ABSTRACT
The fruitless (fru) gene in Drosophila has been proposed to play a master regulator role in the formation of neural circuitries for male courtship behavior, which is typically considered to be an innate behavior composed of a fixed action pattern as generated by the central pattern generator. However, recent studies have shed light on experience-dependent changes and sensory-input-guided plasticity in courtship behavior. For example, enhanced male-male courtship, a fru mutant “hallmark,” disappears when fru-mutant males are raised in isolation. The fact that neural fru expression is induced by neural activities in the adult invites the supposition that Fru as a chromatin regulator mediates experience-dependent epigenetic modification, which underlies the neural and behavioral plasticity.
Male courtship is something more than an innate behavior
fruitless (fru)-mutant males kept in a group often engage in male-to-male courtship, forming a long chain of courting males ().Citation1 This chaining behavior in males has been regarded as a hallmark of fru mutants, based on the assumption that enhanced male-directed courtship is a genetic predisposition in fru-mutant males. Nonetheless, careful observers have noted that male chaining among fru-mutant males is more susceptible to disturbances than the male-to-female courtship in wild-type flies. For instance, if fru mutant males forming a courtship chain in a culture vial are transferred to an observation chamber by aspiration (a disturbing manipulation), the chaining activity among these males is readily suppressed, whereas wild-type males similarly transferred to an observation chamber immediately commence courtship toward a target female in the unfamiliar chamber despite the disturbing manipulation. Another puzzling observation is that males carrying a strong loss-of-function fru mutation show few courtship activities toward a target male under single-pair conditions, in contrast with the vigorous male-to-male courtship with chaining under grouped conditions (D. Yamamoto, unpublished observations). Despite these intriguing observations, however, there have been few serious attempts to explore the mechanistic basis of the easy disturbance of male chaining in fru-mutant males, presumably due to the general reluctance to work on capricious phenotypes.
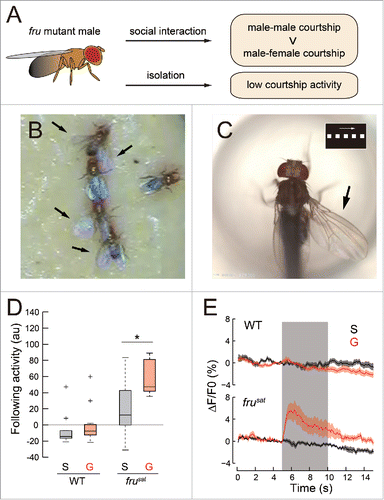
Villella et al.Citation2 were the first to quantitatively analyze the effect of group rearing on courtship chaining in fru-mutant males. They found that the frequency of chain formation among fru-mutant males gradually increased with increasing duration of cohabitation in the vial (up to 5 days). This trend was shared by 17 different fru mutant genotypes,Citation2 including those with no detectable FruM (M stands for male-specific; see below) protein expression in the nervous system, such as fruCitation3 and fru3/Df[ChaM5].Citation3 For almost 20 y after their work, researchers shifted their focus to elucidating how FruM orchestrates gene expression as a transcription factor, how FruM produces neural sex differences and how FruM-expressing neurons contribute to the circuitry for innate courtship behavior.Citation5-8 Then, in 2014, the group effect on fru-mutant courtship activities was revisited by Pan and Baker,Citation4 who documented that male-male chaining in fru mutants is nearly completely suppressed in males raised in isolation since eclosion, and that such fru-mutant males deprived of social experience may rapidly develop chain-forming activities upon grouping with other males for a few days (). Remarkably, they found that fru-mutant males grouped with conspecific females or females of other species acquire a female-directed courtship activity, though the level of heterosexual courtship thus acquired is lower than that of homosexual courtship, which is similarly acquired irrespective of the sex or species of other members in the group ().Citation4 This latter observation implies that the socializing stimulus is sex-specific and species-specific to some extent. Indeed, Pan and BakerCitation4 found that the male-male courtship activity is acquired in an isolated fru-mutant male in the absence of chemical cues provided that he can see other flies, yet the acquisition of male-female courtship activity requires additional cues, possibly olfactory stimuli from females during the acquisition phase. In keeping with this idea, Pan and BakerCitation4 found that olfaction-deficient orco-homozygous fru-mutant males fail to show male-female courtship even after group rearing. They further claimed that olfaction is required for manifesting male-male courtship at the test session after the acquisition phase because the orco mutation prevented fru-mutant males from exhibiting courtship activities. However, our later experiment with a virtual reality paradigm indicated that fru-mutant males do not require olfactory input to manifest acquired courtship activities (see below).Citation9 The orco effect on male courtship will be further discussed in a later section.
