Abstract
Biologic treatment options such as tumor necrosis factor (TNF) inhibitors have revolutionized the treatment of inflammatory diseases, including rheumatoid arthritis. Recent data suggest, however, that full and long-lasting responses to TNF inhibitors are limited because of the activation of the pro-inflammatory TH17/interleukin (IL)-17 pathway in patients. Therefore, dual TNF/IL-17A inhibition is an attractive avenue to achieve superior efficacy levels in such diseases. Based on the marketed anti-TNF antibody adalimumab, we generated the bispecific TNF/IL-17A-binding FynomAb COVA322. FynomAbs are fusion proteins of an antibody and a Fyn SH3-derived binding protein. COVA322 was characterized in detail and showed a remarkable ability to inhibit TNF and IL-17A in vitro and in vivo. Through its unique mode-of-action of inhibiting simultaneously TNF and the IL-17A homodimer, COVA322 represents a promising drug candidate for the treatment of inflammatory diseases. COVA322 is currently being tested in a Phase 1b/2a study in psoriasis (ClinicalTrials.gov Identifier: NCT02243787).
Abbreviations
| AUC | = | Area Under the Curve |
| CHO | = | Chinese Hamster Ovary cells |
| Cl | = | Clearance |
| Cmax | = | maximum serum concentration |
| GLP | = | Good Laboratory Practice |
| GMP | = | Good Manufacturing Practice |
| IL-17 | = | Interleukin-17 |
| iv | = | intravenous |
| NHDF | = | Normal Human Dermal Fibroblasts |
| NOAEL | = | No Observed Adverse Effect Level |
| PBMC | = | Peripheral Blood Mononuclear Cells |
| RA | = | Rheumatoid Arthritis |
| SEC | = | Size-Exclusion Chromatography |
| TNF | = | Tumor Necrosis Factor-α |
| T½ | = | Terminal disposition half-life |
| Vz | = | Volume of distribution. |
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory disease that destroys joints. RA is thus associated with major consequences for the individual, causing loss of function and work disability, and poses significant challenges to society, given its economic consequences.Citation1 Conventional disease-modifying anti-rheumatic drugs (DMARDs) such as methotrexate are still the mainstay of treatment for many inflammatory diseases including RA.Citation2 Recently, biologic agents such as tumor necrosis factor (TNF) inhibitors have become available and are recommended for patients not responding to DMARDs.Citation3,4 However, based on the results of clinical trials, more than half of RA patients treated with TNF blockers do not achieve a substantial response, defined as 50% improvement according to American College of Rheumatology criteria (ACR50) or as a state of low disease activity.Citation5 Furthermore, some RA patients treated with cytokine inhibitors lose their responsiveness over time and need to switch to other agents. Therefore, a high unmet medical need remains and alternative strategies to fight inflammatory and auto-immune disorders are needed to further improve patients' lives.
The pro-inflammatory cytokine interleukin (IL)-17 is secreted by a variety of immune and non-immune cells including CD4+ Th17 T cells, and it has been shown to be an important contributor to the pathogenesis of several autoimmune diseases including RA.Citation6 Importantly, several preclinical and clinical studies suggest that inhibition of both IL-17A and TNF may be superior and more efficacious compared to the corresponding monotherapies. For example, Wim van den Berg and colleagues demonstrated in a collagen-induced arthritis model in mice that the dual inhibition of TNF and IL-17A suppressed arthritic symptoms more effectively than the inhibition of each cytokine alone.Citation7 Importantly, irreversible damage of the joints involving chondrocyte death, cartilage and bone destruction was prevented more effectively by simultaneous inhibition of TNF and IL-17 compared to monospecific blockade. Furthermore, Alzabin and colleagues showed that RA patients receiving TNF blockers had elevated percentage of circulating Th17 cells within a few weeks after onset of therapy, leading to the hypothesis that long-lasting responses to TNF inhibition may be compromised by the induction of pathogenic Th17 cells.Citation8 Therefore, dual TNF/IL-17A inhibition may represent a very promising novel approach for the treatment of rheumatoid arthritis, psoriasis and psoriatic arthritis.
