Abstract
Semaphorin 4D (SEMA4D or CD100) is a member of the semaphorin family of proteins and an important mediator of the movement and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. Blocking the binding of SEMA4D to its receptors can result in physiologic changes that may have implications in cancer, autoimmune, and neurological disease. To study the effects of blocking SEMA4D, we generated, in SEMA4D-deficient mice, a panel of SEMA4D-specific hybridomas that react with murine, primate, and human SEMA4D. Utilizing the complementarity-determining regions from one of these hybridomas (mAb 67-2), we generated VX15/2503, a humanized IgG4 monoclonal antibody that is currently in clinical development for the potential treatment of various malignancies and neurodegenerative disorders, including multiple sclerosis and Huntington's disease. This work describes the generation and characterization of VX15/2503, including in vitro functional testing, epitope mapping, and an in vivo demonstration of efficacy in an animal model of rheumatoid arthritis.
Introduction
Semaphorins are a family of soluble and membrane-bound proteins that were originally defined as axonal-guidance factors (for review, see RefCitation1) guiding the formation of precise connections between neurons and their appropriate target cells during development. Semaphorins have also been shown to affect numerous other physiological processes such as tissue repair, tumor progression, immune cell regulation, and vascular growth and migration. One such multifunctional semaphorin is Semaphorin 4D (SEMA4D or CD100), a 150-kDa transmembrane protein expressed predominantly on lymphocytes (for reviews, see RefCitation2-4). SEMA4D has a large N-terminal propeller-like “sema” domain followed by an Ig-like domain, a lysine-rich domain, a transmembrane domain, and a cytoplasmic tail with consensus tyrosine and serine phosphorylation sites. The molecule is expressed on the cell surface as a disulfide-linked homodimer (300-kDa), and upon cell activation the extracellular portion of SEMA4D can be released from the cell surface via proteolytic cleavage to generate a physiologically active, 240-kDa soluble form of the protein (sSEMA4D).Citation5 SEMA4D is thought to function as both a receptor, which signals through its cytoplasmic domain, and as a ligand.Citation6
SEMA4D, the first semaphorin determined to have immunoregulatory functions,Citation1,7 is expressed abundantly on the surface of most lymphocytes, including T cells, B cells, natural killer (NK) cells, as well as cells of the myeloid lineage such as monocytes, macrophages, and dendritic cells (DCs). T cells express the highest levels of surface SEMA4D and cellular activation stimulates up-regulation of surface expression of SEMA4D on B cells, T cells, and DCs, as well as the generation of soluble SEMA4D via cleavage of the extracellular domain. SEMA4D has also been reported to be present at low levels on the surface of platelets and SEMA4D surface expression increases with platelet activation and is followed by a gradual shedding of the extracellular domain.Citation8 Expression of SEMA4D has also been reported to be up-regulated on oligodendrocytes upon injury.Citation9 In addition, Smith et al., demonstrated that sSEMA4D is present in normal human sera at an average of 10.4 ng/ml, and that it is significantly elevated in multiple sclerosis (MS) patient sera (27.4 ng/ml).Citation10
Three cellular receptors have been identified for SEMA4D: PLXNB1, PLXNB2, and CD72. PLXNB1, the highest-affinity SEMA4D receptor (KD = 1 nM), is expressed on multiple cell types, including DCs, endothelial cells, and neural cells.Citation11 SEMA4D engagement with PLXNB1 has been shown to induce activation and migration of endothelial cells, and to promote migration of tumor cells.Citation12 SEMA4D/PLXNB1 signaling has also been reported to induce apoptosis of neural precursor cells, process extension collapse, and apoptosis of oligodendrocytes.Citation13 PLXNB2, whose best characterized ligand is SEMA4C, has an intermediate affinity for SEMA4D. A recent report indicates that PLXNB2 is expressed on keratinocytes and can activate SEMA4D-positive γδ T cells to contribute to epithelial repair.Citation14 CD72 is a relatively low-affinity (KD = 300 nM) SEMA4D receptorCitation15 expressed on B cells, antigen-presenting cells and platelets. In addition, CD72 is thought to act as a negative regulator of B cell responses by binding the tyrosine phosphatase SHP-1, which can recruit many inhibitory mediators. An interaction between SEMA4D and CD72 leads to the dissociation of SHP-1, and loss of this negative activation signal.Citation16
The results of various studies suggest that SEMA4D may play important physiological and pathological roles in the immune system. SEMA4D has been shown to promote T cell responses and B cell aggregation and survival in vitro. Addition of either SEMA4D-expressing cells or soluble SEMA4D enhanced CD40-induced B cell proliferation and immunoglobulin production in vitro, and accelerated in vivo antibody responses.Citation16,17 Also, the addition of sSEMA4D inhibited immune cell migration, and this effect was reversed by addition of anti-SEMA4D antibodies.Citation18,19
Experiments using SEMA4D-deficient (SEMA4D knockout (KO)) mice demonstrated that SEMA4D may play a non-redundant role in the immune system. SEMA4D knockout mice have reduced antibody responses to T-dependent antigens and impaired T cell priming; both of these functions were restored upon the administration of sSEMA4D.Citation20 Importantly, evaluation of anti-SEMA4D (VX15/2503) in a rat host resistance model demonstrated that the antibody was not immunosuppressive as it did not inhibit the clearance of flu virus from the treated animals.Citation21 In general, the immune suppressive effects of SEMA4D blocking antibody in vivo are much less pronounced than reported for genetic deletion of SEMA4D in embryonic development.
