from substrate and frass (Wynants et al. Citation2017; Mancini et al. Citation2019c). Cooking the larvae in oven at 150 °C for 10 min decreased all the microbial form quantified, indeed only the total viable count was not reduced under the threshold of 1 log cfu/g (about 4 logs of reduction for this enumeration). Safety of edible insects is a mandatory requirement to ensure their employment in food chain, both as food and feed. Cooking, along with other heat treatment, seems one of the more effective methods, even if the cost of this step must be taken into account (Caparros Megido et al. Citation2018; Mancini et al. Citation2019c; Kooh et al. Citation2020).
Table 4. Statistical evaluation of the microbiological determinations in relation to the diet (D), fasting (F), washing (W), and cooking (C).
Table 5. Microbial loads (log cfu/g) in relation to the diet (D), fasting (F, Yes/No), washing (W, Yes/No), and cooking (C, Yes/No).
Principal component analysis
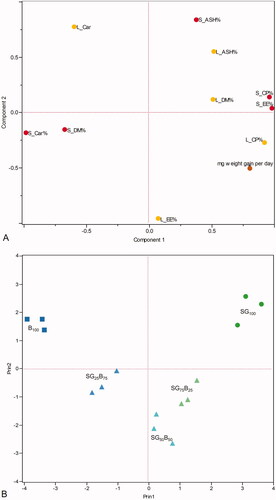
Principal component analysis plots (Figure ) indicate a direct link between chemical composition of substrate and the chemical composition of the larvae, as well as growth performances. The first two principal components gained respectively the 53.19% and 26.72% of the total variability. As plotted in the score plot the samples gathered together in relation to the substrate employed, following the percentages of spent grains and bread. The PC1 indicate to separate the samples in relation to the percentages of two ingredients, from negative value for higher percentage of inclusion of B to positive value for higher percentage inclusion of SG. PC1 interestingly followed strictly the variation of the two ingredients percentages. Similarly, PC2 showed a trend that divided the feeding groups into two main cluster (positive or negative values), with the diets based on only one ingredient (SG100 and B100) on the top part of the plot, and the mixed diets (SG75B25, SG50B50, and SG25B75) plotted in the bottom part. Also, in this case the relative abundance of the two ingredients followed a linear trend of variation. The loading plot showed that the growing performance of the larvae, expressed as mg of weight gain per day, was related to the crude protein and ether extract of the substrates. The opposite trend was shown by the capacity of gain weight and the substrate carbohydrates content. Larvae crude protein content was plotted closely to the growth capacity and the substrate energy components (crude proteins and ether extract), highlighting that the larvae fed the more nutritional feeds performed better and showed increased nutritional values.
Figure 2. Loading plot (A) and Score plot (B) of principal components analysis (PCA) performed on growth performance and proximate compositions of substrates (S) and larvae (L) (SG100: only brewery spent grains; SG75B25: 75% brewery spent grains + 25% bread; SG50B50: 50% brewery spent grains + 50% bread; SG25B75: 25% brewery spent grains + 75% bread; B100: only bread).
Taking into account that the two employed ingredients were former foodstuff or by-products it is important to highlight the capacity of mealworms to positively convert side-stream materials into rich nutritional animal product.
Conclusions
By-products, former foodstuff and side-stream materials might be used as a feed in T. molitor rearing, with effects on the growth performance of the larvae, chemical composition and microbial load. The findings show that mealworm larvae are highly flexible, with the capacity to change the final characteristics in relation to the substrate. The use of a single ingredient in the diet or combinations of different ingredient might be used in response to the producers’ demands and benefit–cost criteria. In general, it appears that high-protein diets result in lower rearing time. Anyhow our data highlighted that mixing a high nutritional ingredient (brewery spent grain) with a low nutritional ingredient (bread) with a proportion of 75:25 could be more effective than use only one ingredient alone. When a single ingredient was tested, bread leftovers showed the worst results in relation to the other diet, while spent grains alone performed similar to the most effective diet (75:25 spent grains:bread). The mixed diet with 75% of spent grains and 25% of bread showed also the better results in terms of nutritional value (highest ether extract and crude protein) and the lowest count of staphylococci.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, SM, upon reasonable request.