Fynomers are small 7-kDa globular proteins derived from the SH3 domain of the human Fyn kinase (Fyn SH3) that can be engineered to bind with antibody-like affinity and specificity to virtually any target of choice through random mutation of 2 loops (RT- and src-loop) on the surface of the Fyn SH3 domain.Citation9 In the past, we have successfully isolated Fynomers binding to mouse serum albumin,Citation10 chymase,Citation11 BACE2,Citation12 and HER2.Citation13 Recently, we described the isolation of high-affinity IL-17A-binding Fynomers and Fc fusions thereof.Citation14
Results
The anti-IL-17A Fynomer 11L5B06 (Fig. S1A, S1C) was genetically fused to the C-terminus of the light chain of the human anti-TNF antibody adalimumab to generate the bispecific anti-TNF/IL-17A FynomAb COVA322 (). The Fynomer was isolated using recombinant IL-17A as target antigen in phage display selections with a repertoire of mutated Fyn SH3-derived domains.Citation11 Fynomer 11L5B06 was produced at high levels (> 35 mg/L) in E.coli shake flask cultures under non-optimized conditions, and inhibited specifically IL-17A in a cell-based assay (Fig. S1B). COVA322 was produced in transiently transfected Chinese hamster ovary (CHO) cells and purified by standard protein A and size-exclusion chromatography (SEC) with a yield of 110 mg/l, which is comparable to the yield of unmodified adalimumab produced in the same expression system. shows the analytical SEC profile of COVA322, which elutes as a single peak, confirming the monomeric nature and the high purity of the FynomAb. The binding properties of COVA322 to human and cynomolgus IL-17A and TNF were analyzed by real-time interaction analysis on a BIAcore chip with immobilized COVA322, revealing picomolar binding affinities to human and cynomolgus TNF and IL-17A (Fig. S2). In addition, COVA322 was able to bind both TNF and IL-17A simultaneously (). Importantly, the fusion of a Fynomer to adalimumab did not change the antibody's TNF binding properties (Suppl. Table 1). TNF inhibition, as determined in a standard L929 murine fibroblast cell assay,Citation15 was comparable between adalimumab and COVA322 (data not shown). To test IL-17A inhibition by COVA322, normal human dermal fibroblasts (NHDF) were stimulated with recombinant IL-17A at a concentration of 64 pM in the presence of IL-1β, as this cytokine pair acts in synergy. In this way, the cell inhibition assay could be performed using very low IL-17A concentrations, providing the ability to determine low IC50 values. The calculated IC50 value of COVA322 for IL-17A inhibition was 121 pM (). Moreover, we confirmed the ability of COVA322 to simultaneously inhibit IL-17A and TNF (IC50 value of 169 pM) in an assay using the human colorectal adenocarcinoma cells HT-29, which were stimulated to produce Gro-α upon addition of both cytokines (). In addition, the ability of COVA322 to neutralize peripheral blood mononuclear cell (PBMC)-derived human IL-17A was evaluated. Purified human PBMCs were stimulated to produce IL-17A as described in Gerhardt et al.Citation16 The IL-17A-containing supernatant of the PBMCs was then used to stimulate HT-1080 cells to produce IL-6. In order to evaluate specifically IL-17A inhibition by COVA322, the experiment was carried out in presence of an excess of adalimumab (anti-TNF antibody) and canakinumab (anti-IL-1β antibody) because the stimulated PBMCs secrete considerable amounts of TNF and IL-1β. shows that COVA322 efficiently neutralizes human PBMC-derived IL-17A. Additionally, the neutralizing activity of COVA322 to the IL-17A homodimer, IL-17A/F heterodimer and the IL-17F/F homodimer was compared. Fig. S3 shows that COVA322 inhibited the IL-17A homodimer in a specific manner (IC50 = 217 pM) and did not cross-react with the IL-17F homodimer. The presence of 2 IL-17-A binding Fynomers () allows COVA322 to avidly bind and selectively inhibit the IL-17A/A homodimer, whereas the IL-17A/F heterodimer is only weakly inhibited.(Fig S3)
Figure 1. Characterization of COVA322 (A) Schematic picture of COVA322 showing that the anti-IL-17A Fynomer (orange circle) was genetically fused to the C terminus of the light chain of the anti-TNF antibody adalimumab. (B) COVA322 was expressed transiently in CHO cells and purified using protein A. The resulting protein was >95 % pure and monomeric, as determined by size exclusion chromatography over a period of at least 2 months in a non-optimized phosphate buffered saline buffer. (C) Dual TNF and IL-17A binding of COVA322 is shown using immobilized IL-17A on a BIAcore chip with subsequent injection of COVA322 and TNF (red) or COVA322 only (black) (D) COVA322 and the control anti-IL-17A antibody secukinumab were tested in a cell assay after stimulation with IL-17A and IL-1β. COVA322 and the control antibody specifically inhibited IL-17A with IC50 values of 121 pM and 470 pM, respectively. Mean values of triplicates are shown, error bars represent standard deviations (SD) (E) Gro-α ELISA levels in the supernatants are shown after the stimulation of HT-29 cells with 200 pM TNF and 400 pM IL-17A. After addition of the indicated concentrations of COVA322, a dose-dependent inhibition of IL-17A and TNF can be observed because the Gro-α levels decline with higher concentrations of the inhibitors. As controls, cells were treated with the single cytokines (IL-17A or TNF) or with medium only. Mean values of triplicates are shown, error bars represent standard deviations (SD). (F) COVA322 mediated inhibition of T-cell derived IL-17A induced IL-6 release from HT-1080 cells, comparable to the control anti-IL-17A antibody secukinumab in the presence of excess of anti-TNF antibody adalimumab (Adal.) and anti-IL-1β antibody canakinumab (Cana.). Mean values of quadruplicates are shown, error bars represent standard deviations (SD).
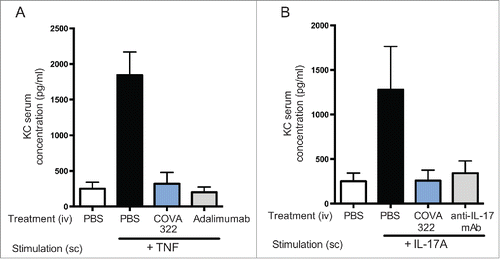
Serum concentrations at different time-points after a single intravenous (iv) injection of COVA322 in mice (Fig. S4) and cynomolgus monkeys () were determined by ELISA. COVA322 and 2 widely marketed anti-TNF antibodies (adalimumab and golimumab) had comparable serum concentrations in mice and cynomolgus monkey over several days, demonstrating that the fusion of a Fynomer to adalimumab did not have an effect on its pharmacokinetic properties. Adalimumab is known to be immunogenic in cynomolgus monkeys following a single iv dose of 5 mg/kg, resulting in a fast clearance after about 14 dCitation17 We also observed a drop in serum concentration after ∼2 weeks, suggesting a similar degree of immunogenicity to that of adalimumab. However, further studies are needed to elucidate the immunogenicity profile of COVA322, in particular in patients. Mice treated with human IL-17A or TNF upregulate the chemokine KC, which can be measured in serum.Citation14 Here, we investigated whether COVA322 can inhibit IL-17A- and TNF-mediated KC up-regulation in vivo. When injected iv, COVA322 completely abrogated KC induction by subcutaneously administered human IL-17A or TNF (), demonstrating the ability of COVA322 to inhibit human IL-17A and TNF in an in vivo environment.
