Abstract
Recombinant single domain antibodies (nanobodies) constitute an attractive alternative for the production of neutralizing therapeutic agents. Their small size warrants rapid bioavailability and fast penetration to sites of toxin uptake, but also rapid renal clearance, which negatively affects their performance. In this work, we present a new strategy to drastically improve the neutralizing potency of single domain antibodies based on their fusion to a second nanobody specific for the complement receptor CD11b/CD18 (Mac-1). These bispecific antibodies retain a small size (˜30 kDa), but acquire effector functions that promote the elimination of the toxin-immunocomplexes. The principle was demonstrated in a mouse model of lethal toxicity with tetanus toxin. Three anti-tetanus toxin nanobodies were selected and characterized in terms of overlapping epitopes and inhibition of toxin binding to neuron gangliosides. Bispecific constructs of the most promising monodomain antibodies were built using anti Mac-1, CD45 and MHC II nanobodies. When co-administered with the toxin, all bispecific antibodies showed higher toxin-neutralizing capacity than the monomeric ones, but only their fusion to the anti-endocytic receptor Mac-1 nanobody allowed the mice to survive a 10-fold lethal dose. In a model of delayed neutralization of the toxin, the anti- Mac-1 bispecific antibodies outperformed a sheep anti-toxin polyclonal IgG that had shown similar neutralization potency in the co-administration experiments. This strategy should have widespread application in the development of nanobody-based neutralizing therapeutics, which can be produced economically and more safely than conventional antisera.
Abbreviations
| ANOVA | = | analysis of variance |
| ELISA | = | enzyme-linked immunosorbent assay |
| FR2 | = | framework 2 |
| IMGT | = | International ImMunoGeneTics |
| LD | = | lethal dose |
| MALDI-TOF | = | matrix assisted laser desorption/ionization-time of flight |
| Poly-IgG | = | sheep anti-toxin polyclonal IgG |
| SEC | = | size exclusion chromatography |
| TetC | = | tetanus toxin fragment C |
| VHH | = | heavy chain variable domain from heavy chain only antibiotics |
Introduction
Since their early discovery, antibodies have been widely used as passive immunotherapeutic agents for protection against pathogens and their toxic products, envenoming by poisonous animals, or drug overdoses.Citation1-3 Polyclonal antibodies were typically used for these purposes, but in the past decades there have been numerous efforts to replace them with monoclonal antibodies in order to improve safety and standardization.Citation4-6 However, the use of monoclonal antibodies as anti-infective or anti-toxin agents is challenging because of the need of targeting the proper epitope, and due to poor opsonization, particularly in the case of toxins that lack repetitive epitopes. Thus far, palivizumab (Synagis®, MedImmune), used in the prevention of respiratory syncytial virus, is the only anti-infective monoclonal approved by the FDA. In addition, conventional therapeutic monoclonal antibodies are still expensive to produce, and, until this limitation is overcome, it does not seem viable to use them for controlling epidemics or the consequences of the aftermath of a bioterror incident.Citation7
An interesting alternative to conventional antibodies is the use of recombinant single-domain antibody fragments (VHHs or nanobodies) derived from the variable domain of the heavy-chain-only antibodies found in camelids.Citation8 These antibodies are devoid of the light chain, and the antigen-binding sites are contained entirely in the VHH domain. There are several biotechnological advantages that position VHHs as promising options for the generation of neutralizing agents. Complex phage display VHH libraries are easy to generate because the original specificity of VHH is not affected by shuffling of the heavy and light chains.Citation9 The selected VHHs are typically highly stable, can be produced in microorganisms with high yields, and can be genetically fused to form bispecific or bivalent constructs.Citation10,11 Their small size (˜15 kDa) is a major asset to facilitate fast distribution and diffusion into tissues, a critical aspect when potent drugs, venoms or toxins need to be rapidly neutralized. However, the minimalistic functionality of VHHs comes with some hurdles to their development as therapeutics. Due to their small size, VHHs have rapid renal clearance, and thus a short half-life (˜2 h),Citation12 and they lack the effector functions of whole antibody molecules. To what extent these features contribute to the neutralization and clearance of the target appears to be case dependent. For instance, VHHs provided full protection of mice challenged with lethal doses of a small (˜7 kDa) scorpion toxin,Citation13 but potent in vitro neutralizing VHHs failed to provide protection when tested in vivo in guinea pigs challenged with food-and-mouth disease virus.Citation14
Different strategies have been devised to improve the performance of neutralizing VHHs. Commonly, one or more VHHs are combined in tandem to produce bivalent or bispecific constructs.Citation15 Due to an increased avidity, the construction of bivalent VHHs typically improves the neutralization potency of VHHs.Citation16,17 The increased molecular size may also contribute to this effect by extending the half-life of the construct, as has been observed by comparison of the neutralizing activity of glycosylated and deglycosylated VHHs against the heat-labile toxin of E. coli produced in yeast.Citation18 The half-life of VHHs has also been prolonged via interaction with the neonatal Fc receptor by fusing them to Fc fragments,Citation19 or to a second VHH with affinity for host immunoglobulinsCitation11 or albumin.Citation20 The potency of neutralizing VHHs has also been improved by recruiting strong effector functions with the help of a host conventional antibody, the so called “clearing antibody.” This was demonstrated by fusing a peptide tag to an anti-Botulinum toxin VHH; upon binding the anti-tag clearing antibody provides Fc receptor mediated effector functions. The use of additional peptide tag on the neutralizing VHH helped to increase the recruitment of the clearing antibody, and thus the opsonization of the toxin.Citation21 This was accompanied by a drastic empowering of the VHH neutralization capacity, but the large size of the clearing antibody may be an impediment to attain a rapid neutralization of the toxin that has reached the tissues, and the use of a second antibody adds complexity to the therapy.