Additional information
Funding
References
- AOAC. 1995. Official methods of analysis. 15th ed. Washington DC, USA: Association of Official Analytical Chemists.
- AOAC. 2012. Official methods of analysis (19th ed.). Washington DC, USA: Association of Official Analytical Chemists.
- Bordiean A, Krzyżaniak M, Stolarski MJ, Peni D. 2020. Growth potential of yellow mealworm reared on industrial residues. Agriculture. 10(12):599.
- van Broekhoven S, Oonincx DGAB, van Huis A, van Loon JJA. 2015. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J Insect Physiol. 73:1–10.
- Caparros Megido R, Poelaert C, Ernens M, Liotta M, Blecker C, Danthine S, Tyteca E, Haubruge É, Alabi T, Bindelle J, et al. 2018. Effect of household cooking techniques on the microbiological load and the nutritional quality of mealworms (Tenebrio molitor L. 1758. Food Res Int. 106(2017):503–508.
- Dreyer M, Hörtenhuber S, Zollitsch W, Jäger H, Schaden L-M, Gronauer A, Kral I. 2021. Environmental life cycle assessment of yellow mealworm (Tenebrio molitor) production for human consumption in Austria – a comparison of mealworm and broiler as protein source. Int J Life Cycle Assess. 26(11):2232–2247.
- Grabowski NT, Klein G. 2017. Microbiological analysis of raw edible insects. J Insects Food Feed. 3(1):7–14.
- Janssen RH, Lakemond CMM, Fogliano V, Renzone G, Scaloni A, Vincken J-P. 2017. Involvement of phenoloxidase in browning during grinding of Tenebrio molitor larvae.Lee B-L, editor. PLOS ONE. 12(12):e0189685.
- Janssen RH, Vincken J-P, van den Broek LAM, Fogliano V, Lakemond CMM. 2017. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J Agric Food Chem. 65(11):2275–2278.
- Kooh P, Jury V, Laurent S, Audiat-Perrin F, Sanaa M, Tesson V, Federighi M, Boué G. 2020. Control of biological hazards in insect processing: application of HACCP method for yellow mealworm (Tenebrio molitor) powders. Foods. 9(11):1528.
- Mancini S, Fratini F, Tuccinardi T, Turchi B, Nuvoloni R, Paci G. 2019a. Effects of different blanching treatments on microbiological profile and quality of the mealworm (Tenebrio molitor). J Insects Food Feed. 5(3):225–234.
- Mancini S, Fratini F, Turchi B, Mattioli S, Dal Bosco A, Tuccinardi T, Nozic S, Paci G. 2019b. Former foodstuff products in Tenebrio molitor rearing: effects on growth, chemical composition, microbiological load, and antioxidant status. Animals. 9(8):484.
- Mancini S, Paci G, Ciardelli V, Turchi B, Pedonese F, Fratini F. 2019c. Listeria monocytogenes contamination of Tenebrio molitor larvae rearing substrate: preliminary evaluations. Food Microbiol. 83:104–108.
- Mancini S, Fratini F, Tuccinardi T, Degl'Innocenti C, Paci G. 2020. Tenebrio molitor reared on different substrates: is it gluten free? Food Control. 110:107014. https://doi.org/10.1016/j.foodcont.2019.107014
- Mancini S, Sogari G, Espinosa Diaz S, Menozzi D, Paci G, Moruzzo R. 2022. Exploring the future of edible insects in Europe. Foods. 11(3):455.
- Mattioli S, Paci G, Fratini F, Dal Bosco A, Tuccinardi T, Mancini S. 2021. Former foodstuff in mealworm farming: effects on fatty acids profile, lipid metabolism and antioxidant molecules. LWT. 147:111644.
- Melis R, Braca A, Sanna R, Spada S, Mulas G, Fadda ML, Sassu MM, Serra G, Anedda R. 2019. Metabolic response of yellow mealworm larvae to two alternative rearing substrates. Metabolomics. 15(8):113–113.
- Merrill AL, Watt BK. 1973. Energy value of food: basis and derivation. Washington DC: USDA United States Department of Agriculture.
