ABSTRACT
The spinal cord contains specialized groups of cells called pattern generators, which are capable of orchestrating rhythmic firing activity in an isolated preparation. Different patterns of activity could be generated in vitro including right-left alternating bursting and bursting in which both sides are synchronized. The cellular and network mechanisms that enable these behaviors are not fully understood. We have recently shown that Ca2+-activated K+ channels (SK channels) control the initiation and amplitude of synchronized bursting in the spinal cord. It is unclear, however, whether SK channels play a similar role in the alternating rhythmic pattern. In the current study, we used a spinal cord preparation from functionally mature mice capable of weight bearing and walking. The present results extend our previous work and show that SK channel inhibition initiates and modulates the amplitude of alternating bursting. We also show that addition of methoxamine, an α1-adrenergic agonist, to a cocktail of serotonin, dopamine, and NMDA evokes robust and consistent alternating bursting throughout the cord.
Introduction
Neuronal bursting is a behavior in which the neuron alternates between periods of repetitive firing and periods of inactivity.Citation1 In the spinal cord, there are specialized burster interneurons, which can be activated in vitro via application of different combinations of neuromodulators.Citation2 These interneurons can then drive the firing of multiple motoneuron pools in a rhythmic, spatially organized manner to produce behavior-relevant bursting activity. Depending on its pattern and location, this rhythmic activity could underlie various motor behaviors such as locomotion, breathing, and chewing.Citation2,3 Even though rhythmic bursting in the spinal cord has been studied extensively, the ionic mechanisms underlying its initiation and modulation remain unclear. Recently, we have shown that the small conductance Ca2+-activated potassium channel (SK channel) controls the initiation and sets the amplitude of synchronized bursting in the adult mammalian spinal cord.Citation4
The gating of SK channels is controlled by intracellular Ca2+ levels ([Ca2+]i).Citation5 The channel subunits interact constitutively with calmodulin, which causes a conformational change and pore opening when [Ca2+]i is increased.Citation6 Potassium (K+) efflux through the channel hyperpolarizes the membrane and regulates the activation of voltage-gated ion channels. Thus, when coupled with voltage-dependent Ca2+-permeable channels, neuronal SK channels form a negative feedback loop that regulates membrane potential and excitability.
In spinal motoneurons, SK channels are expressed in multiple locations and coupled with various Ca2+ sources to perform many functions. On the motoneuron soma, SK channels form large clusters at the cholinergic C-bouton synapses,Citation7,8 and are co-localized with N-type Ca2+channels.Citation9,10 The N-type Ca2+ channels are activated during the action potential spike causing a rise in [Ca2+]i and activation of the neighboring SK channels. This generates a long post-spike hyperpolarization period known as the medium afterhyperpolarization (mAHP),Citation11 which is a major determinant of motoneuron firing rate.Citation12 On the dendrites, however, SK channels are activated by persistent L-type Ca2+channels and by NMDA receptors; their activation causes reduction in the amplitudes of the persistent inward Ca2+ current and excitatory postsynaptic potentials (EPSPs),Citation10,13,14 respectively. Accordingly, their position on the motoneuron dendrites and soma, where the cell's inputs and output arrive/emerge, allows SK channels to modulate synaptic inputs and firing output, thereby giving them substantial control over the input-output relationship of motoneurons.
We have recently shown that SK channel inhibition is required for the initiation of synchronized bursting (in which bursts occur on both sides of the cord simultaneously).Citation4 We have also shown that graded SK inhibition results in a proportional increase of burst amplitude. It is unknown, however, whether this role of SK channels is restricted to synchronized bursting or is a ubiquitous mechanism involved in other bursting patterns, such as alternating bursting (also known as fictive locomotion). While synchronized bursting drives motor behaviors like hopping and jumping, alternating bursting underlies other behaviors, such as walking and running. It has been shown that networks generating the two patterns interact and influence each other, but originate from different generators.Citation15 Therefore, the main goal of the present study is to examine the role of SK channels in regulating the other form of rhythmic bursting in the spinal cord, fictive locomotion.