There is a strong rationale for treatment of solid tumors with VX15/2503. Semaphorins can guide migrationCitation18,22 and trigger cytoskeletal changes in endothelial, tumor, and immune cells within the tumor microenvironment (TME). Immunohistochemical analysis of SEMA4D and PLXNB1 expression patterns by several human tumor types revealed that SEMA4D is overexpressed in head and neck, prostate, colon, breast, and lung cancers, and that many of these tumors also express PLXNB1.Citation23 Moreover, elevated expression of SEMA4D or PLXNB1 correlates with invasive disease and poor prognosis.Citation24 SEMA4D may also be regulated by hypoxia and other cues within the TME.Citation25 In solid tumor animal models, expression of SEMA4D at the invasive tumor edge creates a barrier to immune infiltration and antibody-mediated SEMA4D blockade effectively facilitates access and amplification of anti-tumorigenic immune activity within the tumor. Evans et al. have demonstrated anti-SEMA4D-mediated tumor rejection in several murine tumor models, and increased efficacy in combination with checkpoint blockade inhibitors, corresponding with increased immune activity in the TME.Citation26
In addition to being an inhibitory mediator of immune responses and potential oncology target, SEMA4D is also involved in central nervous system (CNS) physiology and homeostasis. SEMA4D knockout mice are resistant to the development of experimental allergic encephalomyelitis (EAE), a murine model for human MS, due, at least in part, to a reduced generation of immune response to the EAE-inducing antigen.Citation27 Administration of an anti-SEMA4D antibody has been shown to attenuate EAE in multiple rodent models.Citation10,28 In addition to its involvement with the immune response to CNS antigens, SEMA4D may play an important role in several other critical physiological and potentially pathogenic CNS processes, including oligodendrocyte survival,Citation13 and remyelination.Citation10 SEMA4D inhibition may also preserve integrity of the neurovascular unit composed of endothelial cells and astrocytes by protecting endothelial tight junctions.Citation29 This could serve to not only reduce immune and inflammatory cell infiltration into the CNS, but also prevent activation of astrocytes and microglia, a process that has been implicated in a variety of neurodegenerative diseases.
Specific blockade of SEMA4D from interaction with its receptors represents a novel therapeutic strategy for cancer, MS, and other autoimmune and neuroinflammatory / neurodegenerative diseases. Here, we describe the discovery and preclinical characterization of an anti-SEMA4D antibody (VX15/2503) that is currently in clinical development.
Results
Generation of mouse anti-SEMA4D (mAb 67-2) and humanized anti-SEMA4D (VX15/2503)
It is advantageous for functional studies and non-clinical toxicology to generate mouse antibodies that are cross-reactive with mouse, rat, primate, and human SEMA4D. Not surprisingly, standard immunization techniques using wild-type mice did not elicit the desired species cross-reactive anti- SEMA4D response. We, therefore, immunized SEMA4D KO mice in an effort to bypass the tolerance mechanisms of normal mice. Approximately 96 parental hybridomas were selected that reacted with both mouse and human SEMA4D. A variety of assays were used to characterize these hybridomas for specificity and affinity, including enzyme-linked immunosorbent assay (ELISA; specificity, competition, and IC50) and flow cytometry. Generation of SEMA4D-specific antibodies mAb 67-2 and VX15/2503 is illustrated in , and described in detail in the Materials and Methods section.
Figure 1. Generation of mouse anti-SEMA4D (mAb 67-2) and humanized anti-SEMA4D (VX15/2503). SEMA4D KO mice were immunized with SEMA4D and mAb 67-2 was selected as the lead mouse anti-SEMA4D monoclonal antibody. This antibody was humanized and optimized to generate VX15/2503. See Materials and Methods section for details.

Affinity and species reactivity
ELISA, flow cytometry, and immunohistochemistry (IHC) all demonstrated high specificity of mAb 67-2 and VX15/2503 for SEMA4D (data not shown). In addition, binding studies by ELISA using both native and recombinant SEMA4D, and flow cytometric studies using peripheral blood mononuclear cells (PBMCs) confirmed that mAb 67-2 and VX15/2503 were reactive with soluble and cellular SEMA4D from all species tested; mouse, rat, rabbit, cynomolgus macaque, marmoset, rhesus macaque, and human (data not shown).
No binding was observed by ELISA against a panel of 8 to 10 additional irrelevant purified proteins. In addition, no binding was observed via flow cytometry on cell lines that were known to be negative for SEMA4D (data not shown). Affinity was determined quantitatively via Biacore and by a flow cytometry cell-based affinity assay; these results are shown in and . Affinity (KD) of both mAb 67-2 and VX15/2503 for SEMA4D via Biacore on recombinant antigens ranged from 1-5 nM while the affinity for native, cell-associated human SEMA4D was approximately 10-fold greater; KD = 0.45 nM (SEM = 0.13). ELISA and flow cytometry blocking assays confirmed that mAb 67-2 and VX15/2503 recognized the same epitope (data not shown).
Figure 2. Binding of VX15/2503 to native SEMA4D expressed on human peripheral blood T lymphocytes. PBMCs from normal human blood samples were utilized as targets to assess the affinity of VX15/2503 for native, cellular SEMA4D. Using flow cytometry, fluorescently labeled beads as a standard, and modified scatchard analysis, the average KD of VX15/2503 was calculated to be 0.45 nM (SEM = 0.13). The data shown are representative of the results obtained for VX15/2503 binding to CD3+ T lymphocytes from 5 different human PBMC samples.