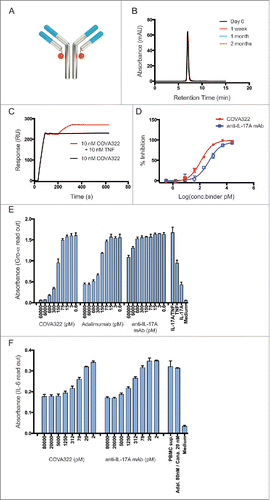
Figure 2. Plasma concentration of COVA322 in cynomolgus monkeys. (A) Bifunctional ELISA was performed to detect intact COVA322. Biotinylated TNF was immobilized in the wells of neutravidin-coated microtiter 96-well plates. Plasma containing COVA322 was added to the wells. For detection, digoxigenin-labeled IL-17A was used, followed by an anti-digoxigenin antibody-HRP conjugate for substrate processing and color development. Monofunctional ELISA was performed to detect specific TNF binding of COVA322, adalimumab or golimumab. Biotinylated TNF was immobilized in the wells of neutravidin-coated microtiter 96-well plates. Plasma containing COVA322 or anti-TNF antibody was added to the wells. TNF binding compound was detected using an anti-human IgG antibody-HRP-conjugate for substrate processing and color development. (B) Plasma concentrations of COVA322 and the anti-TNF antibodies adalimumab and golimumab at different time-points after a single iv injection into cynomolgus monkeys. The COVA322 or anti-TNF antibody concentration in plasma was determined by Bifunctional and Monofunctional ELISA, respectively. Mean plasma concentrations of 3 cynomolgus monkeys are plotted versus time, error bars represent standard deviations (SD). (C) Comparison of the plasma concentrations of COVA322 in cynomolgus monkeys determined by Bifunctional and Monofunctional ELISA. The plasma concentrations obtained from both assays are comparable, indicating that COVA322 is stable in cynomolgus monkeys for at least 220 hours.
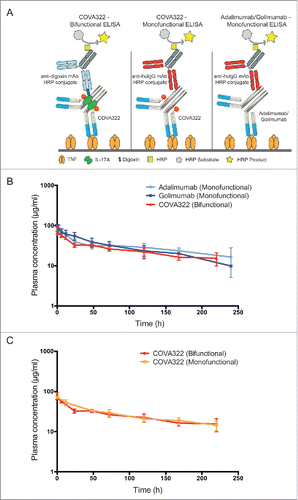
Figure 3. Inhibition of human IL-17A and TNF in vivo. Mice were injected intravenously (iv) with COVA322, anti-IL-17A antibody secukinumab or anti-TNF antibody adalimumab followed by subcutaneous injection of human TNF (A) or human IL-17A (B). Two hours after the administration of the indicated cytokine, blood samples were taken from the mice and KC levels were determined by ELISA. As a control, basal KC levels are shown (mice treated with PBS only (iv), without cytokine stimulation). Mean KC levels of 5 mice per group are shown (± SEM).
COVA322 was produced and purified under GMP conditions with very high yields (3.3 g/l obtained from a 1000 l GMP run). Of note, standard monoclonal antibody production protocols and upstream/downstream purification processes could be applied for the bispecific FynomAb COVA322. The overall process from gene synthesis until drug product (COVA322) took only 14 months, further demonstrating the robustness of the bispecific FynomAb platform. The pivotal nonclinical safety package to support the first-in-human study of COVA322 was a 4-week Good Laboratory Practice (GLP) repeat-dose toxicity study in cynomolgus monkeys testing weekly iv doses of 5, 25 and 100 mg/kg. Toxicokinetic analyses of COVA322 demonstrated IgG-like pharmacokinetics comparable to that of adalimumab in cynomolgus monkeys. Cmax and AUC increased in a dose-proportional manner, and the terminal disposition half-life calculated was ∼2–3 weeks. summarizes all important pharmacokinetic parameters. Importantly, COVA322 was well tolerated following weekly iv infusions in the GLP 4-week repeat-dose toxicity study in cynomolgus monkeys and the no observed adverse effect level (NOAEL) was 100 mg/kg, supporting the dose range for the currently ongoing Phase 1b/2a study in psoriasis patients.