Results and discussion
Induction of stable locomotive alternating activity in the spinal cord of adult mammalian animals in vitro has been shown to be challenging (for review, see Citationref. 15). The oldest age in mice at which alternating rhythmic activity has been induced in vitro in an intact lumbosacral spinal cord preparation is P10-P12.Citation16 At this age, the locomotor neural circuit has developed sufficiently to allow mice to bear weight and walk. Therefore, we used P11 mice or older in these experiments. The firing activity of the ventral roots was recorded at the lower lumbar (L5–L6) and upper sacral segments (S1–S2). Locomotor bursts were pharmacologically induced by administering neuromodulators to the recording solution.
Episodic alternating bursts induced in the functionally-mature murine cord
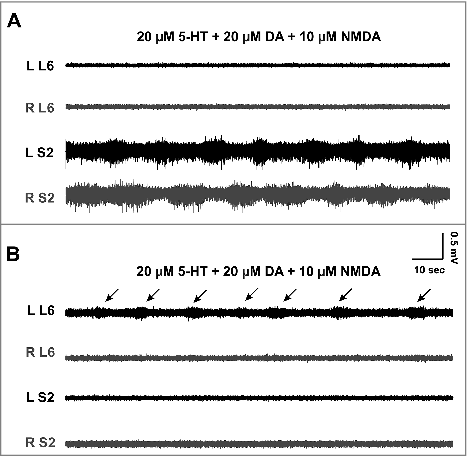
In normal artificial cerebrospinal fluid (nACSF), the ventral roots are usually quiet except for random, low-amplitude spiking activity of no consistent pattern. As reported before,Citation3,16 the addition of a combination of serotonin (5-HT), dopamine (DA), and N-methyl-D-aspartate (NMDA) produces right-left alternating activity (). Our recordings at that age showed that locomotor-like activity was mostly restricted to one segment, was usually unstable, and had low amplitude (0.15 ± 0.08 mV, n = 4) and long burst cycles (24.2 ± 6 sec, n = 4). In addition, the same combination of neuromodulators sometimes failed to produce alternating activity, especially as mice became older ().
Figure 1. Induction of alternating bursting activity in the spinal cord of functionally-mature mouse. A: The activity of the ventral roots at the lower lumbar (L6) and mid-sacral (S2) segments on the left (L) and right (R) sides of a spinal cord obtained from a mouse at P11. The application of serotonin (5-HT), dopamine (DA), and NMDA induces low-amplitude, left-right alternating, rhythmic bursting at the sacral, but not the lumbar, segments. B: Recordings from a P12 mouse showing the effect of the same concentrations of serotonin, dopamine, and NMDA. Only the left side ventral root at L6 is showing low amplitude bursts (arrows), while the right side and sacral roots are silent.
It has been shown that methoxamine, an α1-adrenergic receptor agonist, induces alternating activity in the isolated sacral cord of neonatal rats.Citation17 We thus tested whether methoxamine can stabilize the 5-HT/DA/NMDA-induced rhythm in our preparation. The addition of 100 µM methoxamine to the locomotion cocktail drastically changed the overall activity (, n = 6). At a relatively large time scale, the combination seemed to produce rhythmic synchronous activity across multiple segments of the cord (, left and middle panels). The cycle time of this synchronous activity was 33.7 ± 18.37 sec (n = 6) and the duration of each episode was 7.68 ± 3.05 sec (n = 6). However, on a smaller time scale, this synchronized activity is composed of short bursts that alternate on the left and right sides of the cord (, right panel), with an almost eighty-times-shorter burst cycle (0.31 ± 0.02 sec, n = 6) and a burst duration of 0.16 ± 0.02 sec (n = 6). The cycle and duration of the fast alternating activity was consistent across different experiments (compare the right panel in to ). shows the changes in burst parameters after methoxamine administration expressed as percentage of control (i.e., values before methoxamine administration).