Another interesting observation by Pan and BakerCitation4 was that loss-of-dsx completely inhibits the acquisition of courtship activities by fru-mutant males; they considered that “DsxM is both necessary and sufficient for fruM-independent, experience-dependent courtship.” This phrase is somewhat misleading, however, because wild-type males do not engage in male-male courtship even after group housing and thus this phenomenon is fru-mutant specific; it depends on the absence of fru functions (it is fruitless-less-dependent). Note however, that isolation has generally been thought to enhance the motivation to court in males, and in fact, the standard protocol of mating assays uses singly housed males. A recent study presented quantitative evidence that isolated wild-type males show higher levels of courtship activity than grouped males.Citation10 The inhibitory effect of dsx-loss on the acquired courtship observed by Pan and BakerCitation4 may reflect the developmental requirement of dsx for certain neurons that contribute to the acquired courtship. For example, the P1 cluster, which is the courtship decision-making center, exists only in the male brain, as a result of the absence of DsxF, which eliminates the P1-counterpart in females during development.Citation11 In males, the P1 cluster neurons express, in addition to dsx, fru, the absence of which does not affect survival or death of the P1 cluster in males, such that fru-mutant males retain this neural cluster.Citation11
Physiological substrates for social effects on courtship behavior
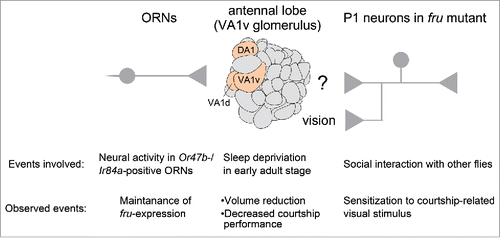
The physiological correlates of social experience in fru-mutant males were recorded by Ca2+-imaging by Kohatsu and Yamamoto,Citation9 who demonstrated that dsx-positive neurons, including the P1 subclass of pC1 cluster neurons in the lateral protocerebrum (lpr),Citation12 acquire visual hypersensitivity by social experience (). Kohatsu and YamamotoCitation9 established a virtual reality paradigm for the quantitative analysis of sensorimotor control of male courtship behavior. In this paradigm, a tethered male fly on a treadmill is visually stimulated with an array of light spots displayed on a computer screen, and induced locomotion and other behavioral acts such as tapping, wing extension and vibration, licking and abdominal bending can be monitored for quantification. Under the tethered conditions, wild-type males do not exhibit courtship acts unless they sense pheromones with their foreleg gustatory receptors by touching the female abdomen, i.e., tapping.Citation13 Once they have touched a female, the wild-type males continue to pursue the moving light spots on the treadmill while vibrating a wing to generate courtship songs.Citation9 Surprisingly, fru-mutant males kept in a group readily started the courtship pursuit of moving light spots without prior touching of a female (). However, such a visually induced courtship pursuit was barely induced in fru-mutant males that were kept singly ().Citation9 GCaMP3.0-aided activity recordings from dsx-expressing neurons in the lpr revealed a robust Ca2+ rise in response to moving light spots in the group-housed fru-mutant males, whereas the same stimuli did not bring about discernible neural responses in isolated fru-mutant males, or in wild-type males that were kept in group-housing or isolation ().Citation9 Thus group housing of fru-mutant males, but not wild-type males, renders the dsx-expressing lpr neurons hypersensitive to visual stimuli, thereby triggering a courtship pursuit in these flies. Inagaki et al.Citation14 measured the effect of housing conditions of wild-type males on the courtship behavior induced via channelrhodopsin (ChR) expressed in P1 neurons, and found that isolated males had a lower threshold for behavior induction than group reared males did. Inagaki et al.Citation14 also showed that the identical ChR activation leads to a smaller Ca2+ influx in grouped males than in isolated males. These observations suggest that group rearing makes lpr neurons (or P1 neurons, in particular) more excitable in fru-mutant males but not in wild-type males. It appears that functional fru is necessary to “calm down” P1 neurons in the male adult so that P1 neurons do not respond to stimuli originating from objects that are not courtship targets. It is tempting to speculate that early social experience upon eclosion initiates this tuning of neural responsiveness, which is fru-dependent and thus disrupted in fru-mutant males. This idea would appear to suggest a Fru function for adults, in addition to the well-recognized roles of Fru for neural sexual differentiation in the late larval to pupal stages.Citation7,8 Alternatively, the courtship circuitry laid out before eclosion in the absence of fru might have a latent defect that manifests itself upon social experience after eclosion as hyperneural sensitivity to sensory input in fru mutant males. A recent finding that fru expression in a few specific classes of olfactory receptor neurons (ORNs) at the adult stage requires ligand-induced activities in these neurons (see below) seems to favor the first hypothesis that FruM in the adult stage mediates experience-dependent tuning of neural responsiveness.