Table 1. Summary of important pharmacokinetic parameters obtained from the non-clinical GLP safety study with cynomolgus monkeys
Discussion
Our research and several preclinical studies by others have recently demonstrated that dual treatment with inhibitors of TNF and IL-17 is significantly more efficacious than treatment with either agent alone.Citation7,8,18 However, one of the major risks for dual cytokine inhibition is immune suppression that is too strong, which may lead to an increased risk of infections and other adverse effects. So far, the results of clinical trials with combinations of biologics have been disappointing. A combination of anakinra and etanercept or the combination of abatacept and etanercept did not result in a significant benefit for the RA patients, but led to an increased infections risk.Citation19,20
Preclinical data indicate that dual TNF/IL-17A inhibition might have a better benefit/risk profile compared to the combinations tried in the past. Alzabin and co-workers showed that the administration of anti-TNF and anti-IL-17 compounds at low doses in a collagen-induced arthritis mouse model did result in a very significant anti-inflammatory effect, whereas the monotherapies had almost no effect, demonstrating that partial inhibition of both cytokines may be sufficient to provide clinical efficacy.Citation5 This finding supports the idea that, with a dual TNF/IL-17A inhibitor, it could be possible to dissociate clinical efficacy from immune suppression. To find the right balance between clinical efficacy and unwanted broad immune suppression, it will be crucial to elaborate the correct dose and the optimal degree of cytokine inhibition in clinical trials. Besides immune suppression, a key challenge for all therapeutic treatment options in inflammatory diseases is the achievement of remission. In a collagen-induced arthritis model in mice, Alzabin and colleagues demonstrated that the dual inhibition resulted in long-lasting responses, as mice were treated for 10 d after onset of disease and then observed for 40 d. At day 40, 7 out of 8 mice in the anti-TNF/IL-17 treatment group were still in remission.Citation5 Importantly, these preclinical studies were performed with a 1:1 ratio of anti-TNF/IL-17 antibodies, i.e., with the same binding site ratio as COVA322.
The concept of using bispecific antibodies was conceived more than 25 y ago. However, only 2 such molecules (catumaxomab,Citation21 and blinatumomab,Citation22) have been approved to date, mainly due to rather disappointing initial clinical studies. Low efficacy, severe adverse effects and immunogenicity were the main reasons bispecific antibodies failed in the past.Citation23 Manufacturing efficiency and appropriate in vivo pharmacokinetic properties were 2 additional major hurdles.Citation24 We achieved a production yield of more than 3 g/l in our 1000 L GMP run, which is, to our knowledge, one of the highest yields reported for a bispecific antibody. Because FynomAbs are fusion proteins of an antibody and a Fynomer, it is critical for the activity of the molecule that the Fynomer is not cleaved in vivo. In our animal experiments, we could demonstrate that COVA322 stays fully functional and intact for at least 10 d in cynomolgus monkeys, and there were no indications of in vivo degradation (e.g., cleavage of Fynomer entity from the antibody) (). The terminal disposition half-life calculated was ∼14–21 days, which is comparable to the estimated half-life of adalimumab in cynomolgus monkeys (adalimumab: EPAR – Scientific Discussion, dated 30th March 2006, online available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Scientific_Discussion/human/000481/WC500050867.pdf).
To obtain COVA322, we fused the Fynomer to the C-terminus of the light chain of the anti-TNF antibody using a standard 15 amino acid glycine serine (GGGGS)3 linker. Earlier work from our group with monospecific anti-IL-17A Fynomer-Fc fusions suggested that this linker length resulted in highest anti-IL-17A activity.Citation14 Indeed, analysis of the pharmacodynamic properties indicated that COVA322 is able to inhibit recombinant TNF and IL-17A with picomolar potency, and it inhibits T cell-derived human IL-17A. Additionally, COVA322 is able to inhibit both TNF and IL-17A in an acute inflammation model in mice. The results described herein further demonstrate that Fynomers represent ideal building blocks to generate bispecific FynomAbs in general.
In summary, by fusing the small (7 kDa) anti-IL-17A Fynomer to the clinically validated anti-TNF antibody adalimumab we generated COVA322, a bispecific FynomAb that may achieve superior efficacy compared to current standard of care in the treatment of inflammatory diseases. COVA322 is currently being evaluated in a first-in-man, single dose escalation, tolerability, safety, pharmacokinetic and efficacy Phase 1b/2a study in psoriasis patients.
Materials and Methods
Isolation and characterization of anti-IL-17A Fynomer 11L5B06
Using Fynomer phage libraries,Citation11 IL-17A-binding Fynomers were isolated using recombinant IL-17A (R&D Systems, 317-ILB-050) as antigen and standard phage display as the selection technology.Citation9,25 Fynomer 11L5B06 was expressed in E.coli bacteria and purified as described earlier.Citation9,25 Endotoxin was removed through filtration with the Acrodisc Mustang E membrane (VWR, MSTG25E3). After filtration, the endotoxin levels of the protein solution were less than 0.1 EU/ml, as determined by the Limulus amebocyte lysate (LAL) test (PYROGENT Single test Gel Clot LAL Assay (Lonza, N189-06)). For the IL-17A cell inhibition assay, 100 µl of a cell suspension containing about 3900 NHDF (PromoCell, NHDF-c, C12300) were distributed per well (96-well plate, TPP or Corning) and cultured for 24 hours at 37°C (medium: Fibroblast Growth Medium, PromoCell, C-23010). The supernatant was aspirated and after mixing different concentrations Fynomer 11L5B06 with IL-17A and TNF-containing medium (final concentrations 1 ng/ml and 50 pg/ml, respectively), 100 µl of the corresponding solution was added per well. As controls, phosphate-buffered saline (PBS) was mixed with the IL-17A/TNF-containing medium and medium with the single cytokines IL-17A or TNF only. As a negative control, PBS was mixed with medium only. After 24 hours incubation at 37°C the IL-6 concentration in the supernatant was determined using an IL-6 ELISA kit following the manufacturer's instructions (IL-6 ELISA kit, R&D Systems, DY206). The percentages of inhibition were plotted and IC50 values were calculated using the software Prism 5.