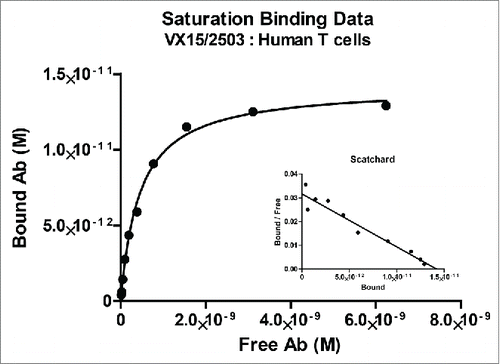
Table 1. Anti-SEMA4D Antibody Affinity for Recombinant SEMA4D1 Produced for Various Species
Functional in vitro characterization
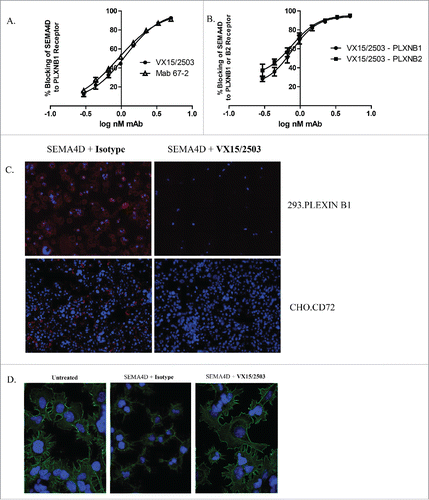
Since SEMA4D is a multifunctional molecule involved in various pathogenic processes, it was important to demonstrate that VX15/2503 blocks binding of SEMA4D to all 3 of its known receptors, PLXNB1, PLXNB2, and CD72. A flow cytometry-based functional assay was developed to quantitate blocking of SEMA4D binding by VX15/2503, a measure of the potency of this antibody. demonstrates that mAb 67-2 or VX15/2503 can block the binding of SEMA4D to PLXNB1 expressed on the surface of PLXNB1-transfected human 293 cells. In this assay, histidine-tagged (His-tagged) SEMA4D was pre-incubated with anti-SEMA4D antibodies at various concentrations and then added to 293.PLXNB1 cells. Cell-bound SEMA4D was detected via flow cytometry following staining employing an anti-6xHis-allophycocyanin (APC)-labeled antibody. Increasing amounts of anti-SEMA4D antibody correlate with a decrease in the amount of APC fluorescence due to a reduction of bound SEMA4D-His; this blocking activity can be quantitated and expressed as an EC50. This assay is currently employed as a potency release assay for VX15/2503 for which the mean EC50 is 1.2 nM. Similar results were observed when 293.PLXNB2 cells were used (). Functional blocking was also visualized by immunofluorescence with either 293.PLXNB1 or CHO.CD72 cells (), demonstrating that VX15/2503 can functionally block the interaction of SEMA4D with any of its 3 receptors. One of the functional consequences of blocking SEMA4D:PLXNB1 is inhibition of actin cytoskeletal “collapse” normally induced by the SEMA4D:PLXNB1 interaction. This is shown in and demonstrates that, in vitro, VX15/2503 can block the downstream functional consequences of SEMA4D interaction with one of its cognate receptors.
Figure 3. VX15/2503 functional blocking of SEMA4D with plexinB1, plexinB2, and CD72. A receptor blocking assay was developed that utilizes flow cytometry, recombinant soluble SEMA4D, and plexinB1 (A.) or plexinB2 (B.) receptor-transfected cells to quantitate blocking of SEMA4D by VX15/2503. Error bars represent intra-assay triplicates and similar assays have been repeated 2 times with similar results. (C.) An immunofluorescence blocking assay using 293.PLXNB1 and CHO.CD72 demonstrates that VX15/2503 completely blocks the binding of SEMA4D to either receptor. Transfected cells were allowed to adhere overnight, and then the antibody / recombinant his-tagged SEMA4D was added. Bound SEMA4D was detected with an anti-6xHis-APC tagged secondary antibody. Blue represents DAPI staining to visualize nuclei and red indicates SEMA4D staining. Representative images are shown and similar experiments were repeated 4x for each cell line. (D.) “Collapse” assay was performed to demonstrate the ability of VX15/2503 to block SEMA4D-induced actin rearrangement. Cells were allowed to adhere overnight and the antibody / recombinant his-tagged SEMA4D was added and then cells were fixed. Actin cytoskeleton and nuclei were stained using phalloidin-Alexa488 (green) and DAPI (blue). Representative images are shown; similar experiments have been repeated 3x with similar results.
Safety characterization
Blocking binding of SEMA4D to its receptors, as described above, was a desired function of VX15/2503. We also wanted to confirm that VX15/2503 did not elicit any untoward functional activity including immune cell depletion or immune cell activation.
VX15/2503 was constructed using a human IgG4 isotype backbone to minimize potential depletion of SEMA4D-expressing immune cells.Citation30 Any residual depleting activity of VX15/2503 was assessed in vitro in complement-mediated cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC) assays. As shown in , VX15/2503 did not elicit CDC or ADCC on SEMA4D-positive targets. In vivo data showing similar results in toxicology studies were published previously.Citation21
Figure 4. Characterization of VX15/2503 stability and in vitro toxicity. To assess in vitro killing, VX15/2503 was tested in non-radioactive CDC (A) and ADCC (B) assays by using alamar blue or the a-Cella tox kit, respectively. Rituximab (Rituxan®) was utilized as a positive control since the Daudi target cells were both CD20 and SEMA4D positive. To assess in vivo half antibody formation, VX15/2503 (Anti-SEMA4D S228P) or VX15/2503 without the S228P mutation (Anti-SEMA4D WT) were injected into SCID mice with additional control IgG4 antibodies (anti-C35) and serum was tested in a bispecific antibody ELISA (C) 48 hours later. (D) Human PBMCs were incubated with VX15/2503 or control mAbs and cytokines were measured using multiplex CBA analysis.