fru is not just a developmental gene
The Drosophila nervous system contains ∼100,000 neurons, ∼2000 of which express fru.Citation15 Some fru-expressing neurons are sex-specific and others exhibit sexual dimorphisms to a varying extent depending on the cell group. Loss of fru functions leads to loss of these sex differences in fru mutants, as a result of sexual fate changes in preimaginal development due to the absence of male-specific FruM as a neural masculinizing factor, while other neurons (that compose the major portion of the nervous system) appear unaffected. In fact, FruM is the sole Fru protein expressed in the postembryonic (preimaginal) nervous system and is dedicated exclusively to the sex determination role of neurons with no known functions. In the nervous system, the fru gene is transcribed in a specific subset of neurons in both sexes but is not translated in the female nervous system.Citation15,16 Apart from this developmental role of Fru, Hueston et al.Citation17 have reported that 2 (Or47b and Ir84a ORNs) of 3 fru-expressing groups of ORNs require neural activities for maintaining fru expression (as detected with fru-GAL4) in the adult stage: even though mutants that were defective in olfactory receptors Or47b and Ir84a in addition to olfactory co-receptor orco exhibited normal expression of fru in pupae (∼40 h APF), the fru expression in these olfactory receptor cells was lost in 3–5 day-old flies (). The loss of fru expression from Or47b-expressing ORNs accompanied no obvious changes in cellular structure and function, including the Or-receptor identity or specification of Or-receptor distribution.Citation17 The fru-positive ORNs expressing Or47b, Ir84a, and Or67d, respectively, innervate 3 sexually dimorphic glomeruli,Citation21 the VA1v (VA1m), VA1d (VL2a) and DA1 glomeruli, in the antennal lobe.Citation18-20 Whereas a neural activity block by a transgenically expressed K+ channel reduced fru expression in Or47b ORNs, the restoration of neural activities by heterologous expression of the receptor protein Or67d in Or47b-neurons in Or47b mutants failed to rescue fru expression in these cells, suggesting that the neural activity alone is insufficient for the maintenance of fru expression. Intriguingly, Hueston et al.Citation17 found that similar heterologous expression of Or88a partially rescued fru expression but that of Or67d could not restore fru expression. Or67d is normally expressed in a fru-positive ORN class and Or88a is normally expressed in a fru-negative ORN class. Importantly, the 2 receptors, Or88a and Or47b, share ligands, including methyl laurate and other fatty acids that are fly-produced attractants,Citation22 whereas Or67d binds 11-cis-vaccenyl acetate (cVA; Kurtovic et al.Citation23), a male pheromone transferred to the female mate during copulation that acts as a repellant for males.Citation24,25 It appears that Or47b ORNs need to be activated via a receptor that is responsive to methyl laurate for the maintenance of fru expression in the adult.