Cloning, expression and purification of COVA322
The anti-IL-17A Fynomer 11L5B06 was genetically fused to the C-terminus of the light chain of the anti-TNF antibody adalimumab via a 15 amino acid glycine serine linker (GGGGS)3 linker. The resulting bispecific FynomAb COVA322 was transiently transfected into FreeStyle CHO-S cells and expressed in serum-free/animal component-free media for 6–10 d (Evitria, Switzerland). The proteins were purified from the supernatants by Protein A affinity chromatography (MabSelect Sure column; GE Healthcare, 11-0034-94) and by SEC (Superdex G200, 30/100 GL column; GE Healthcare, 17-5175-01) on an Äkta Purifier instrument (GE Healthcare). SEC was performed via HPLC (1200 Infinity Series, Agilent) on an Agilent Bio SEC-5 column (Agilent, 5190-2528).
Affinity measurements to TNF and IL-17A
Affinity measurements were performed using a BIAcore T200 instrument (GE Healthcare). For the interaction analysis, a Series S CM5 chip (GE Healthcare, BR-1005-30) was coated with 8000 RU goat anti-human IgG antibody (Jackson ImmunoResearch, 109-005-098) using the amine coupling kit (GE Healthcare, BR-1006-33). The running buffer was PBS containing 0.05% Tween 20, and the interactions were measured at a flow of 30 μl/min. Twenty nM of COVA322 or adalimumab were captured on the anti-human IgG antibody-coated surface, followed by injections of the antigens TNF and IL-17A at different concentrations (0 nM, 1.56 nM, 3.125 nM, 6.25 nM, 12.5 nM, 25 nM, and 50 nM - TNF; 0 nM, 0.39 nM, 0.78 nM 1.56 nM, 3.125 nM, 6.25 nM, 12.5 nM, 25 nM - IL-17A). All kinetic data of the interactions were evaluated using the BIAcore T200 evaluation software.
Dual binding to TNF and IL-17A
Dual binding experiments were performed using a BIAcore T200 instrument (GE Healthcare). Human IL-17 was immobilized on a Series S CM5 chip (GE Healthcare, BR-1005-30) using the amine coupling kit (GE Healthcare, BR-1006-33). The running buffer was PBS containing 0.05% Tween 20, and the interactions were measured at a flow of 30 µl/min. Ten nM of COVA322 were captured on the IL-17A coated surface to show IL-17A binding. In addition, 10 nM of TNF was injected to demonstrate additional TNF binding.
IL-17A inhibition cell assay
The inhibitory potency of COVA322 was determined by stimulating NHDF with recombinant IL-17A in the presence of IL-1β, as these cytokines act in synergy. NHDF cells (PromoCell, NHDF-c, C12300) were seeded in 96-well plates (TPP) in Fibroblast Growth Medium (Promocell, C-23010). The cells were allowed to adhere. Supernatant was exchanged with medium containing 64 pM recombinant IL-17A and 10 fM recombinant IL-1β (R&D Systems, 201-LB-005), with and without different concentrations of COVA322 or a reference anti-IL-17A antibody (secukinumab). As negative control, equal volume of PBS was added to the medium. After 24h, supernatants were collected, filtered (0.45 μm pore size) and used to determine IL-6 concentrations by ELISA according to the manufacturers protocol (DuoSet, R&D Systems, DY206). Data points were measured in triplicates, percent inhibition calculated and IC50 values were determined using the GraphPad Prism 6 software (GraphPad Software). The data shown here indicate a representative result.
Simultaneous inhibition of IL-17A and TNF
HT-29 cells (ATCC, HTB-38) were cultured in McCoy's 5A medium (Invitrogen, 26600023) supplemented with 10% fetal bovine serum (HyClone). Serial dilutions of COVA322, adalimumab and the anti-IL-17A antibody were prepared in medium containing IL-17A (final concentration 400 pM) and TNF (final concentration 200 pM), and were distributed into 96-well plates. HT-29 cells were then directly distributed in the 96-well plates to each well, and were incubated 48 hr at 37°C and 5% CO2. After 48h, supernatants were collected, filtered (0.45 μm pore size) and used to determine GROα concentrations by ELISA according to the manufacturers protocol (DuoSet, R&D Systems, DY275). Data points were measured in triplicates. Data indicate a representative result. IC50 values were calculated using the GraphPad Prism 6 software (GraphPad Software).
Inhibition of human derived IL-17A
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat preparations (obtained from Blutspende Zurich, Switzerland) by Ficoll gradient centrifugation, and were stimulated to produce IL-17A as described in Gerhardt et al.Citation16 Briefly, PBMCs were stimulated at 5 × 106 cells/ml (final concentration) in flasks in the presence of anti-CD3/anti-CD28 (both at a final concentration of 1 μg/ml; eBioscience, 16-0037-85 and 16-0289-85, respectively), transforming growth factor β (5 ng/ml; R&D Systems, 240-B-002), IL-23 (50 ng/ml; R&D Systems, 1290-IL-010), anti-IFNγ antibody (10 μg/ml; R&D Systems, AB-285-NA), and goat anti-mouse IgG cross-linker (4 μg/ml, added 15 min after other reagents; Sigma, 71246). Supernatants were harvested by centrifugation after 48 h. The supernatants were then used to stimulate the production of IL-6 in HT-1080 cells (ATCC, CCL-121). HT-1080 cells were seeded overnight at 20,000 cells/well in media (DMEM (Invitrogen, 41966-029) supplemented with 10% fetal bovine serum (Hyclone), and 1×MEM-NEAA (Invitrogen, 11140035)). Serial dilutions of COVA322 and a reference anti-IL-17A monoclonal antibody (secukinumab) were prepared in media containing 40 nM adalimumab and 40 nM anti-IL-1β monoclonal antibody (canakinumab) (used to neutralize the effects of TNF and IL-1β on HT-1080 cells) and mixed in equal volume with conditioned PBMC supernatant and incubated at 37 °C for 1 h. Media were removed from the cells and replaced with COVA322 and anti-IL-17A antibody solutions, and the cells were incubated overnight. The experiment was performed in quadruplicates. Supernatants were removed, diluted 1:100 and analyzed with IL-6 ELISA (DuoSet, R&D Systems, DY206), in accordance with the manufacturer's instructions.
