ABSTRACT
Ingestion of leaves of the European yew tree (Taxus baccata) can result in fatal cardiac arrhythmias and acute cardiogenic shock. This cardiotoxicity derives from taxine alkaloids that block cardiac voltage-gated sodium and calcium channels. Prompt initiation of venoarterial extracorporeal membrane oxygenation is essential to bridge these critically ill patients to recovery, as there is no antidote available. We here report a 39-year old patient with toxic cardiogenic shock after yew poisoning, who was successfully rescued by venoarterial extracorporeal membrane oxygenation and had a full neurological recovery. This report emphasizes the role of intoxications as reversible causes of cardiac arrest and adds further evidence to the body of existing literature thus encouraging the early use of venoarterial extracorporeal membrane oxygenation in patients with yew poisoning and cardiogenic shock.
KEYWORDS:
Introduction
The potentially fatal effects of the European yew tree (Taxus baccata) were known since ancient times [Citation1] and derive from taxine alkaloids that inhibit sodium and calcium channels in cardiomyocytes [Citation2]. We report the case of a 39-year old male, who developed an acute cardiogenic shock after ingestion of multiple yew branches and leaves, and barely survived after prompt resuscitation with venoarterial extracorporeal membrane oxygenation (VA-ECMO).
Case report
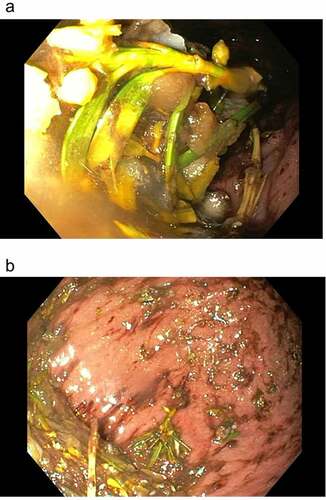
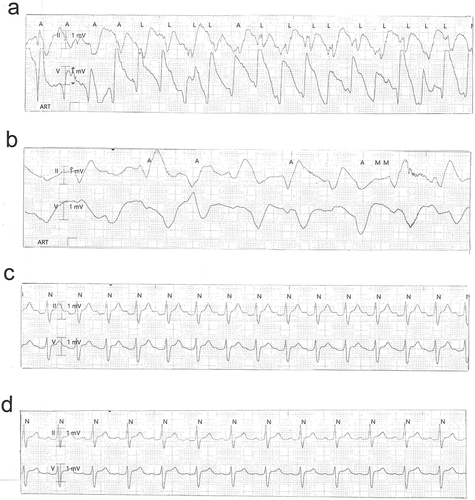
The 39-year old patient presented to our emergency department two hours after having deliberately ingested approximately 30 g of Taxus baccata leaves and branches. He had collapsed in his residential home for psychiatric patients, clarified himself that he had ingested yew in a suicidal attempt, and was brought to our hospital by emergency medical services. His past medical history included schizophrenia and chronic hepatitis C. His premedication consisted of zolpidem, sertraline, olanzapine, clozapine, trazodone, oxazepam, and paliperidon. On arrival in the emergency department, he was somnolent (Glasgow coma scale 8), had a heart rate of 117/min, an oxygen saturation of 95%, and a body temperature of 36.9°C. A faint peripheral pulse was palpable but no blood pressure readings could be obtained. He was immediately transferred to ICU. Five minutes after arrival on the ICU, we started with cardiopulmonary resuscitation because of pulseless electric activity. During cardiopulmonary resuscitation, we administered adrenaline (4 mg), esketamine (250 mg), midazolam (5 mg), rocuronium (100 mg), magnesium (4.8 mmol), sodium bicarbonate 8.4% (100 ml), balanced crystalloid fluid (1500 ml), and noradrenaline (0.70 µg/kg/min). The initial electrocardiogram showed an irregular arrhythmia at a rate of 107/min with bizarre (non-typical bundle branch block) QRS prolongation >600 ms followed by irregular tachy- as well as bradyarrhythmias (). Emergency echocardiography during cardiopulmonary resuscitation showed no pericardial effusion, and no relevant cardiac contractions. Defibrillation was ineffective (). As there is no validated antidote for yew intoxication, we immediately performed extracorporeal life support via VA-ECMO, which was implanted during