Notably, the Or47b ORN-target VA1v glomerulus is one of only a few glomeruli in the antennal lobe that exhibit anatomical plasticity in response to environmental stimuli ().Citation26,27 For example, sleep-deprivation by mechanical disturbances or by artificial dopaminergic activation on the day of eclosion resulted in atrophy of VA1v, but the same treatments were without effect when administered to elder flies, e.g., 5-day-old flies.Citation26 Thus, it seems that the Or47b ORN – VA1v pathway in young adult flies is highly susceptible to changes in internal and external conditions, responding with altered fru expression and/or volumetric changes. Fatty acid ligands for the Or47b receptor are contained in cuticles of both sexes and of other species, and therefore, they cannot be cues for the recognition of the sex and species.Citation28 Fatty acids are synthesized from nutrients by fat body cells in flies.Citation29 Oenocytes use these fatty acids as precursors for synthesizing cuticular hydrocarbons, some of which function as pheromones.Citation25,30 Genetic ablation of oenocytes yielded flies without cuticular hydrocarbons (oe- flies), which were found to be hyper-attractive; wild-type males preferred oe- females and males rather than normal flies.Citation31 These findings suggest that fatty-acid pheromones common to both sexes act as attractants for males, whereas some hydrocarbon pheromones, with negative or positive roles, provide clues for the males to differentiate females from males and conspecifics from other species in choosing an appropriate courtship target.Citation25 It should be noted, however, that these chemical cues are dispensable for triggering courtship, because wild-type males may initiate courtship in response to a moving inorganic dummy without any chemical attractants,Citation32-34 although such courtship attempts are generally brief. Intriguingly, the aberrant courtship toward oe- males was suppressed by removing functional Or47b from the courter males.Citation35 Thus Or47b stimulates male-male courtship under certain conditions. Fine-tuning of the excitability of Or47b ORNs could affect the prevalence of male-male courtship in a given male fly, and the fly's early experience likely modulates the olfactory tuning. Hypersensitivity of Or47b might elicit courtship toward an inappropriate target, as the courter male would be primed for courtship by fatty acids on his own cuticles. Alternatively, Or47b hyperactivation in young adults might trans-synaptically induce a persistent elevation of the excitability of higher-order neurons in the courtship circuitry, such as those composing the P1 cluster, a courtship-triggering center (cf. Clowney et al.Citation36 for olfactory contributions to P1 activities). According to Hueston et al.,Citation17 one of the outcomes of Or47b activation in young adults is the persistent expression of fru in Or47b ORNs, as mentioned above. An attractive scenario is that the fru thus expressed fine-tunes the excitability of Or47b ORNs, perhaps to “calm down” these cells which would otherwise become hyperactive as a result of the intense interactions with other flies under grouped conditions, and such hyperactivity would result in a loss of acuity in detecting odorant quality and quantity. It is conceivable that, in fru-mutant males, this negative feedback regulation of the excitability of Or47b ORNs would not operate because of the absence of Fru, making the P1 neurons hypersensitive to “non-specific” sensory stimuli, leading to an inappropriate activation of courtship repertoires by such stimuli ().Citation9
Does fru boost fitness in elder males?
Why should the excitability of Or47b ORNs be modulated by the experience of individual flies? A recent work by Lin et al.Citation37 provides an answer to this question. Addressing an earlier report that sexually experienced males have a competitive advantage over naïve males,Citation38 Lin et al.Citation37 demonstrated that even without prior sexual experience, older males have an advantage over young males in competitive mating, and that this elderly-fly advantage is ascribable to an age-dependent sensitization of Or47b ORNs, which supports a higher courtship activity in older males than younger males.Citation37 Lin et al.Citation37 further showed that the age-dependent sensitization of Or47b ORNs was induced by juvenile hormone (JH), a key player in the reproductive maturation of insects. It is envisaged that plasticity in the Or47b ORN responsiveness to fatty acids evolved under selective pressure to favor mature males, which are more highly fit than young males. It is noteworthy that elder (4-day-old) males retain a high level of fertility throughout 3 successive copulations, whereas younger (1-day-old) males become practically infertile after 3 successive copulations.Citation39 It is therefore possible that the higher courtship activity in elder males would be profitable for the population because it boosts the number of offspring. Males can increase their fitness by mating with a younger female, and younger females lay more eggs than older females, particularly in the presence of the Sex peptide (SP),Citation40 a seminal fluid component transferred from the male to the female during copulation, as SP promotes egg laying only in young females.Citation41 Because Or47b has been implicated in the discrimination of younger female mates from older ones by males, the higher acuity of Or47b-expressing neurons may confer a mating advantage on older males over younger males.Citation42
How does Fru encode experience?