Comparison of in vitro IL-17A/A, IL-17A/F and IL-17F/F inhibition
The ability of COVA322 to inhibit IL-17A/A compared to the inhibition of IL-17A/F and IL-17F/F was determined in a similar cell assay as described in the section “IL-17A inhibition cell assay.” NHDF were stimulated with recombinant IL-17 in presence of IL-1β, as these cytokines act in synergy. NHDF cells (PromoCell, NHDF-c, C12300) were seeded in 96-well plates (TPP) in Fibroblast Growth Medium (Promocell, C-23010). The cells were allowed to adhere. Supernatant was exchanged with medium containing either 200 pM recombinant IL-17A/A, 4 nM IL-17A/F, or 10 nM IL-17F/F combined with 10 fM recombinant IL-1β (R&D Systems, 201-LB-005), with and without different concentrations of COVA322. As negative control, equal volume of PBS was added to the medium. After 24h, supernatants were collected, filtered (0.45 μm pore size) and used to determine IL-6 concentrations by ELISA according to the manufacturers protocol (DuoSet, R&D Systems, DY206). Data points were measured in triplicates, percent inhibition calculated and IC50 values were determined using the GraphPad Prism 6 software (GraphPad Software). The data shown here indicate a representative result.
Pharmacokinetic studies in mice
COVA322 or adalimumab were injected iv into 5 mice (C57BL/6, Charles River) at a dose of 10 mg/kg. After the indicated time-points, ∼20 μl of blood were taken from the vena saphena with the capillary Microvette CB 300 (Sarstedt). The blood samples were centrifuged for 10 min at 9500 × g and the serum was stored at −20° C until ELISA analysis was performed. Using dilution series with known concentrations of COVA322 or adalimumab, the concentration in serum was determined by ELISA using both TNF and IL-17A as capturing agents. 50 μl biotinylated IL-17A (50 nM) (R&D Systems, biotinylated using NHS-PEO4-biotin (Pierce, 21455) according to the manufacturer's instructions) or 50 μl biotinylated TNF (10 nM) were added to streptavidin-coated wells (Reactibind, Pierce) and, after blocking with PBS containing 4% milk (Rapilait, Migros, Switzerland), 50 μl of a dilution series of the serum samples were added. After incubation for 1 h and washing, bound COVA322 or adalimumab were detected with protein A-HRP conjugate (Sigma). Peroxidase activity was detected by addition of QuantaRed enhanced chemifluorescent HRP substrate (Pierce, 15159). This mouse study was approved by the Veterinaeramt des Kantons Zurich, Zurich, Switzerland.
Pharmacokinetic studies in cynomolgus monkeys
COVA322 or the reference antibodies adalimumab and golimumab were injected iv into 3 male cynomolgus monkeys at a dose of 3 mg/kg (Accelera S.r.l., Nerviano, Italy). Blood was taken from peripheral veins at the indicated time-points after end of dosing (0.6 ml of collection volume per sample). EDTA solution (7.5%) was used as anticoagulant. After blood collection, the samples were centrifuged at 4°C for 10 min at 1200 g, and plasma was stored at −80°C until analysis by ELISA.
“Bifunctional ELISA,” detecting intact and full-length COVA322: Neutravidin-coated wells (Biomat MCP44-11) were blocked with 150 μl/well of PBS, 5% BSA. The wells were washed with wash buffer (PBS, 0.1 % Tween 20) and then incubated with 100 μl/well of a solution containing 0.2 μg/ml of biotinylated TNF-α (PeproTech, biotinylated using a biotin labeling kit from ANP Technologies according to the manufacturer's instructions) diluted in assay buffer (PBS, 1 % BSA). After three washes with 200 μl/well of wash buffer, samples diluted 1:4 in assay buffer were dispensed to the wells (100 μl/well) and incubated for 1 hour under gentle shaking. The plate was washed 3 times (200 μl/well) with wash buffer prior to the addition of 100 μl/well of a solution containing 64 ng/ml of digoxigenylated IL-17A (PrepoTech, labeled with digoxigenin using a Digoxigenin Labeling Kit, purchased from Solulink, by following the supplier's recommended protocol) diluted in assay buffer. A further incubation for 1 hour at RT, under gentle shaking was then carried out. After 3 washes (200 μl/well) with wash buffer, the plate was incubated with 100 μl/well of the anti-digoxigenin HRP-conjugated antibody (Roche), diluted 1:10000 in Assay Buffer. The plate was washed 3 times (200 μl/well) with wash buffer and 100 μl/well of TMB (Sigma) were immediately added and incubated for 5-10 minutes at room temperature in the dark. The enzymatic reaction was stopped and optical density was measured at 450 nm using a Victor2V (Wallac Perkin Elmer) microtiter plate reader.