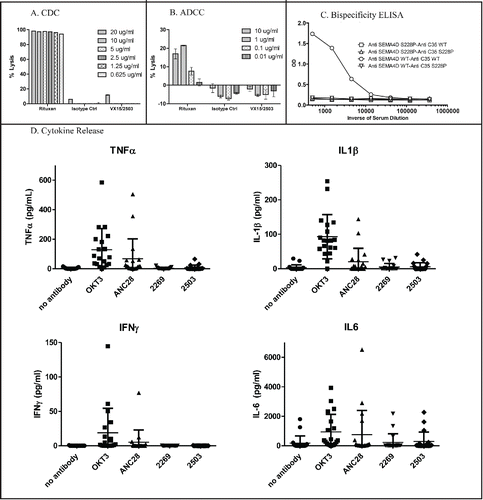
Antibodies of human IgG4 isotype are prone to form half-antibody structures due to the flexibility in the hinge region where the disulfide bonds form. In order to reduce this possibility, a serine to proline mutation is often introduced at position 241 (S241P) in the hinge region to “stabilize” it.Citation31 VX15/2503 contains this mutation and, based on published results, should not form half or bispecific antibodies. An in vivo study in SCID mice was done to demonstrate that VX15/2503 does not form bispecific antibodies in vivo. Mice were injected with wild-type or S241P mutant versions of VX15/2503 and a control antibody (anti-C35). Serum was obtained and analyzed for the presence of bispecific antibodies using a bispecific ELISA. Positive control for the experiment was sera from mice injected with a wild-type version of VX15/2503 and a wild-type (no S241P mutation) anti-C35. demonstrates that bispecific antibodies were generated in the positive control mice, but not in any other combination, including VX15/2503 (anti-SEMA4D S241P) with wild-type IgG4 anti-C35, which would most closely represent the clinical situation.
Given the Tegenero TGN1412 incident in 2006, when a life threatening cytokine release syndrome occurred during a first-in-human study,Citation32 it has become increasingly important to test new human therapeutic antibodies (especially those that bind to immune cells) for their potential to induce cytokine release in vitro. Using an in vitro method similar to Romer et al,Citation33 VX15/2503 was evaluated in a cytokine release assay after incubation with human PBMCs from 19 different healthy donors. As shown in , even though positive control antibodies OKT3 (anti-CD3) and ANC28 (anti-CD28) induced release of tumor necrosis factor (TNF)α, interleukin (IL)1β, interferon (IFN)γ, and IL6 when incubated with human PBMCs, VX15/2503 did not cause a significant cytokine release in 19 donors as compared to the IgG4 isotype control antibody. In addition, VX15/2503 did not elicit a proliferative response in human PBMCs from 6 different donors when assayed using carboxyfluoroscein succinimidyl ester (CFSE) (data not shown). Finally, a GLP immunohistology study (data not shown) was conducted to characterize the reactivity pattern of VX15/2503 with a panel of 33 fresh-frozen human tissues. No significant off-target binding was noted. This work demonstrated that lymphoid cells were recognized by the antibody in human blood, gastric antrum, gastric body, duodenum, ileum, colon, lymph node, spleen, tonsil, and uterus (endometrium). In addition, the glandular epithelium of one of 3 human donor uteri was reactive with VX15/2503.
Epitope mapping
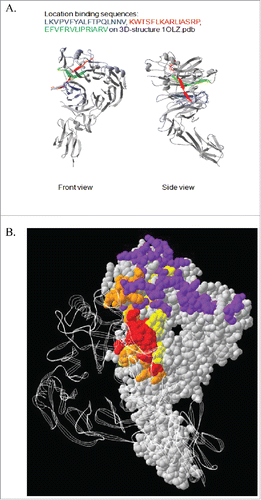
To define the SEMA4D epitope recognized by VX15/2503, epitope mapping was performed as described in the Materials and Methods section. The epitope defined appeared to be formed by a non-linear, discontinuous sequence. As shown in , 3 amino acid sequences in human SEMA4D were identified that putatively comprise the discontinuous epitope of VX15/2503. These sequences are LKVPVFYALFTPQLNNV, KWTSFLKARLIASRP, and EFVFRVLIPRIARV. All three sequences are part of the SEMA domain of the protein and, when analyzed in conjunction with the crystal structure of SEMA4D,Citation34 they are all located within one larger region that can form a single discontinuous conformational epitope on SEMA4D. Additional fine mapping using alanine scanning suggested that the most important of these 3 peptides was likely EFVFRVLIPRIARV. Interestingly, this peptide, as well as the other 2, is located within the homodimerization site of SEMA4D. Since homodimerization is known to be important in semaphorin-plexin signaling, this suggests VX15/2503 could interfere with homodimerization as a potential mechanism to block SEMA4D signaling. Furthermore, this peptide is also located at the border of the binding interface of SEMA4D and its ligand, PlexinB1,Citation35 suggesting VX15/2503 could also sterically hinder SEMA4D-PlexinB1 interactions. The cumulative epitope mapping data for VX15/2503 supports the conclusion that the epitope recognized is a functionally relevant region of SEMA4D.
Figure 5. Epitope Mapping of VX15/2503 : SEMA4D interaction. Epitope mapping studies were performed utilizing CLIPS Epitope Mapping technology at Pepscan. Using the entire protein sequence of human SEMA4D, surface immobilized, spatially constrained peptide microarrays, and an ELISA based detection method using CDD camera quantification, the SEMA4D protein was examined for either linear or conformational epitopes of VX15/2503. Three amino-acid sequences were identified (A.) that putatively comprise the discontinuous epitope of VX15/2503. (B.) A combination ribbon / space-filling structural diagram of a SEMA4D dimer that shows the VX15/2503 epitope overlapping the dimerization and plexin-binding domains; colors indicate PLXNB1 binding site, SEMA4D dimerization domain, VX15/2503 epitope, Overlap between VX15/2503 epitope and SEMA4D dimerization domain.