In this work, we explored a new strategy for increasing the neutralizing potency of single domain antibodies by fusing them to an effector domain. This helps to improve the permanence of the administered nanobody and the immunocomplex removal, while keeping a small size that favors a rapid diffusion and high yield expression in bacteria. As effector domain, we assayed a VHH specific for Mac-1 (CD11b/CD18). This surface integrin receptor, which is expressed on most innate immune cells types, plays an essential role in the elimination of complement opsonized microorganisms.Citation22 The principle was tested in a mouse model of lethal toxicity caused by the tetanus neurotoxin produced by Clostridium tetani. The toxin, one the most powerful toxins known,Citation23 has the classical structure of A-B components, where the A subunit is responsible for the cytotoxic activity, and the B component contains both the membrane translocation domain (N-terminus) and the cell receptor binding domain (TetC). The neutralizing potency of the anti-TetC VHHs in monomeric or bispecific form combined with VHHs against Mac-1 or other cell receptors was tested.
Results
Selection of TetC specific VH/VHHs
The VH/VHH library was panned on microtiter plates coated with TetC (100 ng/well) using inputs of 1012 transducing units as described. There was a strong enrichment after the third round of panning, being the outputs of the 3 consecutive rounds: 3.4 × 104, 4.0 × 105 and >1×108 transducing units. Twenty-four colonies from the 3rd output were randomly picked and cultured to generate soluble monodomain antibody supernatants. Ten clones showing the highest ELISA readouts against TetC and negligible reactivity with BSA were selected and sequenced (). Seven of the amino acid sequences were identical and are represented by clone T3 in the figure. This clone has an unusually short CDR2 of only 2 amino acids. T3 and T23 have the hallmark residues of VHH domains in framework 2 (FR2) at positions 42, 50 and 52 (International ImMunoGeneTics (IMGT) amino acid numbering), but T5 have V, L and W at those positions, respectively, which are characteristic of VH domains.Citation24 These amino acids and a distinctive short CDR3 indicate that T5 derives from a VH(3) segment that recombined with a D-JH encoding a Trp103Arg substitution.Citation25,26 The DNA sequences of the 3 clones displayed in were cloned in the pINQ-BtH6 vector and the antibody fragments were expressed in the periplasm of E. coli. The antibodies were purified to homogeneity on Ni-NTA agarose with sequence-dependent yields; typically, between 5–20 mg/L of purified end product were obtained in shake flasks.
Figure 1. Amino acid sequence alignment of the anti-TetC variable domains. The deduced amino acid sequences are given in single-letter code. Residues are numbered according to the IMGT numbering system. Dots are used to denote identical residues and dashes to indicate gaps. Dotted boxes frame the CDRs and solid-line boxes outline the characteristic hallmark residues that differ between VHs and VHHs.

The three VH/VHHs define non-overlapping epitopes on TetC and interfere with its binding to the receptor ganglioside GT1b
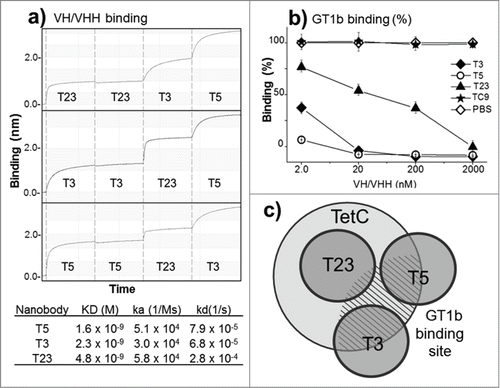
Epitope binning of the 3 antibody fragments was performed using dual bio-layer interferometry in the BLItZ System. GST-TetC was covalently immobilized on the AR2G sensors and was sequentially exposed in each step to each of the VH/VHHs at 100 μg/mL. The addition of the first antibody fragment was repeated in the second step to confirm saturation of the sensor binding capacity (). The sensorgrams show that T3, T5, and T23 bind to non-overlapping epitopes and that changing the order of these antibodies does not affect this result. Affinity measurement by bio-layer interferometry showed that the 3 monodomain antibodies have nanomolar affinity. The reactivity of the nanobodies was also characterized in terms of their inhibitory effect on the interaction of TetC with the GT1b ganglioside. GT1b mediates the binding and internalization of the toxin to neurons by interaction with 2 rather distant sites of TetC, known as the R and W sites.Citation27 The graphs in show that T3, T5 strongly inhibited the binding of TetC to GT1b along the range of concentrations tested, while T23 only did so at high concentrations. The schematic representation of the epitopes defined by the 3 antibody fragments with regard to their relative overlap and in vitro neutralization behavior is display in .
Figure 2. Epitope mapping and in vitro inhibition of TetC binding to GT1b. (a) Epitope binning and affinity measurement to TetC by bio-layer interferometry: The vertical dotted lines separate the steps (30 s) in the sequential exposure of nanobodies to the sensor. (b) Inhibition of GT1b binding of TetC coupled to peroxidase with the 3 VH/VHHs; VHH-TC9 against triclocarbanCitation29 was used as a negative control, all measurements are triplicates. (c) Schematic representation of the VHH epitopes according to the binning analysis and GT1b binding inhibition. The GT1b binding site is represented by the dashed area. The error bars represent the SD of mean value.