ongoing resuscitation with an automated chest compression system (LUCAS-2, Jolife AB, Lund Sweden). We used a 23 Fr drainage cannula in the left femoral vein and a 19 Fr return cannula in the right femoral artery with an antegrade perfusion catheter. Cardiopulmonary resuscitation with chest compressions was done for 39 minutes. In this period, we managed to perform tracheal intubation, central venous access, arterial line, emergency echocardiography, (ineffective) defibrillation, and implantation of VA-ECMO as a rescue therapy. Lactic acid levels rose to a maximum of 4.4 mmol/L 35 min after arrival on ICU and rapidly turned normal with start of VA-ECMO. It was not necessary to introduce a left ventricular assist device. The initial laboratory analysis showed a high white blood cell count of 25 G/l, a normal C-reactive protein of 0.8 mg/dl (normal range <5 mg/dl), and slightly elevated aspartate and alanine aminotransferase levels of 145 and 87 U/l, respectively (normal range < 50 U/l). We next conducted a gastroscopy, which revealed plenty of ingested yew leaves and branches (). Gastric juice was removed together with the copious foliage (using endoscopic baskets), and activated charcoal was given via a nasogastric tube to reduce further resorption of toxins. Cardiac electrophysiology slowly recovered within the next hours, as shown in the sequential electrocardiograms (). The initial pH of 7.372 decreased to a nadir of 7.276 after 10 hours and rapidly recovered to normal ranges thereafter. High-sensitivity cardiac troponin T levels reached a maximum of 161 pg/ml on day 2 (normal range <14 pg/ml), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) was slightly elevated to 134 pg/ml (normal range <100 pg/ml). Echocardiography on day 2 showed a left ventricular ejection fraction of 40%, no regional hypokinesis, and no pericardial effusion. Therefore, VA-ECMO support was stopped after 39 hours, and vascular surgeons removed cannulas. We liberated the patient from mechanical ventilation after seven days. He had no neurological deficit, and perfectly recalled what had happened. Neuronal specific enolase was within reference range, indicating that no relevant cerebral ischemia was present throughout treatment. On day 7, echocardiography showed full cardiac recovery with normal diameters of the cardiac chambers, normal left ventricular ejection fraction (60%), and normal right ventricular function (tricuspid annular plane systolic excursion 25 mm). The patient was discharged from ICU to psychiatry after 12 days and was dismissed from hospital after 23 days.
Figure 1. Electrocardiogram after yew poisoning showing an irregular arrhythmia at a rate of 107/min with bizarre QRS prolongation.
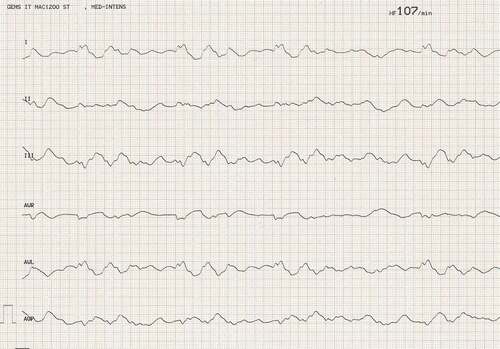
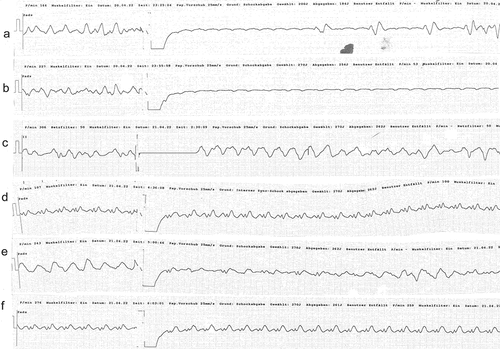
Figure 2. Ineffective defibrillation after ingestion of Taxus baccata (a) Irregular wide complex tachycardia terminating to arterial flutter without ventricular response. Seconds later, broad ventricular complexes appear that quickly degenerate into the irregular wide complex tachycardia. (b) Irregular wide complex tachycardia terminating to arterial flutter without ventricular response. (c-f) Ineffective defibrillation of wide complex tachycardia.