Internalization
Antibody-induced shedding or internalization of a target antigen can have important pharmacokinetic and pharmacodynamic consequences.Citation36 To address this, we tested human PBMCs in vitro for evidence of SEMA4D internalization or shedding after addition of VX15/2503. As previously mentioned, SEMA4D exists in both cell membrane-associated and soluble cell-free forms. To address the question of whether cellular SEMA4D (cSEMA4D) is released from the cell or internalized following binding by the anti-SEMA4D antibody VX15/2503, we incubated human PBMCs with VX15/2503 in vitro and used flow cytometry and fluorescent microscopy to measure internalization. Our results suggest that the VX15/2503 - SEMA4D complex is internalized and does not appear to be released as a soluble form. These results were similar to those reported by Beers, et alCitation37 with regard to the fate of CD20 following exposure to rituximab (Rituxan®). shows that quenched samples of cultured peripheral blood T cells exhibited an increase in the mean percent of SEMA4D internalization over time in the presence of VX15/2503, reaching a maximum of roughly 60% after 24 hours (). The kinetics of internalization appear to be similar among the 3 donors tested (data not shown). These results were confirmed by fluorescence microscopy using the same samples (); similar results were also obtained using rat and cynomolgus macaque PBMCs incubated with VX15/2503 (data not shown).
Figure 6. Internalization of VX15/2503 : SEMA4D complex upon binding cellular SEMA4D. PBMCs were incubated with Alexa 488 fluorochrome-labeled VX15/2503 or human IgG4 isotype control antibody at 37°C for up to 24 hours. The cells were washed to remove labeled antibody and incubated at 4°C in the presence or absence of an anti-Alexa 488 quenching antibody. Samples were subsequently stained with anti-human CD3-Alexa 647 to label CD3+ T cells and analyzed via flow cytometry. The quenching antibody blocks any fluorescent signal from the cell surface, so any signal remaining after quenching represents internalized antigen. A comparison was made between the GMFI values from the quenched and unquenched samples; this allowed for a calculation of the percent SEMA4D internalized using GMFI values from the Alexa 488 channel. In (A.), the % internalization data represent the mean internalization from 3 different human donors. Microscopy and imaging was performed to visualize internalization (B.); images were pseudocolored to represent DAPI (blue), CD3 (red), and VX15/2503 (green) and 2 of 4, 60X representative images are shown stained with either isotype or VX15/2503. The supernatant from the same experiment was analyzed for levels of total soluble SEMA4D (free or bound by VX15/2503) and those levels are shown for each condition in the table in part (C). The ELISA data represents the mean from 2 different human donors and similar results were obtained in a second experiment (data not shown), <LLQ indicates [sSEMA4D] <0.08 ng/ml.
![Figure 6. Internalization of VX15/2503 : SEMA4D complex upon binding cellular SEMA4D. PBMCs were incubated with Alexa 488 fluorochrome-labeled VX15/2503 or human IgG4 isotype control antibody at 37°C for up to 24 hours. The cells were washed to remove labeled antibody and incubated at 4°C in the presence or absence of an anti-Alexa 488 quenching antibody. Samples were subsequently stained with anti-human CD3-Alexa 647 to label CD3+ T cells and analyzed via flow cytometry. The quenching antibody blocks any fluorescent signal from the cell surface, so any signal remaining after quenching represents internalized antigen. A comparison was made between the GMFI values from the quenched and unquenched samples; this allowed for a calculation of the percent SEMA4D internalized using GMFI values from the Alexa 488 channel. In (A.), the % internalization data represent the mean internalization from 3 different human donors. Microscopy and imaging was performed to visualize internalization (B.); images were pseudocolored to represent DAPI (blue), CD3 (red), and VX15/2503 (green) and 2 of 4, 60X representative images are shown stained with either isotype or VX15/2503. The supernatant from the same experiment was analyzed for levels of total soluble SEMA4D (free or bound by VX15/2503) and those levels are shown for each condition in the table in part (C). The ELISA data represents the mean from 2 different human donors and similar results were obtained in a second experiment (data not shown), <LLQ indicates [sSEMA4D] <0.08 ng/ml.](/cms/asset/00bba257-f704-4569-b497-7051c5660ef8/kmab_a_1102813_f0006_c.gif)
To evaluate the presence of soluble SEMA4D in the culture fluids of human PBMCs incubated with VX15/2503, we utilized a total extracellular SEMA4D ELISA that detects both free sSEMA4D as well as the VX15/2503-sSEMA4D complex (Leonard et al, submitted Clinical Cancer Research). Shedding of sSEMA4D or VX15/2503: sSEMA4D complex following incubation of cells with VX15/2503 would result in an increased ELISA signal. As shown in , the results indicate that no VX15/2503-mediated shedding occurred even though human PBMC controls (untreated, or sham-treated cultures) did secrete sSEMA4D into the culture fluid over time. Indeed the data denote that, following treatment with VX15/2503, the amount of total sSEMA4D detected in the culture medium actually decreased compared to controls, reaffirming that cellular SEMA4D was internalized following antibody exposure. These cumulative data suggest that cell membrane-associated SEMA4D is internalized, rather than shed, following binding by VX15/2503.
CIA
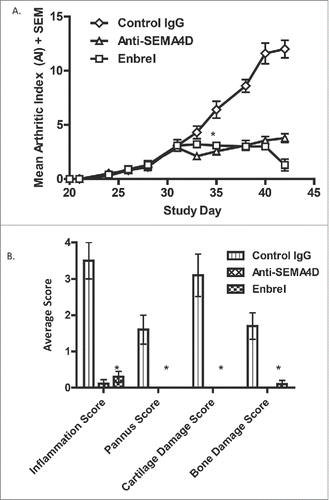
Anti-SEMA4D has been demonstrated to be efficacious in ameliorating disease in several animal models of oncology,Citation26 MSCitation10 and Huntington's disease.Citation38 We also tested the role of SEMA4D in a therapeutic model of collagen-induced arthritis (CIA) in mice. Disease was induced by immunization with collagen / complete Freund's adjuvant (CFA) and with a boost of collagen/ incomplete Freund's adjuvant (IFA) on Day 21. Treatment was initiated with either mAb 67-2, isotype control antibody, or etanercept (Enbrel®; an approved fusion protein therapeutic for rheumatoid arthritis (RA)) 10 days later, when mice had reached a mean arthritic index (AI) score of 3. As shown in , anti-SEMA4D treatment reduced clinical scores and inhibited the progression of the disease to a degree similar to Enbrel®. In addition, anti-SEMA4D administration was very effective at reducing the score of several common histological parameters, including inflammation, pannus formation, and cartilage / bone damage ().