Given that FruM is required for the fine-tuning of excitability of Or47b ORNs, what is the molecular mechanism of the action of FruM? A body of evidence supports the idea that FruM regulates transcription. First, FruM has a BTB domain and zinc finger motifs, both of which are hallmarks of a class of transcription factors.Citation43,44 Second, FruM binds to over 100 sites on polytene chromosomes and undergoes immunoprecipitation with chromatin factors including bonus (TIF1s), histone deacetylase 1 (HDAC1) and heterochromatin protein 1a.Citation45 Third, a FruM fragment fused to DNA-methylase (DamID) labels specific segments of enhancers on the genome.Citation46 Fourth, a large proportion of putative FruM targets recovered by ChIP-seq in cultured cells coincided with the genes marked by the above-mentioned Fru-DamID experiment.Citation47 Fifth, the combination of gel-mobility shift assay, reporter assay, and CRISPR-Cas9-mediated targeted mutagenesis in flies demonstrated that FruM (more specifically, the FruBM isoform) binds to the robo1 promoter and represses its transcription.Citation48
What could be the possible link between the presumed FruM function for activity tuning of neurons in the adult stage and the FruM function in constructing sex-specific circuitries during development? A clue to this link came from the finding that fru-expressing neurons in a male fly exhibited a remarkable elevation in Ca2+-responsive transcription factor activity when the male fly was grouped with 10 females in the same chamber, compared with a male separated from females by a nylon mesh in the chamber so that he was exposed to female odors but had no direct contact with females.Citation49 In this experiment, nuclear factor of activated T cells (NFAT), a Ca2+-responsive transcription factor, was used to cumulatively monitor neural-activity-dependent transcription.Citation49 These findings point to the possibility that FruM might modulate the transcription of activity-dependent genes that affect the neural excitability, synaptic efficacy and/or JH susceptibility, when its expression is modulated in an experience-dependent manner as observed in Or47b ORNs in the adult.
Notably, a recent study in the mouse cerebellum revealed that the nucleosome remodeling and deacetylase (NuRD) complex, to which HDAC1 contributes, is recruited to the promoter of activity-dependent genes, thereby triggering their inactivation.Citation50 Mouse mutants for the core NuRD subunit (Chd4) exhibited hyperresponsivity of granule neurons to sensorimotor stimuli,Citation50 reminiscent of the hypersensitivity of lpr neurons to visual stimuli and concomitant courtship pursuits in fru-mutant males in Drosophila.Citation9 There also exists a precedent case in which the presence or absence of a sex-specific neuronal type was dependent on the state of sensory experience: the exposure of isolated male mice to the scent of female urine for 2 months or longer eliminated a class of vomeronasal organ neurons responsive to this scent, and re-isolation restored these neurons in male mice.Citation51 These observations in mice and flies invite the interesting hypothesis that fru expressed in young adult flies upon exposure to sib flies might play a role in down-regulating activity-dependent genes via chromatin remodeling to suppress indiscriminative courtship throughout the life of a fly. This hypothesis can be tested by, for example, comparing the PolII occupancy at FruBM target genes between fru mutant males kept in isolation and those in a group, with the aid of the TaDa method.Citation52
Concluding remarks
Some of the effects of early experience on behavior and physiology throughout the lifetime of animals seem to be mediated by changes in the chromatin state. For example, increased pup licking and grooming and arched-back nursing by rat mothers alter the offspring epigenome at a glucocorticoid receptor gene promoter in the hippocampus.Citation53 The pups may thereby be reprogrammed so that their stress sensitivity becomes more appropriate for the anticipated social conditions they will encounter later in life.Citation54 In light of this phenomenon, what are the possible “benefits” for males to change their courtship activities upon group housing? It might be that crowded conditions drive the male to invest more effort in courtship in order to compete with rival males for a potential mate. Such an outcome would be reminiscent of the finding that males deliver more sperm to a female under conditions where sperm competition is stronger.Citation55,56 It is envisaged that, under such conditions, the courtship-promoting sensory pathways that lack the ability to discriminate the target sex, such as the Or47b-mediated courtship-promoting sensory pathway that is activated by ligands shared by 2 sexes, need to be downregulated to prevent fruitless courtship toward males, and FruM is likely required for this type of gain adjustment of neural excitability. We infer that FruM plays a similar role in the CNS, particularly in the courtship-triggering center P1 neurons, as expected from the hypersensitivity of lpr neurons to visual stimuli in group-reared frusat males, yet this possibility remains to be further explored experimentally. Thus FruM provides a blue print for the gendered neural circuitry during development (nature) and tunes the circuitry for better adaptation to varying social conditions after adult emergence (nurture).