Figure 2. Effect of methoxamine on alternating activity in the lumbosacral cord. A: Left: The addition of the α1-adrenergic agonist (methoxamine) to the 5-HT/DA/NMDA cocktail, in the same experiment as , produces a seemingly synchronized high-amplitude activity in all ventral roots. Middle: Each one of these episodes of synchronous activity is a cluster of short bursts. Right: This clustered activity is composed of short alternating bursts between the left and right sides; with the lumbar bursts in phase with sacral bursts on the same side of the cord (notice the different time scales). B: An episode of fast, alternating activity produced by the same chemical cocktail in a spinal cord of a P15 mouse. Note that burst duration and cycle are similar to the experiment in (A). C: The burst parameters after methoxamine administration, plotted as a percentage of the value before adding the drug. The bars represent the mean ± standard deviation. * Statistically significant, p value < 0.05.
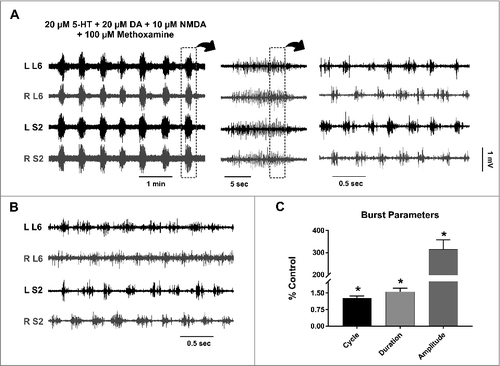
Interestingly, the frequency and duration of the episodic synchronous activity (, left panel) are similar to those of disinhibited bursting in the sacral cord of fully-mature adult mice shown in Mahrous and Elbasiouny (2017).Citation4 This suggests that the two rhythmic patterns might share a significant amount of neuronal circuitry.
Effect of SK modulators on alternating bursting activity
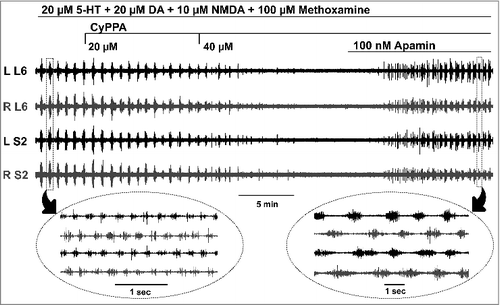
Serotonin, dopamine, and methoxamine have been reported to inhibit SK channels.Citation18–20 To test whether SK channels regulate fictive locomotor activity in the spinal cord, SK modulators were administered to the recording solution after stable bursting had been established. shows episodic alternating bursting activity in the lumbosacral cord induced by 5-HT, DA, NMDA, and methoxamine. The application of CyPPA, an SK channel activator,Citation21 at 20 µM reduced the burst amplitude. When the concentration of CyPPA was increased to 40 µM, the bursting was completely inhibited, indicating that SK inhibition is needed to induce bursting (n = 3). Similar results were obtained using another SK channel activator SKA-19,Citation22 (n = 3). To further confirm, a specific SK channel inhibitor, apamin, was further added to the recording solution, which restored the rhythmic activity (, n = 6). The locomotor bursts generated after adding apamin had longer duration (compare the insets in ), consistent with the role of SK channels in terminating the burst.Citation23
Figure 3. Locomotor bursts are inhibited by SK activators and restored by SK blockers. The application of the SK activator (CyPPA) inhibits the episodic alternating bursting activity in a dose-dependent manner. After the bursting has completely ceased, the addition of the SK blocker, apamin, restores the rhythmic alternating activity. The insets show, on a different time scale, representative alternating bursts from two different episodes of activity.
Isolated spinal cord preparations have been long used to study alternating bursting using combinations of 5-HT/DA/NMDA,Citation3,16,24 and synchronized bursting using disinhibition.Citation25,26 Interestingly, our data show that adding methoxamine to a cocktail that normally induces alternating bursts, resulted in slow synchronous activity that resembled disinhibited bursting.Citation4 However, a fast alternating bursting was established within these synchronous episodes (), which we referred to as episodic alternating pattern. The new drug combination containing methoxamine induced the episodic alternating pattern reliably in spinal cord preparations from functionally mature mice (up to postnatal day 15).
The results are also consistent with our recently published data, which showed that SK channels regulate another form of bursting in the spinal cord.Citation4 This suggests that SK channels act as a global mechanism for regulation of bursting activity in the spinal cord. Therefore, SK channels could be an important pharmacological target to modulate the motor output of the spinal cord. This could ultimately have therapeutic implications for the treatment of neurological disorders in which motor function is impaired.