The monomeric nanobodies failed to neutralize high doses of the toxin in vivo, and their neutralization effect on low doses was gradually lost after administration
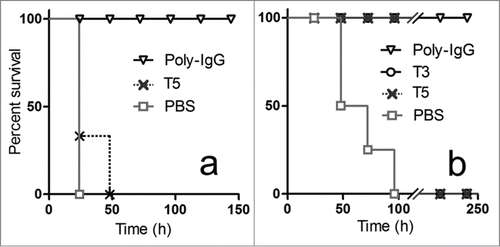
Based on their GT1b binding inhibition and good level of expression, VH T5 and VHH T3 were selected for the in vivo studies. Initially, the VH/VHHs in their monomeric form were tested for their ability to protect the mice from different doses of the toxin. For these studies, 0.63 nmoles (10 µg) or 6.3 nmoles (100 µg) of VH/VHH were co-administered with a low and high dose of the toxin. Using a high dose of toxin, 10 × LD100, all control mice died after 24 h, and none of the VH/VHHs fully protected the mice by day 4, although some animals treated with 6.3 nmoles of the antibody fragments were still alive after 24 h (shown in for T5). In parallel, a polyclonal anti-serum obtained from sheep vaccinated with a commercially available veterinarian anti-tetanus vaccine was used for comparison. Co-administration of the toxin with the equivalent to 3.3 nmoles (˜500 µg) of total sheep IgG (Poly-IgG) fully protected the mice by day 4, which could not be accomplished with 0.33 nmoles of Poly-IgG. When a low dose of toxin was used, 1 × LD100, the 5 control mice died by day 4, but all animals treated with 0.63 (10 µg) or 6.3 nmoles (100 µg) of T3 or T5 survived, as well as all mice co-administered with 0.33 or 3.3 nmoles of Poly-IgG. While day 4 is the commonly accepted end point of the neutralization test for tetanus toxin,Citation28 we noticed that in the group of mice treated with 0.63 nmoles of antibody fragments, the tetanus symptoms relapsed gradually afterwards and all animals were dead by day 10. This reversion of the neutralization effect was not observed when the toxin was co-administered to the animals with the polyclonal sheep IgG. The rapid clearance of the uncombined VH/VHHs and the lack of effector functions of the VH/VHHs bound to the toxin likely account for the gradual loss of the neutralization effect. Indeed, despite the good affinity of the antibodies, once the initial large excess of VH/VHHs is lost through renal filtration and metabolic serum turnover, the toxin-VH/VHH immunocomplexes may start dissociating, and hence releasing some active toxin.
Figure 3. In vivo neutralization of high (a) and low (b) doses of toxin with monomeric antibody fragments. Groups of 5 mice were co-administered with: (a) 10 × LD100 of the toxin preparation and 6.3 nmoles of T5, or 3.3 nmoles of Poly-IgG, or PBS ; or (b) 1 × LD100 of the toxin preparation and 0.63 nmoles of T5, 0.63 nmoles of T3, 0.33 nmoles of Poly-IgG, or PBS.
The combination of 2 of the most promising anti-toxin nanobodies in a bivalent construct did not provide a significant increase in neutralization potency
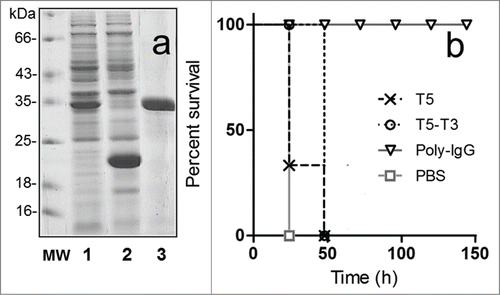
We next tried to improve the neutralization ability of the nanobodies by increasing the avidity of the toxin-VH/VHH interaction using bivalent constructs.Citation16,17 To this end we combined VH T5 with VHH T3 using the flexible spacer -GGGSGGGS-; having non-overlapping epitopes, this could allow the simultaneously binding of these 2 nanobodies to the toxin. Indeed, the increased avidity of the bivalent antibody was reflected by the kinetic measurements performed by biolayer interferometry, T5–T3 KD = 0.76 nM versus T5 KD = 1.6 nM or T3 KD = 2.3 nM. A control antibody, T5–TC9 was also built using the unrelated VHH TC9 specific for the bactericide triclocarban.Citation29 The expression level of the bivalent antibodies was lower than that of the monomeric domains, but still about 5 mg/L of purified end product were obtained in shake flasks. The neutralization activity of these constructs was tested under challenging conditions, using a 10 × LD100 of the toxin preparation. Despite the increased avidity and larger size we did not observed a major change in the potency of the T5 – T3 construct. As observed in the bivalent antibody (T5 – T3) performed slightly better than VH T5 in the monomeric form (p < 0.05), but all animals were dead by day 2. This modest improvement seems to be mostly related to its increased size, because the control nanobody T5 – TC9 provided identical protection (not shown).
Figure 4. Expression of the bivalent nanobody T5–T3 and in vivo neutralization assay. (a) SDS-PAGE 12% analysis of the cell culture extract of VH-VHH T5–T3 (1) or VH T5 (2), and the affinity purified T5–T3 (3). (b) Survival rate of groups of 5 mice co-administered with 10 × LD100 of the toxin preparation and PBS or PBS containing 6.3 nmoles of T5, 6.3 nmoles of T5–T3, or 3.3 nmoles of Poly-IgG.