Discussion
Cardiotoxicity of the European yew derives from a mixture of the major alkaloids taxine A and B, which block cardiac voltage-gated sodium and calcium currents [Citation2]. Taxines are contained in most parts of the Taxus baccata including the bark, leaves, and the hard central seed, whereas the surrounding fleshy red pulp, the aril, does not contain any toxins. The lethal oral dose of yew leaves was estimated to be in the range of 0.6 to 1.3 g yew leaves/kg body weight [Citation2]. Clinical manifestations of yew intoxication often begin with abdominal discomfort, nausea, dizziness, and vomiting starting within a few hours after exposure [Citation2]. The major clinical hazard of taxine A and B is that they can induce cardiac arrhythmias ranging from atrioventricular conduction blocks to refractory ventricular fibrillation [Citation3–10]. The clinical severity depends on quantity, type as well as route of exposure. Taxine A and B are volatile and difficult to detect. Therefore, paclitaxel can be used as a specific biomarker for yew poisoning in primary high performance liquid chromatography with mass spectrometry (HPLC-MS) screening, when patient history and gastroscopy are inconclusive [Citation11].
A literature search on PubMed using the mesh-terms ECMO AND (taxine OR yew OR taxus) revealed a handful of other case reports published involving patients which were resuscitated using VA-ECMO after intentional ingestion of yew leaves and branches leading to cardiogenic shock [Citation3–10]. Additional therapeutic approaches included albumin dialysis, digoxin immune Fab, and lipid-emulsion therapy [Citation8,Citation10,Citation12,Citation13]. However, these approaches are not feasible in patients undergoing cardiopulmonary resuscitation.
Thus, the present report strengthens the notion that prompt VA-ECMO can provide a bridging to full recovery for patients with yew poisoning and cardiogenic shock [Citation3–10], and emphasizes the role of intoxications as reversible causes of cardiac arrest. The impressive ineffectiveness of defibrillation and/or overdrive pacing against taxine-derived arrhythmias is in line with prior publications [Citation4,Citation14,Citation15]. The outcome of patients with acute cardiogenic shock due to yew poisoning treated with VA-ECMO appears to be excellent and the present case adds to the body of existing literature encouraging the early use of VA-ECMO in these cases. Prompt primary toxin-elimination using endoscopic tools and activated charcoal reduce toxin resorption and thus may shorten the necessary time of extracorporeal cardiopulmonary support in critically ill patients.
Ethics approval and informed consent
The Institutional Review Board of the Medical University of Graz approved this clinical study (EK-Nr.: 34-342 ex 21/22). Written informed consent to participate and written informed consent to publish was obtained from the patient. He approved the use of clinical data, images and other clinically related material for scientific purposes and publication.
Authors’ contributions
NS and PE wrote the manuscript. MM, SP, CH, GH, ACR and PE were directly involved in clinical management of the patient. MM, SP, GH, ACR, SH and CH critically revised the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects related to accuracy and integrity of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, [PE], upon reasonable request. The data are not publicly available due to privacy restrictions.
Additional information
Funding
References
- Gaius Julius C. De bello Gallico VI 31, 5:“Catuvolcus rex dimidiae partis Eburonum, qui una cum Ambiorige consilium inierat, aetate iam confectus, cum laborem aut belli aut fugae ferre non posset, omnibus precibus detestatus Ambiorigem, qui eius consilii auctor fuisset, taxo, cuius magna in Gallia Germaniaque copia est, se exanimavit.”