“Monofunctional ELISA,” detecting the anti-TNF binding antibody part of COVA322, adalimumab and golimumab: Neutravidin-coated 96-well plates (Biomat MCP44-11) were blocked with 150 μl/well of blocking buffer (PBS, 5 % BSA) for 60 minutes at RT under gentle orbital shaking set at 300 rpm. Wells were washed with 200 μl/well of wash buffer (PBS, 0.1 % Tween 20) and then incubated with 100 μl/well of a solution of 0.2 μg/ml of biotinylated TNF-α diluted in assay buffer (PBS, 1 % BSA) for 60 minutes at RT under gentle shaking. After three washes with 200 μl/well of wash buffer, samples diluted 1:4 in assay buffer were dispensed to the wells (100 μl/well) and incubated for 1 hour under gentle shaking. The plate was washed 3 times (200 μl/well) with wash buffer prior to the addition of 100 μl/well of a solution of 44 ng/mL of HRP-conjugated anti-Human IgG monkey-adsorbed antibody (Abcam) diluted in assay buffer. A further incubation for 1 hour at RT under gentle shaking was then carried out. The plate was washed 3 times (200 μl/well) with wash buffer, and 100 μl/well of TMB (Sigma) were immediately added and incubated for 5-10 minutes at room temperature in the dark. The enzymatic reaction was stopped and optical density was measured at 450 nm using a Victor2V (Wallac Perkin Elmer) microtiter plate reader.
In vivo pharmacodynamics activity of COVA322
Human IL-17A and human TNF bind and stimulate their murine receptors resulting in an increase of serum KC-chemokine (CXCL1) levels.Citation14 To test COVA322 in vivo, C57BL/6NCrl mice (Charles River) were injected iv with 40 μg COVA322, 40 μg secukinumab, 40 μg adalimumab or PBS only. One hour post administration, 3 μg/mouse of human IL-17A, or 0.25 μg human TNF, or PBS were injected subcutaneously. At two hours after treatment, blood was collected (Microvette CB 300, Sarstedt) and sera were stored frozen until analysis. Five mice were used per treatment group.
KC levels were determined using the Quantikine Mouse CXCL1/KC Immunoassay (R&D Systems, MKC00B) according to the manufacturer's instructions. Briefly, 25 μl sera were mixed with 25 μl calibrator diluent as described. Then, 50 μl sample diluent were added. Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software) and unpaired t test assuming Gaussian distribution. A p-value < 0.05 was considered as statistically significant. The mouse study was approved by the Veterinaeramt des Kantons Zurich, Zurich, Switzerland.
Non-clinical GLP safety studies
Toxicology studies were conducted at Covance Laboratories GmbH (Muenster, Germany) in accordance with GLP and International Conference on Harmonization guidelines. COVA322 was administered by iv infusion (over 30 minutes) to 3 groups (3 or 5/sex/group) of cynomolgus monkeys at weekly doses of 5, 25, and 100 mg/kg. Another group (4/sex) received vehicle and served as controls. At the end of the dosing phase, 2 animals per sex in the 100 mg/kg group and in the control group were maintained on study for a 17-week recovery period. Evaluation of clinical signs, body weight, body temperature, neurobehavioral observations, ophthalmology, cardiovascular function, respiratory rate, clinical pathology, immunophenotyping, natural killer cell function and cytokine/total complement concentration in serum were performed. Complete necropsies were performed on all animals with a recording of organ weights, macroscopic and microscopic abnormalities for all tissues. Finally, samples for toxicokinetic assessment were collected throughout the study. Toxicokinetic evaluation was performed by non-compartmental analysis using Phoenix WinNonlin version 6.3 (Certara Company, USA).
Disclosure of Potential Conflicts of Interest
All authors are or have been employees of Covagen AG.
Author Contributions
MS, WL, RW, WZ, NB, SB, RS isolated the anti-IL-17A Fynomer and characterized COVA322 in vitro. IAT, UvdB, SKF performed all mice experiments. WL, IAT, BSch and ML designed and executed preclinical development including non-clinical toxicology testing. MS, JB and DG wrote and edited the manuscript. MS, JB, DG designed and planned the study.