Fusion of the neutralizing single domain antibodies to a Mac-1 (CD11b/CD18) binding domain dramatically increases their potency
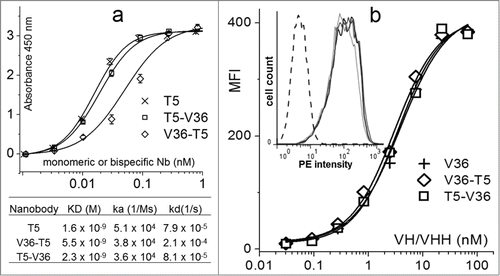
As mentioned before, rapid clearance and lack of effector functions may be disadvantages for the use of nanobodies as neutralizing agents. We reasoned that this could be counteracted by the addition of a small domain specific for cell receptors that are involved in opsonized phagocytosis and immune complex clearance. As proof of concept, we used the nanobody VHH V36, an anti-Mac-1 (CD11b/CD18) VHH, and compared its performance for the construction of neutralizing bispecific nanobodies with other VHHs to mouse cell receptors. The VHHs V36, G7, and 49 specific for the CD11b chain of Mac-1, the mouse Ly-5 leukocyte common antigen (CD45), and the β chain of the class II MHC, respectively, were recently isolated and characterized in our laboratory.Citation30 The bispecific antibodies were prepared and their functionality tested by ELISA and flow cytometry. shows the ELISA titration of equal amounts of nanobodies against TetC. When VH T5 was used at the N-terminus of the bispecific nanobody, its molar reactivity was identical to that of monomeric T5, which was not the case when T5 was at the C-terminus. This was in agreement with the affinity values of these antibodies. In the case of VHH V36, the reactivity against Mac-1 was assayed by FACS on the macrophage cell line J774.A1. In this case, the N-terminal or C-terminal position of the VHH did not affect the binding of V36 to its cognate target. Based on these results, the bispecific construct T5–V36 was used for the neutralization experiment. The biochemical properties of the biespecific nanobodies were also characterized by size exclusion chromatography (SEC) and matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) (Fig. S1, supplementary information). The mass of the antibodies measured by MALDI-TOF was in agreement with their calculated size, and SEC experiments showed that most nanobodies occurred in the monomeric form and did not aggregate, with the only exception of T5–G7, which tended to dimerize, forming dimers of about 60 kDa.
Figure 5. Comparison of the reactivity of the monomeric and bispecific nanobodies. (a) Analysis of the nanobody reactivity against TetC by ELISA and bio-layer interferometry. (b) Flow cytometry mean fluorescent intensity (MFI) of the J774.A1 cell line stained with the biotinylated monomeric or bispecific nanobodies and streptavidin phycoerythrin. The insert shows the histogram for the 1 nM concentration of V36 (black), V36-T5 (dark gray), T5–V36 (light gray), and TC9 (negative control, dashed line). The error bars represent the SD of mean value.
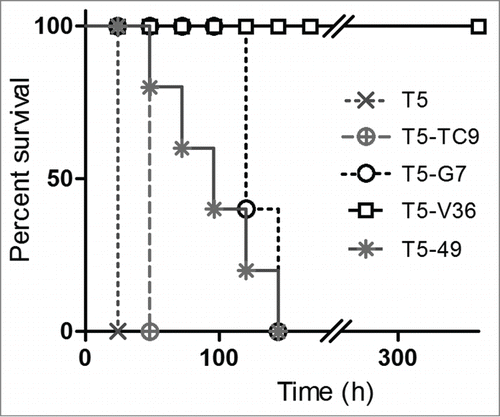
When the bispecific antibodies were used in protection experiments (), the monomeric T5 again failed to confer protection to a high toxin challenge, and its combination with the irrelevant VHH (TC9) to triclocarban only marginally helped to increase the survival. Fusion of T5 to VHHs that bind to non-endocytic cell receptors highly expressed in leukocytes, such as CD45 (VHH G7) and MHC II (VHH 49), improved the neutralization potency of T5, most probably by decreasing their kidney clearance through the interaction with cells bearing these receptors, as demonstrated by the biodistribution analysis (see below). However, the combination with VHH V36, which reacts with the endocytic receptor CD11b/CD18, dramatically increased the neutralization power of T5, enabling the whole group of mice to survive a 10 × LD100 of the toxin, not only up to the end point of the test by day 4, but up to day 15. At that point, all animals looked healthy and their observation was ended.
Figure 6. In vivo neutralization assay with bispecific antibodies. Survival rate of groups of 5 mice co-administered with 10 × LD100 of the toxin preparation and 6.3 nmoles of the monomeric or bispecific antibodies in PBS. The Log-rank (Mantel-Cox) test indicated significant differences in the neutralizing activity of the nanobodies as follows: T5 < T5 – TC9 (p < 0.0002), T5 – TC9 < T5 – 49 (p < 0.0002), T5 – 49 < T5 – G7(p<0.02), T5 – G7 <T5 – V36 (p < 0.0001).
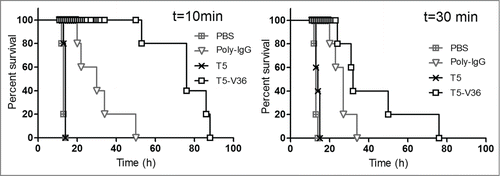
We then mimicked the conditions that are found during an intoxication episode, i.e., when the toxin is produced locally by an infectious focus and the anti-toxin agent is supplied intravenously to allow rapid neutralization. This is major variation from the administration of the preformed toxin-antibody immunocomplex, and it better reflects the bioavailability of the neutralizing agent. Indeed, CD11b/CD18 is highly expressed on circulating leukocytes of myeloid origin and the use of the intravenous route would allow the rapid interaction of the bispecific antibody with these cells, which in term will affect its biodistribution. To this end, 5 × LD100 of the toxin were injected subcutaneously and then, at different time points, the toxin was neutralized by i.v. injection of 6.3 nmoles (200 µg) of recombinant antibodies or 3.3 nmoles of Poly-IgG. These doses of T5–V36 and Poly-IgG were fully protective when co-administered with 10 × LD100 of toxin (), but were not enough to warrant the survival of the mice when their administration was delayed (). Interestingly though, T5–V36 outperformed the polyclonal IgG antibody, which was more evident when the antibodies were administrated shortly after intoxication. This probably reflects a more efficient biodistribution and better tissue penetration due to the small size of the nanobody. At a later time, this advantage starts to be useless to neutralize the toxin, most probably because some of it has already being internalized by the neurons. It is noteworthy that the monomeric version of T5 had almost no effect when administered under similar conditions, highlighting the importance of improving the permanence and effector function of neutralizing monodomain antibodies.