Figure 7. Treatment with anti-SEMA4D inhibits progression in a murine model of collagen-induced arthritis. (A.)To induce disease, DBA/1J mice were immunized subcutaneously (s.c.) on day 0 with an emulsion of collagen / CFA and challenged (s.c.) on day 21 with collagen / IFA. Treatment with either control IgG, mAb 67-2 (anti-SEMA4D), or Enbrel® 2x/week was started after mice reached an AI of 3 (day 31). (B.) Mice were sacrificed at endpoint (day 42) and paws were fixed, stained with toluidine blue and analyzed and scored histologically as described in the Material and Methods section. * Error bars represent S.E.M. and significance was calculated versus the control Ig group using Student's t-test, p≤0.001 for both Anti-SEMA4D and Enbrel groups for day 35 and later in 7A and at day 42 in 7B.
Discussion
SEMA4D is a multifunctional target involved in a variety of physiological systems. Blocking the activity of this molecule represents a novel therapeutic strategy. Our results describe the generation and preclinical characterization of the first humanized anti-SEMA4D monoclonal antibody (mAb) that is now being evaluated in clinical trials.
Mouse mAbs reactive with mouse antigens are often needed for early preclinical proof-of-concept studies in development programs. It is, however, often difficult to select such antibodies due to inherent tolerance mechanisms. Here, we demonstrate the use of KO mice and a unique immunization protocol to generate functional mouse anti-SEMA4D antibodies. A combination of immunization with recombinant protein in adjuvant and transfected live tumor cells worked effectively to generate functional mAbs, perhaps owing to the different form of antigen in each immunization. Although uncommon to utilize live tumors as an immunogen for generation of mAbs, it has previously been shown that injection of mice with live tumor cells expressing a foreign antigen can effectively induce immune responses.Citation39,40
The work reported here also illustrates several important steps in the translational progression of a mouse mAb from discovery to the clinic. For optimal transition from mouse studies through GLP toxicology studies and into the clinic, it is ideal that the mAb selected binds to the target antigen from rodent, primate and human tissues with similar affinities so that the antibody binding profile in humans can be more accurately estimated. As illustrated for VX15/2503 and SEMA4D, both recombinant and native antigens should be tested, as well as soluble and membrane bound forms. Affinity was determined via Biacore employing recombinant SEMA4D and by flow-based methods for native cell surface associated antigen. In the case of VX15/2503, it appears that the affinity for native, membrane-bound antigen (app. 0.5 nM) is higher than for recombinant soluble antigen (1-5 nM). It is unknown at this stage whether this is due to conformational differences between soluble and membrane bound SEMA4D or different conformational or posttranslational modifications of recombinant and native SEMA4D. Another possibility is that the cell-based assay measures avidity more than affinity, but we used rituximab (Rituxan®) in this assay as a control antibody and obtained a result (KD = 9.95nM) very close to the published “affinity” from the product insert (8 nM).
To minimize depletion of SEMA4D-expressing target cells, a human IgG4 isotype backbone was utilized.Citation30 VX15/2503 was tested in vitro () and demonstrated absence of ADCC or CDC. In the specific case of VX15/2503 and SEMA4D, the use of an IgG4 isotype was a precautionary safety measure since CDC/ADCC was not detected in vitro even with other anti-SEMA4D antibody isotypes, including a human IgG1 version of VX15/2503 or the original mouse IgG1 antibody, mAb 67-2 (data not shown). The IgG4 isotype has the potential for inter-antibody exchanges that can result in formation of bispecific antibodies,Citation31 which can result in unwanted PK or PD, and immunogenicity concerns. In order to reduce this possibility, we incorporated the previously described S241P mutationCitation41 in the hinge region of VX15/2503 and confirmed that this molecule does not form bispecific antibodies in vivo.
In addition to a full GLP toxicology package for VX15/2503,Citation21 we examined cytokine release by in vitro analysis using human PBMCs as an additional safety measure. We employed a modified version of the assay described by Romer et al,Citation33 which demonstrated low background and ample signal-to-noise ratio. Indeed, patients dosed with VX15/2503 have not shown any signs of cytokine release syndrome, with 500 doses having been delivered in total in Phase 1 oncology and MS clinical studies.
The blocking activity of VX15/2503 was demonstrated in vitro by showing that the antibody can block target:receptor complex formation, as well as the functional consequences of such binding. VX15/2503 blocks the binding of SEMA4D to any of its 3 receptors, PLXNB1, PLXNB2, or CD72, and inhibits SEMA4D-induced cytoskeletal reorganization in a cell collapse assay. These robust functional assays provided the framework for subsequent potency assays. The assay described in has been validated and utilized successfully as a GMP potency assay for VX15/2503 antibody release and stability. It is interesting to note that the average EC50 of VX15/2503 is 1.2 nM, very similar to the affinity (KD).
We also explored the biology of the interaction of VX15/2503 with SEMA4D via epitope mapping and internalization experiments. Since VX15/2503 does not detect SEMA4D in western blots, the epitope may be conformational rather than linear. We, therefore, employed a more advanced CLIPS epitope mapping technology.Citation42 Detailed knowledge of an antibody's binding site is important during the development process and can provide information that helps delineate the molecular mechanism of action. Results of our SEMA4D epitope mapping for VX15/2503 () suggest that VX15/2503 interferes with SEMA4D-receptor interactions by both binding to the SEMA4D homodimerization domain and by sterically interfering with the PLXNB1 binding site. We also confirmed that, as expected, the murine mAb 67 binds the same SEMA4D epitope as VX15/2503.