Figure 1. fru-dependent behavioral and physiological plasticities. (A) Development of a behavioral phenotype via social experience in fru mutants. (B and C) When kept in a group, frusat males exhibit courtship chaining under freely moving conditions (B), or courtship-like pursuit under tethered conditions in response to a moving visual target displayed on a computer screen (C). The inset in (C) shows the visual pattern used as a target. Arrows indicate the wing extended to vibrate for song generation. (D) Quantification of courtship following activities directed toward an artificial visual target in wild-type and frusat males. (E) Ca2+ activities recorded from dsx-expressing neurons in the lateral protocerebrum during the presentation of an artificial visual target. The background shading indicates the period during which the target was displayed on the screen. “G” and “S” in (D) and (E) denote that males were reared in a group of 10 individuals and in isolation for 6–9 d after eclosion, respectively. Panels (D) and (E) were reproduced, with permission, from ref.Citation9.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Gill K. A mutation causing abnormal courtship and mating behavior in males of Drosophila melanogaster. Am Zool 1963; 3:507
- Villella A, Gailey DA, Berwald B, Ohsima S, Barnes PT, Hall JC. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics 1997; 147:1107-30; PMID:9383056
- Lee G, Hall JC. Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. J Neurosci 2001; 21:513-26; PMID:11160431
- Pan Y, Baker BS. Genetic identification and separation of innate and experience-dependent courtship behaviors in Drosophila. Cell 2014; 156:236-48; PMID:24439379; http://dx.doi.org/10.1016/j.cell.2013.11.041
- Baker BS, Taylor BJ, Hall JC. Are complex behaviors specified by dedicated regulatory genes? Reasoning from Drosophila. Cell 2001; 105:13-24; PMID:11300999; http://dx.doi.org/10.1016/S0092-8674(01)00293-8
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science 2008; 322:904-9; PMID:18988843; http://dx.doi.org/10.1126/science.1159276
- Pavlou HJ, Goodwin SF. Courtship behavior in Drosophila melanogaster: towards a ‘courtship connectome’. Curr Opin Neurobiol 2012; 23:1-8; PMID:23265962; http://dx.doi.org/10.1016/j.conb.2012.09.002
- Yamamoto D, Koganezawa M. Genes and circuits of courtship behavior in Drosophila males. Nat Rev Neurosci 2013; 14:681-92; PMID:24052176; http://dx.doi.org/10.1038/nrn3567
- Kohatsu S, Yamamoto D. Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nat Com 2015; 6:6457; http://dx.doi.org/10.1038/ncomms7457
- Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods; 6:297-303; PMID:19270697; http://dx.doi.org/10.1038/nmeth.1310
- Kimura K-I, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and Doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 2008; 59:759-69; PMID:18786359; http://dx.doi.org/10.1016/j.neuron.2008.06.007
- Koganezawa M, Haba D, Matuo T, Yamamoto D. The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr Biol 2010; 20:1-8; PMID:20036540; http://dx.doi.org/10.1016/j.cub.2009.11.038
- Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 2011; 69:498-508; PMID:21315260; http://dx.doi.org/10.1016/j.neuron.2010.12.017
- Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson D. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods 2014; 11:325-32; PMID:24363022; http://dx.doi.org/10.1038/nmeth.2765
- Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expressions patterns of the fruitless gene in the Drosophila central nervous system. J Neurobiol. 2000; 43:404-26; PMID:10861565; http://dx.doi.org/10.1002/1097-4695(20000615)43:4%3c404::AID-NEU8%3e3.0.CO;2-D
- Usui-Aoki K, Ito H, Ui-Tei K, Takahashi K, Lukacsovich T, Awano W, Nakata H, Piao ZF, Nilsson EE, Tomida J, Yamamoto D. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat Cell Biol 2000; 2:500-6; PMID:10934470; http://dx.doi.org/10.1038/35019537