Figure 7. Delayed neutralization assay. Survival rate of groups of 5 mice were challenged s.c. with 5 × LD100 of the toxin preparation, and 10 and 30 min later, treated i.v. with PBS, or PBS containing 6.3 nmoles of T5, T5–V36, or 3.3 nmoles of Poly-IgG. The Log-rank (Mantel-Cox) test indicated significant differences in the neutralizing activity of the nanobodies as follows: PBS<Poly-IgG (p < 0.0001), Poly-IgG <T5 – V36 (p < 0.0001) at 10 min, and PBS <Poly-IgG (p < 0.0001), Poly-IgG <T5 – V36 (p < 0.05) at 30 min.
Rapidly after administration the T5–V36 accumulates in lymphoid and other leukocyte rich tissues
The biodistribution of T5–V36 was compared to that of T5, T5–TC9 and T5–G7 using the technetium-99m labeled nanobodies and measuring the radioactivity in various dissected organs at different times (Fig. S2 supporting information). As expected the monomeric nanobodies T5 accumulated more rapidly in the kidney than T5–TC9 (p < 0.001). T5–V36 and T5–G7 showed a slower filtration rate than the control bivalent antibody (p < 0.001), supporting the presumption that the interaction with their cell targets decreased their clearance. Probably due to its occurrence as a dimer and the larger abundance of its target, T5–G7 exhibited a slower kidney filtration than T5–V36 after 1 or 3 h of administration (p < 0.001). In the individual organs, it was evident the stronger accumulation of T5–V36 and T5–G7 activity in lymphoid and leukocyte rich tissues. This enrichment was particularly high for T5–G7 in the spleen, liver, and lungs, but curiously, in bones the accumulation of T5–V36 was significantly higher than that of T5–G7 at all times (p < 0.001), but particularly at 24 h. To better understand the accumulation pattern of these 2 bispecific antibodies, the relative abundance of CD11b+ and CD45+ cells in these tissues was studied by flow cytometry (Figure S3 supporting information). The number of CD45+ cells was higher than the number of CD11b+ cells in all cell preparations, which is in line with the larger accumulation of T5–G7, but this was not the case with bone marrow where, despite the larger abundance of CD45+ cells, T5–V36 accumulates a higher levels. Overall, the biodistribution analysis showed a slower clearance and larger accumulation of T5–G7 in most tissues, indicating that the main feature that explains the outstanding neutralizing potency of T5–V36 is not the relative abundance of the bispecific antibodies, but the interaction with Mac-1.
Discussion
Starting from a vaccinated llama, it was possible to isolate high affinity monodomain antibodies to the cell receptor binding domain of the tetanus toxin. Despite their good affinity, the monomeric or bivalent form of the nanobodies failed to offer significant protection in the high-dose co-administration or post-administration experiments. Although this may depend on the toxin size, mode of action and affinity of the nanobodies, this is a typical limitation affecting the utility of neutralizing nanobodies. As discussed before, different approaches have been used to overcome this problem,Citation11,19-21 but, for the first time, we present a strategy that directly bridges the toxic compound to an endocytic receptor highly expressed in neutrophils, monocytes and macrophages. This contributes to the efficient removal of the toxin and permanence of the nanobody, while the bispecific construct still has a minimum size (˜30 kDa) that favors its rapid biodistribution and is compatible with a high production yield in bacteria. The proof of concept presented here can be further expanded to address other cell receptors involved in the internalization of immunocomplexes, and may be applied to the development of neutralizing therapeutic nanobodies, such as those targeting deregulated inflammatory cytokines (i.e., tumor necrosis factor, interleukin-6) or tumor growth factors.
Material and Methods
Production of recombinant tetanus toxin fragment C (TetC)
The DNA coding sequence of TetC (the non-toxic C-terminal 47 kDa polypeptide fragment (amino acids 863–1315) of tetanus toxin) was codon optimized for E. coli expression and was synthesized by IDT (Coralville, IA). This gene was cloned in the pGEX 5 × plasmid (G&E HealthCare, Piscataway, NJ) and expressed as an N-terminal fusion to GST in BL21 cells. Purification was performed on an agarose-glutathione column from Sigma (St. Louis, MO) and acidic elution (Tris-glycine buffer, pH 2.7) or thrombin digestion were used to obtain homogeneous preparations of GST-TetC or TetC, respectively.
Library construction
Blood was drawn from a 3 y old llama that had been vaccinated regularly (every 6 month) with 5 mL of a clostridial vaccine (Clostrisan 9+T, Santa Elena, Montevideo, Uruguay), which includes 10 Clostridium species/strains including C. tetani. A VHH phage display library was built as previously described.Citation29 Briefly, about 108 lymphocytes were obtained from 150 mL of blood using Histopaque 1077 (Sigma) gradients. The RNA was extracted and reverse transcribed using the primer JH. The cDNA was then used as template for PCR amplification of the VH and VHH genes using the forward primers, VH1, VH3, VH4 and the reverse primer JH.Citation31 The amplified DNA was digested with SfiI and cloned in the pComb3X, a kind gift from Professor Barbas (The Scripps Research Institute, La Jolla, USA). The ligated vector was electroporated into highly competent ER2738 E. coli cells (Lucigen Corporation, WI). The cells were cultured and the phagemid-borne phage library displaying the VH/VHH repertoire was rescued by superinfection with helper phage M13KO7 (Pharmacia Biotech, Uppsala, Sweden).