Based on the results of completed animal studies of mAb 67-2 showing anti-tumor activity in various tumor models,Citation26 disease modification results in EAE,Citation10 and a murine model of Huntington's disease,Citation38 the anti-SEMA4D antibody VX15/2503 is being investigated clinically in several indications, including oncology, MS, and Huntington's disease. Additionally, it was recently reported that a commercial rat anti-SEMA4D antibody could ameliorate symptoms of arthritis in a CIA mouse model of inflammatory arthritis.Citation43 Our data support this observation and further demonstrate that the murine version of an antibody currently in clinical development can also ameliorate the severity of CIA. Anti-SEMA4D worked as well as an approved therapeutic (Enbrel®) to reduce the mean arthritic index and joint histology disease endpoints, including inflammation, pannus destruction, and cartilage and bone damage in a CIA mouse model. The exact mechanism of action is likely multifaceted since SEMA4D could be relevant at several stages of the autoimmune process. SEMA4D is expressed by T cells, one of the effector cells in arthritis, and regulates immune invasion and migration.Citation44 Also, SEMA4D-deficient mice are resistant to the development of other autoimmune diseases such as EAE.Citation27 Importantly, SEMA4D is produced by osteoclasts and can suppress bone formation by PLXNB1-expressing osteoblasts,Citation45 which may play a large role in the progression of RA.Citation46 These observations and the data presented here suggest that SEMA4D may also represent a possible therapeutic target for the treatment of RA.
Overall, this work highlights the discovery and initial characterization of an anti-SEMA4D antibody, VX15/2503, currently in clinical development for oncology, MS, and Huntington's disease. This mAb has been extensively characterized in numerous preclinical, functional, toxicology, and disease model studies. The multiple functions of SEMA4D in basic physiology and disease suggest that blockade of SEMA4D may serve as a unique treatment strategy in additional autoimmune disease indications, including RA.
Materials and Methods
Generation of mouse anti-SEMA4D and humanized anti-SEMA4D
To generate mouse anti-SEMA4D mAbs, BALB/c mice (Jackson Labs, ME) were immunized with both recombinant antigen and live tumor cells. The recombinant antigen was purified from the supernatant (SN) of Chinese hamster ovary (CHO) cells stably transfected with mouse or human SEMA4D- His (the extracellular domain of SEMA4D with a C-terminal 6X his tag for purification). The Line 1 cell line (derived from a spontaneous lung tumor in a BALB/c mouse) was transfected with an expression plasmid encoding the full-length human SEMA4D cDNA and a stable line expressing human SEMA4D was isolated. SEMA4D-deficient mice on the BALB/c background were primed by immunization with 50 μg purified mouse SEMA4D-His emulsified in CFA. One week following this immunization, the mice were injected intramuscularly with 20,000 live Line1-human SEMA4D cells. Serum anti-SEMA4D titer (reactive with both mouse and human recombinant SEMA4D) was determined 13 days post tumor injection; 19 days after Line1-human SEMA4D injection, mice were sacrificed and splenocytes harvested and fused with the P3X63Ag8.653 fusion partner (ATCC# CRL-1580) following standard procedures to generate hybridomas. Hybridoma clones were screened by ELISA for binding to human and mouse SEMA4D.
A large panel of mouse SEMA4D specific antibodies was generated. Most of these antibodies also bind with high affinity to human SEMA4D. mAb 67-2 was chosen as the progenitor antibody for the generation of a humanized anti-SEMA4D antibody. Prior to generating a humanized antibody, the VH and VK genes were cloned from the clone 67-2 hybridoma (producing murine mAb 67-2) and their sequences were determined. The VH gene was cloned into a mammalian expression vector that contained the human gamma 4 heavy chain constant region coding sequence, creating a full-length chimeric heavy chain. The VK gene was cloned into a mammalian expression vector containing the human kappa constant region coding sequence, creating a full-length chimeric light chain. To produce the chimeric antibody, the expression vectors containing the chimeric heavy chain and the chimeric light chain were co-transfected into CHO-S cells (a clonal isolate of CHO). The mAb that was produced was secreted from the cells and harvested after a 3-to 6-day expression period. The resulting mAb was purified using protein A, and characterized. This chimeric mAb (mAb 2368) was demonstrated to be specific for SEMA4D by flow cytometry and ELISA, and blocked binding of the murine mAb 67-2 to SEMA4D. Collectively, these data demonstrated that the correct VH and VK genes encoding the 67-2 hybridoma mAb were isolated.
Subsequently, the mAb 67-2 variable region genes were employed to create a humanized mAb. In the first step of this process, amino acid sequences of the VH and VK of mAb 67-2 were compared against the available database of human immunoglobulin gene sequences in order to define optimally matched human germline immunoglobulin gene sequences. For the light chain, the best human match was the VK4-B3 gene, and, for the heavy chain, the best human match was the VH1-69 gene. Humanized variable domain sequences were then designed where the CDR1, 2 and 3 of the 67-2 light chain were grafted onto framework sequences of the VK4-B3 gene, and the CDR 1, 2 and 3 sequences of the 67-2 VH were grafted onto framework sequences of the VH1-69 gene. A conceptual 3D model was then generated to identify framework positions where retention of the mouse amino acid would potentially be advantageous for stability or binding. The humanized VH and VK genes were generated synthetically and subsequently cloned into vectors containing the human gamma 4 and human kappa constant domains respectively. The pairing of the human VH and the human VK created the IgG4 kappa mAb VX15/2503. VX15/2503 was constructed as a human IgG4 to avoid Fc-mediated effector functions and possible resulting depletion of SEMA4D-positive cells. The serine at position 241 in the core hinge region of the IgG4 domain of VX15/503 was mutated to introduce a proline, a modification previously shown to eliminate Fab-arm exchange.