- Hueston CE, Olsen D, Li Q, Okuwa S, Peng B, Wu J, Volkan PC. Chromatin modulatory proteins and olfactory receptor signaling in the refinement and maintenance of fruitless expression in olfactory receptor neurons. PLoS Biol 2016; 14:e1002443; PMID:27093619; http://dx.doi.org/10.1371/journal.pbio.1002443
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol 2005; 15:1535-47; PMID:16139208; http://dx.doi.org/10.1016/j.cub.2005.07.034
- Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GSXE, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 2011; 478:236-40; PMID:21964331; http://dx.doi.org/10.1038/nature10428
- Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell 2005; 121:795-807; PMID:15935765; http://dx.doi.org/10.1016/j.cell.2005.04.026
- Kondoh Y, Kaneshiro KY, Kimura K-I, Yamamoto D. Evolution of sexual dimorphism in the olfactory brain of Hawaiian Drosophila. Proc Biol Sci 2003; 270:1005-13; PMID:12803889; http://dx.doi.org/10.1098/rspb.2003.2331
- Dweck HKM, Ebrahim SAM, Thoma M, Mohamed AAM, Keesey IW, Trona F, Lavista-Llanos S, SvatoŠ A, Sachse S, Knaden M, Hansson BS. Pheromones mediating copulation and attraction in Drosophila. Proc Natl Acad Sci U S A 2015; 112:21:E2829-35; PMID:25964351; http://dx.doi.org/10.1073/pnas.1504527112
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 2007; 446:542-6; PMID:17392786; http://dx.doi.org/10.1038/nature05672
- Butterworth FM. Lipids of Drosophila: a newly detected lipid in the male. Science 1969; 163:1356-7; PMID:5765118; http://dx.doi.org/10.1126/science.163.3873.1356
- Jallon JM. A few chemical words exchanged by Drosophila during courtship and mating. Behav Genet 1984; 14:441-78; PMID:6441563; http://dx.doi.org/10.1007/BF01065444
- Kayser MS, Yue Z, Sehgal A. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science 2014; 344:269-74; PMID:24744368; http://dx.doi.org/10.1126/science.1250553
- Devaud JM, Acebes A, Ferrus A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci 2001; 21:6274-82; PMID:11487650
- van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol; 2007; 17:606-12; PMID:17363256; http://dx.doi.org/10.1016/j.cub.2007.02.043
- de Renobales M, Blomquist GJ. Biosynthesis of medium chain fatty acids in Drosophila melanogaster. Arch Biochem Biophys 1984; 228:407-14; PMID:6421238; http://dx.doi.org/10.1016/0003-9861(84)90004-3
- Bonsquet F, Nojima T, Houot B, Chauvel I, Chaudy S, Dupas S, Yamamoto D, Ferveur J-F. Expression of desat1 in neural and nonneural tissues separately affects perception and emission of sex pheromones in Drosophila. Proc Natl Acad Sci U S A 2012; 109:249-54; PMID:22114190; http://dx.doi.org/10.1073/pnas.1109166108
- Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 2009; 461:987-91; PMID:19829381; http://dx.doi.org/10.1038/nature08495
- Agrawal S, Safarik S, Dickinson M. The relative roles of vision and chemosensation in mate recognition of Drosophila melanogaster. J Exp Biol 2014; 217:2796-805; PMID:24902744; http://dx.doi.org/10.1242/jeb.105817
- Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A 2012; 109:10065-70; PMID:22645338; http://dx.doi.org/10.1073/pnas.1207107109
- Bath DE, Stowers JR, Hörmann D, Poehlmann A, Dickson BJ, Straw AD. FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat Methods 2014; 11:756-62; PMID:24859752; http://dx.doi.org/10.1038/nmeth.2973
- Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamaoto T, Amrein H, Levine JD, Anderson JD. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci 2011; 14:757-62; PMID:21516101; http://dx.doi.org/10.1038/nn.2800
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 2015; 87:1036-49; PMID:26279475; http://dx.doi.org/10.1016/j.neuron.2015.07.025
- Lin HH, Cao DS, Sethi S, Zeng Z, Chin JSR, Chakraborty TS, Shepherd AK, Nguyen CA, Yew JY, Su C-Y, Wang JW. Hormonal modulation of pheromone detection enhances male courtship success. Neuron 2016; 90:1272-85; PMID:27263969; http://dx.doi.org/10.1016/j.neuron.2016.05.004