Panning of the anti-TetC library
Microtiter ELISA (Maxisorp, Nunc) plates were coated with 1 μg/mL of TetC overnight at 4°C. After coating, wells were blocked with 300 μL of 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). The antibody library (1012 transducing units) was mixed with PBS-0.05% Tween 20 (PBST) containing 1% of BSA, and dispensed into 3 microtiter wells coated with TetC. After incubating for 2 h at 4°C, plates were washed 10 times with PBST, incubated for 30 min in PBST at 4°C, and washed again 10 times, after which the bound phages were eluted by incubation with 100 μL per well of 100 mM glycine-HCl, pH 2.2 for 10 min. The eluted phages were immediately neutralized by addition of 2 M Tris base. Phage outputs were counted by plating on ampicillin plates a fixed amount of ER2738 infected with 10-fold serial dilutions of phage.
ELISA Protocol
ELISA plates (Nunc Maxysorp) were coated with 100 μL/well of 1 μg/mL TetC in PBS overnight at 4°C and blocked with 3% BSA. Individual colonies from the counting plates were cultured in 1 mL of LB medium containing ampicillin, induced with 2 mM IPTG after 3 h, and grown overnight. Hundred µL of supernatant were added to TetC or BSA coated wells, and incubated for 1 h, followed by washing and incubation with anti-HA HRP conjugated antibody (Roche, Boulder, CO). After extensive washing, the peroxidase activity was developed by adding 100 μL of peroxidase substrate (0.4 mL of, 6 mg of 3,3′,5,5′-tetramethylbenzidine in 1 mL of DMSO + 0.1 mL of 1% H2O2 in water, in a total of 25 mL of 0.1M acetate buffer, pH 5.5) and incubated for 20 min at room temperature. The enzyme reaction was stopped by the addition of 50 μL of 2 N H2SO4, and the absorbance was read at 450 nm on a Fluostar Optima Reader (BMG, Ortenberg, GE).
Production of VH/VHH clones in a High Expression System
The phagemids bearing the selected anti-TetC monodomain antibodies were then digested with SfiI restriction enzyme, the VH/VHH DNA was separated by agarose gel electrophoresis and gel purified using Genejet extraction kit (Thermo, Waltham, Massachusetts). This fragment was cloned in the pINQ-BtH6 vector between 2 different SfiI restriction sites. pINQ-BtH6 is a derivative of pET 28a(+) vector (Novagen, Madison, WI), that contains the coding sequences of the OmpA signal peptide upstream of the first SfiI site, and the coding sequence of the peptide GLNDIFEAQKIEWHE and the 6 × His tag downstream of the second SfiI site. The peptide GLNDIFEAQKIEWHE is biotinylated in vivo when the BL21(DE3) cells are co-transformed with the plasmid pCY214 [GenBank:AAD22470.1] that overproduces the E. coli biotin holoenzyme BirA.Citation32 The ligation reaction was transformed into BL21(DE3) E. coli strain containing the pCY214 vector and plated on kanamycin/chloramphenicol plates. Individual clones were grown in LB (kanamycin 40 μg/mL, chloramphenicol 35 μg/mL, arabinose 0.2%) and antibody expression was induced with 4 µM of IPTG (final concentration) for 4 h at 37°C. To obtain the biotinylated version of the antibodies, biotin 100 μM was included. Cells were pelleted, and the periplasmic proteins were extracted by sonication. Antibody purification was performed on Ni-NTA columns in the ÄKTA purification system (GE Healthcare, Uppsala, Sweden) according to the manufacturer's instructions.
Construction of bispecific nanobodies
For this purpose, 2 single domain antibodies were genetically linked using the spacer sequence (G3S)2. The DNA coding for the N-terminal (Nb1) and the C-terminal (Nb2) nanobodies were PCR amplified with forward (Fw) and reverse (Rv) primers as follows, Nb1: Bi-VHH1-Fw and Bi-VHH1-Rv; Nb2: Bi-VHH2-Fw, and Bi-VHH2-Rv, using pfu polymerase (Thermo Scientific, Waltham, MA, USA). The 400 bp DNA fragments were purified using GeneJET PCR purification Kit (Thermo Scientific) and fused by overlap PCR using the primers Bi-Overlap-Fw and Bi-Overlap-Rv. The amplified DNA was then digested with SfiI, gel purified after agarose electrophoresis, and cloned into the pINQ-BtH6 vector. Transformation in E. coli and expression was performed as described.
Binding analysis of VH/VHH interactions by bio-layer interferometry
For epitope binning, recombinant GST-TetC at a concentration of 50 μg/mL was immobilized on ForteBio Second-Generation amine reactive biosensor probes (AR2G) using N-hydroxysuccinimide/1-ethyl-3-(3dimethylaminopropyl)carbodiimide(NHS/EDC) linkage. The sensors were blocked using 1 M ethanol-amine, pH 8.5. All antibody fragments were used at 100 μg/mL. In each subsequent step the previously added antibodies were included to rule out the eventual displacement of the already bound antibodies. Binding interactions were analyzed in a ForteBio BLITz machine (Fortebio, Inc., Menlo Park, CA), and were measured as a wavelength shift in nanometers. Kinetic rate constants were measured with the ForteBio streptavidin sensor (SA) using the advance kinetic mode. The in vivo biotinylated nanobodies were immobilized on the sensor at 50 μg/mL, and were exposed for 300 s to various concentrations of TetC in the 0–100 nM range, followed by a 600 s dissociation step in PBS while shaking at 2200 rpm. Interferometry data were globally fitted to a 1:1 binding ratio for calculating kinetic parameters using the BlitzPro Software, version 1.1 (ForteBio Inc.).