Methods
Animals
Data in the current study were obtained from 8 Mice (5 male and 3 female B6SJL mice, Jackson Laboratory, Bar Harbor, ME), 11–15 days old (mean age = 11.88 ± 1.55, n = 8). All surgical and experimental procedures were reviewed and approved by the Wright State University Animal Care and Use Committee.
In vitro spinal cord preparation
For isolation of the spinal cord, the animal was deeply anesthetized with intraperitoneal injection of Euthasol (1 mg/Kg). Supplemental amounts of anesthetic were given as needed until the animal did not respond to toe and tail pinching. The mouse was then decapitated and the skin of the back was opened. The spinal column was quickly cut and transferred to a dissection dish full of modified cerebrospinal fluid (mACSF, see below) at room temperature. The spinal cord was isolated by laminectomy and longitudinal incision of the dura mater. The cord was transected at the upper lumbar segments (around L1), and all roots were cut close to the side of the cord except for the ventral roots at L5 to S2, which were kept for recording. The lumbosacral cord was then transferred to a recording chamber and continuously perfused with normal artificial cerebrospinal fluid (nACSF, see below) at a rate of 2.5–3 ml/min. The L5-S2 ventral roots were mounted on bipolar wire electrodes.
Solutions and drugs
The mACSF was composed of the following (in mM): 118 NaCl, 3 KCl, 1.3 MgSO4, 5 MgCl2, 1.4 NaH2PO4, 1.5 CaCl2, 24 NaHCO3, and 25 glucose, and aerated continuously with carbogen (95% O2/5% CO2). The osmolarity of the solution was ∼ 310 mOsm. The nACSF was composed of the following (in mM): 128 NaCl, 3 KCl, 1.5 MgSO4, 1 NaH2PO4, 2.5 CaCl2, 22 NaHCO3, and 12 glucose. The osmolarity of the solution was ∼ 295 mOsm. The pH of both mACSF and nACSF was 7.35 – 7.4 when aerated with carbogen. All of the components of the physiological solutions were purchased from Thermo Fisher Scientific (Waltham, MA), and all of the drugs were purchased from Sigma (St. Louis, MO)
Electrophysiology
Extracellular recordings were obtained from the ventral roots using a multi-channel differential amplifier (Kinetic Software, GA). The recordings were digitized using a Power1401-3 data acquisition interface (Cambridge Electronic Design, Cambridge, UK) at 20 kHz, and acquired by Spike2 software. The data were high-pass filtered at 300 Hz and low-pass filtered at 3 kHz.
Alternating rhythmicity was defined as right-left alternating temporary increase in ventral root activity at a particular segment. Burst amplitude was measured as the peak amplitude of the rectified trace. Burst cycle was defined as the time period from the start of one burst until the start of the next one. Burst duration was measured as the time during which the root activity remained increased above the baseline. Burst parameters were calculated as the average from at least 10 bursts, and then a grand mean was calculated for multiple experiments.
Statistical analysis
Data were analyzed using GraphPad Prism (Version 7.01; GraphPad Software, La Jolla, CA). The non-parametric Mann-Whitney test was used to assess changes in burst parameters. Data are reported as the mean ± standard deviation. P value < 0.05 was considered statistically significant.
Disclosure
The authors declare no competing financial interests.
Acknowledgment
Funding to this work was provided by NINDS-NIH (grant # NS091836) for S.E.; A.M. was supported by the Biomedical Sciences PhD Program at Wright State University.
References
- Izhikevich EM. Neural excitability, spiking and bursting. International Journal of Bifurcation and Chaos. 1999;10:1171–266.
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi:10.1146/annurev.neuro.29.051605.112910. PMID:16776587.
- Guertin PA. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol. 2012;3:183. PMID:23403923.
- Mahrous AA, Elbasiouny SM. SK channel inhibition mediates the initiation and amplitude modulation of synchronized burst firing in the spinal cord. J Neurophysiol. 2017;118:161–175 doi:10.1152/jn.00929.2016. PMID:28356481
- Meech RW. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi:10.1146/annurev.bb.07.060178.000245. PMID:352237.