- Saleem S, Ruggles PH, Abbott WK, Carney GE. Sexual experience enhances Drosophila melanogaster male mating behavior and success. PLoS One 2014; 9:e96639; PMID:24805129; http://dx.doi.org/10.1371/journal.pone.0096639
- Baxter CM, Barnett R, Dukas R. Effects of age and experience on male mate choosiness. Ethology 2014; 121:353-63; http://dx.doi.org/10.1111/eth.12344
- Aigaki T, Fleischmann I, Chen PS, Kubli E. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron 1991; 7:557-63; PMID:1931051; http://dx.doi.org/10.1016/0896-6273(91)90368-A
- Fricke C, Green D, Mills WE, Chapman T. Age-dependent female responses to a male ejaculate signal after demographic opportunities for selection. Proc Biol Sci 2013; 280:20130428; PMID:23843383; http://dx.doi.org/10.1098/rspb.2013.0428
- Zhuang L, Sun Y, Hu M, Wu C, La X, Chen X, Feng Y, Wang X, Hu Y, Xue L. Or47b plays a role in Drosophila males preference for younger mates. Open Biol 2016; 6:160086; PMID:27278650; http://dx.doi.org/10.1098/rsob.160086
- Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci U S A. 1996; 93:9687-92; PMID:8790392; http://dx.doi.org/10.1073/pnas.93.18.9687
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 1996; 87:1079-89; PMID:8978612; http://dx.doi.org/10.1016/S0092-8674(00)81802-4
- Ito H, Sato K, Koganezawa M, Ote M, Matsumoto K, Hama C, Yamamoto D. Fruitless recruits two antagonistic chromatin factors to establish single-neuron sexual dimorphism. Cell 2012; 149:1327-38; PMID:22682252; http://dx.doi.org/10.1016/j.cell.2012.04.025
- Neville MC, Nojima T, Ashley E, Parker DJ, Walker J, Southhall T, Van de Sande B, Marques AC, Fischer B, Brand AH, et al. Male-specific Fruitless isoforms target neurodevelopmental genes to specify a sexually dimorphic nervous system. Curr Biol 2014; 24:229-41; PMID:24440396; http://dx.doi.org/10.1016/j.cub.2013.11.035
- Vernes SC. Genome wide identification of fruitless targets suggests a role in upregulating genes important for neural circuit formation. Sci Rep 2014; 4:4412; PMID:24642956; http://dx.doi.org/10.1038/srep04412
- Ito H, Sato K, Kondo S, Ueda R, Yamamoto D. Fruitless represses robo1 transcription to shape male-specific neural morphology and behavior in Drosophila. Curr Biol 2016; 26:1532-42; PMID:27265393; http://dx.doi.org/10.1016/j.cub.2016.04.067
- Masuyama K, Zhang Y, Rao Y, Wang JW. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J Neurogenet 2012; 26:89-102; PMID:22236090; http://dx.doi.org/10.3109/01677063.2011.642910
- Yang Y, Hill KK, Hemberg M, Reddy NC, Cho HY, Guthrie AN, Oldenborg A, Heiney SA, Ohmae S, Medina JF, et al. Chromatin remodeling inactivates activity genes and regulates neural coding. Science 2016; 353:300-5; PMID:27418512; http://dx.doi.org/10.1126/science.aad4225
- Xu PS, Lee D, Holy TE. Experience-dependent plasticity drives individual differences in pheromone-sensing neurons. Neuron 2016; 91:878-92; PMID:27537487; http://dx.doi.org/10.1016/j.neuron.2016.07.034
- Southall TD, Gold KS, Egger B, Davidson CM, Caygill EE, Marshall OJ, Brand AH. Cell-type-specific profiling of gene expression and chromatin binding without cell isolation: assaying RNA Pol II occupancy in neural stem cells. Dev Cell 2013; 26:101-12; PMID:23792147; http://dx.doi.org/10.1016/j.devcel.2013.05.020
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Secckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci 2004; 7:847-54; PMID:15220929; http://dx.doi.org/10.1038/nn1276
- Meaney M. Environmental programming of phenotypic diversity in female reproductive strategies. Adv Genet 2007; 59:173-215; PMID:17888799; http://dx.doi.org/10.1016/S0065-2660(07)59007-3
- Lüpold S, Manier MK, Ala-Honkola O, Belote JM, Pitnick S. Male Drosophila melanogaster adjust ejaculate size based on female mating stats, fecundity, and age. Behav Ecol 2011; 22:184-91; http://dx.doi.org/10.1093/beheco/arq193
- Parker GA, Pizzari T. Sperm competition and ejaculate economics. Biol Rev Camb Philos Soc 2010; 85:897-934; PMID:20560928; http://dx.doi.org/10.1111/j.1469-185X.2010.00140.x