In vitro GT1b neutralizing assay
GT1b ganglioside was purchased from Sigma (St. Louis, MO). One hundred microliters of GT1b, at a final concentration of 10 µg/mL in methanol, were dispensed in microtiter ELISA plate wells (Nunc Maxysorp) and incubated at room temperature overnight to allow complete methanol evaporation. The next day, wells were blocked with 1% BSA in PBS, and 50 µL of TetC conjugated to peroxidase (1 µg/mL) were added together with decreasing concentrations of VHHs. The nanobody TC9, a VHH against the herbicide triclocarbanCitation29 was used as a negative control. After 1 h incubation at room temperature, the plates were washed and developed as described.
Preparation of the test toxin and neutralization assay
C. tetani (Harvard strain) was grown in synthetic mediaCitation33 in shake flasks for 6 days, and kept afterwards at 4°C for 24 h to allow bacterial lysis and toxin release. The supernatant was collected by centrifugation and concentrated 10-fold on Amicon Ultra-15 Centrifugal Filter units (Millipore). The sterile filtrate (0.22 µm) was supplemented with an equal volume of glycerol and was stored a −20°C. The in vivo neutralization assay was performed as described by PeelCitation28 with some modifications. Briefly, equal volumes of toxin and neutralizing antibodies were mixed and incubated for 1 h. Two hundred µL of this mixture were injected subcutaneously to CD1 outbred mice (18–20g) using groups of 5 animals for each condition. Death was recorded every 24 h for 4 days, which was considered the end point of the test. The minimal lethal dose causing the death of 100% of the animals (LD100) after 4 d was established in the absence of protecting antibodies following this procedure. For the in vivo delayed neutralization assay, groups of mice (n = 5) were experimentally challenged by subcutaneous injection of 5 × LD100 in 100 µL. Ten or 30 min later, 100 µL of PBS, or PBS containing 6.3 nmoles of monomeric or bispecific nanobodies, or 3.3 nmoles of sheep anti-tetanus toxin IgG, was injected in the tail vein. Death was recorded hourly.
Ethics statement for animal work
All activities involving animals were performed with special care to establish high standards of biosafety and assure animal welfare. All protocols for animal experimentation were carried out in accordance with procedures authorized by the University's Ethical Committee for Animal Experimentation, Uruguay.
Statistics
Survival analysis was performed by computing pair-wise comparisons of the neutralization activity of the antibodies using a log-rank (Mantel-Cox) test. Differences in the biodistribution of the nanobodies were evaluated using the 2-way analysis of variance (ANOVA). Data analysis was performed using Prism software version 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Disclosure of Potential Conflicts of Interest
The authors declare no competing financial interest
Supplemental_Material.docx
Download MS Word (692.4 KB)Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This work was supported with funds provided by grants CSIC (UDELAR) 984, FCE 6812 ANII (Agencia Nacional de Investigación e Innovación, Uruguay) and TW05718 Fogarty Center NHI. MAR is a recipient of scholarships from ANII and CSIC, Uruguay.
References
- Colburn WA. Specific antibodies and Fab fragments to alter the pharmacokinetics and reverse the pharmacologic/toxicologic effects of drugs. Drug Metab Rev 1980; 11:223-62; PMID:7011759; http://dx.doi.org/10.3109/03602538008994026
- Sewall H. Experiments on the Preventive Inoculation of Rattlesnake Venom. J Physiol 1887; 8:203-10; PMID:16991478; http://dx.doi.org/10.1113/jphysiol.1887.sp000253
- Von Behring E, Kitasato S. Ueber das Sustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Dtsch Med Wochenschr 1890; 16:1113-4; http://dx.doi.org/10.1055/s-0029-1207589
- Adekar SP, Takahashi T, Jones RM, Al-Saleem FH, Ancharski DM, Root MJ, Kapadnis BP, Simpson LL, Dessain SK. Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain. PLoS One 2008; 3:e3023; PMID:18714390; http://dx.doi.org/10.1371/journal.pone.0003023
- Chen Z, Moayeri M, Zhao H, Crown D, Leppla SH, Purcell RH. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc Natl Acad Sci U S A 2009; 106:13487-92; PMID:19651602; http://dx.doi.org/10.1073/pnas.0906581106
- Krautz-Peterson G, Chapman-Bonofiglio S, Boisvert K, Feng H, Herman IM, Tzipori S, Sheoran AS. Intracellular neutralization of shiga toxin 2 by an a subunit-specific human monoclonal antibody. Infect Immun 2008; 76:1931-9; PMID:18285498; http://dx.doi.org/10.1128/IAI.01282-07
- Sethuraman N, Stadheim TA. Challenges in therapeutic glycoprotein production. Curr Opin Biotechnol 2006; 17:341-6; PMID:16828275; http://dx.doi.org/10.1016/j.copbio.2006.06.010
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature 1993; 363:446-8; PMID:8502296; http://dx.doi.org/10.1038/363446a0
- Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem 2013; 82:775-97; PMID:23495938; http://dx.doi.org/10.1146/annurev-biochem-063011-092449