- Montalbán A, Sánchez CJ, Hernández F, Schiavone A, Madrid J, Martínez-Miró S. 2022. Effects of agro-industrial byproduct-based diets on the growth performance, digestibility, nutritional and microbiota composition of mealworm (Tenebrio molitor L.). Insects. 13(4):323.
- Moruzzo R, Riccioli F, Espinosa Diaz S, Secci C, Poli G, Mancini S. 2021. Mealworm (Tenebrio molitor): potential and challenges to promote circular economy. Animals. 11(9):2568.
- Mussatto SI, Dragone G, Roberto IC. 2006. Brewers’ spent grain: generation, characteristics and potential applications. J Cereal Sci. 43(1):1–14.
- Naser El Deen S, Lamaj F. 2021. Productivity and larval growth of Tenebrio molitor reared on differently composed diets of similar nutritional composition. J Insects Food Feed. 7(8):1207–1217.
- Niermans K, Woyzichovski J, Kröncke N, Benning R, Maul R. 2019. Feeding study for the mycotoxin zearalenone in yellow mealworm (Tenebrio molitor) larvae—investigation of biological impact and metabolic conversion. Mycotoxin Res. 35(3):231–242.
- Oonincx DGAB, de Boer IJM. 2012. Environmental impact of the production of mealworms as a protein source for humans – a life cycle assessment. PLOS One. 7(12):e51145.
- Oonincx DGAB, van Broekhoven S, van Huis A, van Loon JJA. 2015. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products.Papadopoulos NT, editor. PLOS One. 10(12):e0144601.
- Osimani A, Milanović V, Cardinali F, Garofalo C, Clementi F, Pasquini M, Riolo P, Ruschioni S, Isidoro N, Loreto N, et al. 2018. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): from feed to frass. Int J Food Microbiol. 272:49–60.
- R Core Team 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Austria http://www.r-project.org/.
- Rumbos CI, Karapanagiotidis IT, Mente E, Psofakis P, Athanassiou CG. 2020. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci Rep. 10(1):11224.
- Thévenot A, Rivera JL, Wilfart A, Maillard F, Hassouna M, Senga-Kiesse T, le Féon S, Aubin J. 2018. Mealworm meal for animal feed: environmental assessment and sensitivity analysis to guide future prospects. J Clean Prod. 170:1260–1267.
- Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch‐Ernst KI, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, et al. 2021a. Safety of frozen and dried formulations from whole yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFS2. EFSA Journal 2021;19(8):6778.
- Turck D, Castenmiller J, de Henauw S, Hirsch‐Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, et al. 2021b. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to regulation (EU) 2015/2283. EFS2. EFSA Journal 2021;19(1):6343.
- Vandeweyer D, Crauwels S, Lievens B, van Campenhout L. 2017. Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllodes sigillatus) from different rearing companies and different production batches. Int J Food Microbiol. 242:13–18.
- van Huis A, van Itterbeeck J, Klunder H, Mertens E, Halloran A, Muir G, Vantomme P. 2013. Edible insects. Future prospects for food and feed security. Rome: FAO (Food and Agriculture Organization of the United Nations). https://www.fao.org/3/i3253e/i3253e.pdf
- Wynants E, Crauwels S, Lievens B, Luca S, Claes J, Borremans A, Bruyninckx L, van Campenhout L. 2017. Effect of post-harvest starvation and rinsing on the microbial numbers and the bacterial community composition of mealworm larvae (Tenebrio molitor). Innovative Food Sci Emerg Technol. 42:8–15.
- Wynants E, Frooninckx L, van Miert S, Geeraerd A, Claes J, van Campenhout L. 2019. Risks related to the presence of Salmonella sp. during rearing of mealworms (Tenebrio molitor) for food or feed: survival in the substrate and transmission to the larvae. Food Control. 100:227–234.
- Zhang X, Tang H, Chen G, Qiao L, Li J, Liu B, Liu Z, Li M, Liu X. 2019. Growth performance and nutritional profile of mealworms reared on corn stover, soybean meal, and distillers’ grains. Eur Food Res Technol. 245(12):2631–2640.