Silacci et al Supplemental Data
Download PDF (142.4 KB)Acknowledgments
We would like to thank Dr Nadja Prang for her valuable help and advise on the manufacturing process for COVA322.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Radner H, Smolen JS, Aletaha D. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Therapy 2014; 16:R56; http://dx.doi.org/10.1186/ar4491
- Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Therapy 2009; 11 Suppl 1:S1; http://dx.doi.org/10.1186/ar2666
- Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, Emery P, Gaujoux-Viala C, Gossec L, Nam J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014; 73:492-509; PMID:24161836; http://dx.doi.org/10.1136/annrheumdis-2013-204573
- Geiler J, Buch M, McDermott MF. Anti-TNF treatment in rheumatoid arthritis. Curr Pharmaceutical Design 2011; 17:3141-54; http://dx.doi.org/10.2174/138161211798157658
- Villeneuve E, Haraoui B. To switch or to change class-the biologic dilemma in rheumatoid arthritis. Nat Rev Rheumatol 2010; 6:301-5; PMID:20386564; http://dx.doi.org/10.1038/nrrheum.2010.45
- Benedetti G, Miossec P. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur J Immunol 2014; 44:339-47; PMID:24310226; http://dx.doi.org/10.1002/eji.201344184
- Koenders MI, Marijnissen RJ, Devesa I, Lubberts E, Joosten LA, Roth J, van Lent PL, van de Loo FA, van den Berg WB. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1beta, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum 2011; 63:2329-39; PMID:21520013; http://dx.doi.org/10.1002/art.30418
- Alzabin S, Abraham SM, Taher TE, Palfreeman A, Hull D, McNamee K, Jawad A, Pathan E, Kinderlerer A, Taylor PC, et al. Incomplete response of inflammatory arthritis to TNFalpha blockade is associated with the Th17 pathway. Ann Rheum Dis 2012; 71:1741-8; PMID:22550316; http://dx.doi.org/10.1136/annrheumdis-2011-201024
- Grabulovski D, Kaspar M, Neri D. A novel, non-immunogenic Fyn SH3-derived binding protein with tumor vascular targeting properties. J Biol Chem 2007; 282:3196-204; PMID:17130124; http://dx.doi.org/10.1074/jbc.M609211200
- Bertschinger J, Grabulovski D, Neri D. Selection of single domain binding proteins by covalent DNA display. Protein Eng Des Sel 2007; 20:57-68; PMID:17242027; http://dx.doi.org/10.1093/protein/gzl055
- Schlatter D, Brack S, Banner DW, Batey S, Benz J, Bertschinger J, Huber W, Joseph C, Rufer A, van der Klooster A, et al. Generation, characterization and structural data of chymase binding proteins based on the human Fyn kinase SH3 domain. MAbs 2012; 4:497-508; PMID:22653218; http://dx.doi.org/10.4161/mabs.20452
- Banner DW, Gsell B, Benz J, Bertschinger J, Burger D, Brack S, Cuppuleri S, Debulpaep M, Gast A, Grabulovski D, et al. Mapping the conformational space accessible to BACE2 using surface mutants and cocrystals with Fab fragments, Fynomers and Xaperones. Acta Crystallogr D Biol Crystallogr 2013; 69:1124-37; PMID:23695257; http://dx.doi.org/10.1107/S0907444913006574
- Brack S, Attinger-Toller I, Schade B, Mourlane F, Klupsch K, Woods R, Hachemi H, von der Bey U, Koenig-Friedrich S, Bertschinger J, et al. A bispecific HER2-targeting FynomAb with superior antitumor activity and novel mode of action. Mol Cancer Therapeutics 2014; 13:2030-9; http://dx.doi.org/10.1158/1535-7163.MCT-14-0046-T
- Silacci M, Baenziger-Tobler N, Lembke W, Zha W, Batey S, Bertschinger J, Grabulovski D. Linker length matters, fynomer-Fc fusion with an optimized linker displaying picomolar IL-17A inhibition potency. J Biol Chem 2014; 289:14392-8; PMID:24692552; http://dx.doi.org/10.1074/jbc.M113.534578
- Urech DM, Feige U, Ewert S, Schlosser V, Ottiger M, Polzer K, Schett G, Lichtlen P. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-TNF{α} single-chain Fv antibody (ESBA105) designed for local therapeutic use. Ann Rheum Dis 2010; 69:443-9; PMID:19293161; http://dx.doi.org/10.1136/ard.2008.105775
- Gerhardt S, Abbott WM, Hargreaves D, Pauptit RA, Davies RA, Needham MR, Langham C, Barker W, Aziz A, Snow MJ, et al. Structure of IL-17A in complex with a potent, fully human neutralizing antibody. J Mol Biol 2009; 394:905-21; PMID:19835883; http://dx.doi.org/10.1016/j.jmb.2009.10.008
- Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, Lowman HB, Fielder PJ, Prabhu S. Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{α} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab Dispos 2010; 38:600-5; PMID:20071453; http://dx.doi.org/10.1124/dmd.109.031310
- Fischer JA, Hueber AJ, Wilson S, Galm M, Baum W, Kitson C, Auer J, Lorenz SH, Moelleken J, Bader M, et al. Combined Inhibition of Tumor Necrosis Factor α and Interleukin-17 As a Therapeutic Opportunity in Rheumatoid Arthritis: Development and Characterization of a Novel Bispecific Antibody. Arthritis Rheumatol 2015; 67:51-62; PMID:25303306; http://dx.doi.org/10.1002/art.38896
- Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, Bekker P, Study G. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004; 50:1412-9; PMID:15146410; http://dx.doi.org/10.1002/art.20221
- Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, Chen D, Becker JC. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis 2007; 66:228-34; PMID:16935912; http://dx.doi.org/10.1136/ard.2006.055111
- Thomaidis T, Worns MA, Galle PR, Mohler M, Schattenberg JM. Treatment of malignant ascites with a second cycle of catumaxomab in gastric signet cell carcinoma - a report of 2 cases. Oncology Res Treatment 2014; 37:674-7; http://dx.doi.org/10.1159/000365597
- Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2014; 16(1):57-66; PMID:25524800; http://dx.doi.org/10.1016/S1470-2045(14)71170-2
- Muller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs 2010; 24:89-98; PMID:20199124; http://dx.doi.org/10.2165/11530960-000000000-00000
- Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 2007; 25:1290-7; PMID:17934452; http://dx.doi.org/10.1038/nbt1345
- Viti F, Nilsson F, Demartis S, Huber A, Neri D. Design and use of phage display libraries for the selection of antibodies and enzymes. Methods Enzymol 2000; 326:480-505; PMID:11036659; http://dx.doi.org/10.1016/S0076-6879(00)26071-0