- Frenken LG, van der Linden RH, Hermans PW, Bos JW, Ruuls RC, de Geus B, Verrips CT. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J Biotechnol 2000; 78:11-21; PMID:10702907; http://dx.doi.org/10.1016/S0168-1656(99)00228-X
- Harmsen MM, Van Solt CB, Fijten HP, Van Setten MC. Prolonged in vivo residence times of llama single-domain antibody fragments in pigs by binding to porcine immunoglobulins. Vaccine 2005; 23:4926-34; PMID:15992972; http://dx.doi.org/10.1016/j.vaccine.2005.05.017
- Cortez-Retamozo V, Lauwereys M, Hassanzadeh Gh G, Gobert M, Conrath K, Muyldermans S, De Baetselier P, Revets H. Efficient tumor targeting by single-domain antibody fragments of camels. Int J Cancer 2002; 98:456-62; PMID:11920600; http://dx.doi.org/10.1002/ijc.10212
- Hmila I, Saerens D, Ben Abderrazek R, Vincke C, Abidi N, Benlasfar Z, Govaert J, El Ayeb M, Bouhaouala-Zahar B, Muyldermans S. A bispecific nanobody to provide full protection against lethal scorpion envenoming. FASEB J 2010; 24:3479-89; PMID:20410443; http://dx.doi.org/10.1096/fj.09-148213
- Harmsen MM, van Solt CB, Fijten HP, van Keulen L, Rosalia RA, Weerdmeester K, Cornelissen AH, De Bruin MG, Eblé PL, Dekker A. Passive immunization of guinea pigs with llama single-domain antibody fragments against foot-and-mouth disease. Vet Microbiol 2007; 120:193-206; PMID:17127019; http://dx.doi.org/10.1016/j.vetmic.2006.10.029
- Els Conrath K, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem 2001; 276:7346-50; PMID:11053416; http://dx.doi.org/10.1074/jbc.M007734200
- Hmila I, Abdallah RB, Saerens D, Benlasfar Z, Conrath K, Ayeb ME, Muyldermans S, Bouhaouala-Zahar B. VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI'. Mol Immunol 2008; 45:3847-56; http://dx.doi.org/10.1016/j.molimm.2008.04.011
- Vance DJ, Tremblay JM, Mantis NJ, Shoemaker CB. Stepwise engineering of heterodimeric single domain camelid VHH antibodies that passively protect mice from ricin toxin. J Biol Chem 2013; 288:36538-47; http://dx.doi.org/10.1074/jbc.M113.519207
- Harmsen MM, van Solt CB, Fijten HP. Enhancement of toxin- and virus-neutralizing capacity of single-domain antibody fragments by N-glycosylation. Appl Microbiol Biotechnol 2009; 84:1087-94; http://dx.doi.org/10.1007/s00253-009-2029-1
- Richard G, Meyers AJ, McLean MD, Arbabi-Ghahroudi M, MacKenzie R, Hall JC. In vivo neutralization of α-cobratoxin with high-affinity llama single-domain antibodies (VHHs) and a VHH-Fc antibody. PLoS One 2013; 8:e69495; http://dx.doi.org/10.1371/journal.pone.0069495
- Coppieters K, Dreier T, Silence K, de Haard H, Lauwereys M, Casteels P, Beirnaert E, Jonckheere H, Van de Wiele C, Staelens L, et al. Formatted anti-tumor necrosis factor α VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum 2006; 54:1856-66; http://dx.doi.org/10.1002/art.21827
- Mukherjee J, Tremblay JM, Leysath CE, Ofori K, Baldwin K, Feng X, Bedenice D, Webb RP, Wright PM, Smith LA, et al. A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PloS one 2012; 7:e29941; http://dx.doi.org/10.1371/journal.pone.0029941
- van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol 2007; 9:2095-102; http://dx.doi.org/10.1111/j.1462-5822.2007.00981.x
- Gill DM. Bacterial toxins: a table of lethal amounts. Microbiol Rev 1982; 46:86-94
- Vu KB, Ghahroudi MA, Wyns L, Muyldermans S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol Immunol 1997; 34:1121-31; http://dx.doi.org/10.1016/S0161-5890(97)00146-6
- Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol 2007; 77:13-22; http://dx.doi.org/10.1007/s00253-007-1142-2
- Saerens D, Kinne J, Bosmans E, Wernery U, Muyldermans S, Conrath K. Single domain antibodies derived from dromedary lymph node and peripheral blood lymphocytes sensing conformational variants of prostate-specific antigen. J Biol Chem 2004; 279:51965-72; http://dx.doi.org/10.1074/jbc.M409292200
- Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem 2001; 276:32274-81; http://dx.doi.org/10.1074/jbc.M103285200
- Peel MM. Measurement of tetanus antitoxin. II. Toxin neutralization. J Biol Stand 1980; 8:191-207; http://dx.doi.org/10.1016/S0092-1157(80)80035-7
- Tabares-da Rosa S, Rossotti M, Carleiza C, Carrion F, Pritsch O, Ahn KC, Last JA, Hammock BD, González-Sapienza G. Competitive selection from single domain antibody libraries allows isolation of high-affinity antihapten antibodies that are not favored in the llama immune response. Anal Chem 2011; 83:7213-20; http://dx.doi.org/10.1021/ac201824z
- Rossotti M, Tabares S, Alfaya L, Leizagoyen C, Moron G, Gonzalez-Sapienza G. Streamlined method for parallel identification of single domain antibodies to membrane receptors on whole cells. Biochimica et biophysica acta 2015; 1850(7):1397-404
- Zarebski LM, Urrutia M, Goldbaum FA. Llama single domain antibodies as a tool for molecular mimicry. J Mol Biol 2005; 349:814-24; PMID:15890359; http://dx.doi.org/10.1016/j.jmb.2005.03.072
- Beckett D, Kovaleva E, Schatz PJ. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci 1999; 8:921-9; PMID:10211839; http://dx.doi.org/10.1110/ps.8.4.921
- Feeney RE, Mueller JH, Miller PA. Growth requirements of clostridium tetani: III. A “Synthetic” Medium. J Bacteriol 1943; 46:563-71; PMID:16560741