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–69. doi:10.1146/annurev-physiol-020911-153336. PMID:21942705.
- Wilson JM, Rempel J, Brownstone RM. Postnatal development of cholinergic synapses on mouse spinal motoneurons. J Comp Neurol. 2004;474:13–23. doi:10.1002/cne.20089. PMID:15156576.
- Deardorff AS, Romer SH, Deng Z, Bullinger KL, Nardelli P, Cope TC, Fyffe RE. Expression of postsynaptic Ca2+-activated K+ (SK) channels at C-bouton synapses in mammalian lumbar -motoneurons. J Physiol. 2013;591:875–97. doi:10.1113/jphysiol.2012.240879. PMID:23129791.
- Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–63. PMID:8350136.
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol. 2007;97:3314–30. doi:10.1152/jn.01068.2006. PMID:17360829.
- Ransom BR, Barker JL, Nelson PG. Two mechanisms for poststimulus hyperpolarisations in cultured mammalian neurones. Nature. 1975;256:424–5. doi:10.1038/256424a0. PMID:1170503.
- Eccles JC, Eccles RM, Lundberg A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958;142:275–91. doi:10.1113/jphysiol.1958.sp006015. PMID:13564435.
- Nanou E, Alpert MH, Alford S, El Manira A. Differential regulation of synaptic transmission by pre- and postsynaptic SK channels in the spinal locomotor network. J Neurophysiol. 2013;109:3051–9. doi:10.1152/jn.00067.2013. PMID:23554432.
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–9. doi:10.1038/nn1449. PMID:15852011.
- Beato M, Nistri A. Interaction between disinhibited bursting and fictive locomotor patterns in the rat isolated spinal cord. J Neurophysiol. 1999;82:2029–38. PMID:10561384.
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999;816:493–9. doi:10.1016/S0006-8993(98)01199-8. PMID:9878874.
- Gabbay H, Lev-Tov A. Alpha-1 adrenoceptor agonists generate a “fast” NMDA receptor-independent motor rhythm in the neonatal rat spinal cord. J Neurophysiol. 2004;92:997–1010. doi:10.1152/jn.00205.2004. PMID:15084642.
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci. 2007;27:13192–204. doi:10.1523/JNEUROSCI.1279-07.2007. PMID:18045913.
- Grunnet M, Jespersen T, Perrier JF. 5-HT1A receptors modulate small-conductance Ca2+-activated K+ channels. J Neurosci Res. 2004;78:845–54. doi:10.1002/jnr.20318. PMID:15521063.
- Wagner EJ, Ronnekleiv OK, Kelly MJ. The noradrenergic inhibition of an apamin-sensitive, small-conductance Ca2+-activated K+ channel in hypothalamic gamma-aminobutyric acid neurons: pharmacology, estrogen sensitivity, and relevance to the control of the reproductive axis. J Pharmacol Exp Ther. 2001;299:21–30. PMID:11561059.
- Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, Strøbaek D, Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol. 2007;151:655–65. doi:10.1038/sj.bjp.0707281. PMID:17486140.
- Coleman N, Nguyen HM, Cao Z, Brown BM, Jenkins DP, Zolkowska D, Chen YJ, Tanaka BS, Goldin AL, Rogawski MA, et al. The riluzole derivative 2-amino-6-trifluoromethylthio-benzothiazole (SKA-19), a mixed KCa2 activator and NaV blocker, is a potent novel anticonvulsant. Neurotherapeutics. 2015;12:234–49. doi:10.1007/s13311-014-0305-y. PMID:25256961.
- el Manira A, Tegner J, Grillner S. Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. J Neurophysiol. 1994;72:1852–61. PMID:7823105.
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–33. doi:10.1016/0165-0270(87)90126-9. PMID:2890797.
- Bracci E, Ballerini L, Nistri A. Spontaneous rhythmic bursts induced by pharmacological block of inhibition in lumbar motoneurons of the neonatal rat spinal cord. J Neurophysiol. 1996;75:640–7. PMID:8714641.
- Bracci E, Ballerini L, Nistri A. Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord. J Neurosci. 1996;16:7063–76. PMID:8824342.