- Wilson CR, Sauer J, Hooser SB. Taxines: a review of the mechanism and toxicity of yew (Taxus spp.) alkaloids. Toxicon. 2001 Feb-Mar;39(2–3):175–185.
- Ward C, Meeks D, Trimlett R, et al. Taxine alkaloid poisoning successfully supported with venoarterial extracorporeal membrane oxygenation: a case report. Eur Heart J Case Rep. 2022 Feb 11;6(2):ytac039.
- Hermes-Laufer J, Meyer M, Rudiger A, et al. Extracorporeal life support as bridge to recovery in yew poisoning: case reports and literature review. ESC Heart Fail. 2021 Feb;8(1):705–709.
- Baum C, Bohnen S, Sill B, et al. Prolonged resuscitation and cardiogenic shock after intoxication with European yew (Taxus baccata): complete recovery after intermittent mechanical circulatory support. Int J Cardiol. 2015 Feb 15;181:176–178.
- Panzeri C, Bacis G, Ferri F, et al. Extracorporeal life support in a severe Taxus baccata poisoning. Clin Toxicol (Phila). 2010 Jun;48(5):463–465.
- Vardon Bounes F, Tardif E, Ruiz S, et al. Suicide attempt with self-made Taxus baccata leaf capsules: survival following the application of extracorporeal membrane oxygenation for ventricular arrythmia and refractory cardiogenic shock. Clin Toxicol (Phila). 2017 Sep;55(8):925–928. Epub 2017 May 11. PMID: 28494178.
- Farag M, Badowski D, Koschny R, et al. Extracorporeal life support and digoxin-specific Fab fragments for successful management of Taxus baccata intoxication with low output and ventricular arrhythmia. Am J Emerg Med. 2017 Dec;35(12):1987.e3–1987.e7. Epub 2017 Sep 18. PMID: 28941873.
- Thooft A, Goubella A, Fagnoul D, et al. Combination of veno-arterial extracorporeal membrane oxygenation and hypothermia for out-of-hospital cardiac arrest due to Taxus intoxication. Cjem. 2014 Nov;16(6):504–507. PMID: 25358285.
- Ajouri J, Muellenbach RM, Rolfes CB, et al. Kardiogener Schock nach Eibennadelintoxikation: digitalisantidot, va-ECMO und Albumindialyse in der Therapie der suizidalen Eibennadelintoxikation [Cardiogenic shock following yew needle poisoning: digoxin immune fab, va-ECMO and albumin dialysis for the treatment of a suicidal yew leaf poisoning]. Anaesthesist. 2022 Mar;71(3): 210–213. German. Epub 2021 Oct 4. PMID: 34608518.
- Reisinger A, Rabensteiner J, Hackl G. Diagnosis of acute intoxications in critically ill patients: focus on biomarkers – part 2: markers for specific intoxications. Biomarkers. 2020 Mar;25(2):112–125.
- Hayes BD, Gosselin S, Calello DP, et al. Lipid emulsion workgroup. systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clin Toxicol (Phila). 2016 Jun;54(5):365–404. Epub 2016 Apr 1. PMID: 27035513.
- Rutkiewicz A, Schab P, Kubicius A, et al. Yew poisoning - pathophysiology, clinical picture, management and perspective of fat emulsion utilization. Anaesthesiol Intensive Ther. 2019;51(5):404–408. PMID: 31769262.
- Chan M GN, Gue YX, Gorog DA. Fatal heart block from intentional yew tree (Taxus baccata) ingestion: a case report. Eur Heart J Case Rep. 2019 Dec 23;4(1):1–4.
- Yersin B, Frey JG, Schaller MD, et al. Fatal cardiac arrhythmias and shock following yew leaves ingestion. Ann Emerg Med. 1987 Dec;16(12):1396–1397. PMID: 3688609.