SEMA4D affinity determinations
Surface Plasmon Resonance (Biacore): Surface plasmon resonance kinetic analysis was performed using a Biacore 2000 system with a CM5 sensor chip and HBS-EP running buffer (HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v Surfactant P20). Goat anti-mouse IgG Fc (for mAb 67-2) or goat anti-human IgG Fc (for VX15/2503) (Jackson ImmunoResearch) was immobilized on the chip surface via amine coupling. mAb 67-2 or VX15/2503 was captured and recombinant antigen was injected at a concentration range from 50 to 0 nM; samples were assayed in duplicate. The dataset was evaluated using BiaEvaluation software and globally fit to a 1:1 model. Sensograms were subjected to reference surface subtraction and subtraction of buffer response (zero analyte concentration). Recombinant 6x histidine-tagged SEMA4D was expressed either transiently or as a stable transfectant in CHO cells and was purified from culture supernatants using a standard Ni column.
Affinity for Cellular SEMA4D: A flow cytometry-based method similar to Heider et alCitation47 was used to measure the affinity of VX15/2503 for SEMA4D expressed on the surface of CD3+ T cells from human PBMCs. Human PBMCs were isolated from whole blood and incubated with various concentrations of VX15/2503 for 1 hour at 4°C, washed, and then incubated with a monoclonal FITC-anti human IgG4 Fc secondary antibody for 30 minutes at 4°C. Quantum FITC MESF beads (Bang's Labs) were utilized to generate a standard curve to convert geometric mean fluorescence intensity (GMFI) to molecules of equivalent soluble fluorochrome (MESF). A modified Scatchard analysis was employed by using GraphPad PRISM® and nonlinear saturation analysis to calculate the binding affinity (KD) of VX15/2503 to cellular SEMA4D. Five different human PBMC samples were assayed.
Flow blocking assay
To measure the ability of VX15/2503 to block the binding of SEMA4D to its receptor PLXNB1, a titration of VX15/2503 or mAb 67-2 antibody from 1.5 μg/mL to 88 ng/mL was combined with 0.8 ng/mL marmoset SEMA4D-his overnight. Recombinant marmoset SEMA4D protein is produced and purified from a stably transfected cell line that has been banked and well characterized. This protein was utilized more than the human protein in these characterization assays mainly due to reagent production rates and availability, and significant differences have not been observed with human versus marmoset recombinant protein in these assays.
The next day the mAb/SEMA4D-his was added to 2 × 106/ml 293PLXNB1 cells in a 96-well round-bottom tissue culture plate (Costar) and incubated for 30-45 minutes at 4°C to allow binding of SEMA4d-his to 293PLXNB1 cells. After washing, cells were stained with anti-6xHis-APC (Abcam, Ab72579) at 1:50 dilution for 30 minutes. Cells were then washed 2x to remove unbound antibody, and stained with propidium iodide (PI) (Sigma) immediately prior to analysis to discriminate dead cells in sample. Cells were acquired on a FACS Canto flow cytometer, gating on the PI-negative cell population. Analysis of APC fluorescence was completed using FlowJo flow cytometry software and APC GMFI was used to calculate % blocking of SEMA4D-his binding on 293PLXNB1 cells. GraphPad Prism software was used for calculation of EC50 values.
Immunofluorescent blocking assays using 293.PLXNB1 or CHO.CD72
Immunofluorescence of SEMA4D Binding to 293.PLXNB1: Twenty-thousand 293F cells stably transfected with human PlexinB1 were seeded onto 48-well Poly-D-Lysine plates (BD BioCoat™) in 200 µL complete medium and allowed to adhere overnight at 37°C, 5% CO2. Recombinant marmoset SEMA4D-His was diluted to 40 µg/mL alone or with 200 μg/ml blocking antibody in complete medium and allowed to equilibrate at room temperature for 30 minutes. Ten microliters of SEMA4D/antibody mixture was added per well of 293F.PlexinB1 cells to give a final concentration of 2 µg/ml SEMA4D +/− 10 μg/ml antibody and allowed to incubate at 37°C, 5% CO2 for 30 minutes. Media and reagents were removed from the wells and 200 µL of anti-6xHis-APC (Abcam, AD1.1.10, 2 µg/mL) in complete medium was overlaid and allowed to bind at room temperature under foil for 30 minutes. Media and reagents were removed from the wells and cells were washed once with 200 μl of phosphate-buffered saline (PBS). The cells were then fixed with 1% paraformaldehyde in PBS at room temperature for 10 minutes under foil. Cells were washed with PBS containing 0.1% Triton-X100 to permeabilize the washed cells. DAPI solution (500 ng/ml in PBS/Triton) was added to each well and allowed to incubate at room temperature for 10 minutes to visualize nuclei. Cells were washed with PBS/Triton and overlaid with 200 µL PBS per well for scanning. The plate was imaged with a 20x objective using a Turboscan system built on to an Olympus IX50 and captured using an Exi Aqua monochrome camera fitted with DAPI and Cy5 filter sets.
Immunofluorescence of SEMA4D Binding to CHO.CD72 Twenty-thousand CHO cells stably transfected with human CD72 were seeded onto 48-well Poly-D-Lysine plates (BD BioCoat™) in 200 µL complete medium and allowed to adhere overnight at 37°C, 5% CO2. Recombinant marmoset SEMA4D-His and antibody were diluted to 800 µg/mL in complete medium and allowed to equilibrate at room temperature for 30 minutes. Ten microliters of SEMA4D/antibody mixture was added per well of CHO-CD72 cells to give a final concentration of 40 µg/ml and allowed to incubate at 37°C, 5% CO2 for 30 minutes. Media and reagents were removed from the wells and 200 µL of anti-6xHis-APC (Abcam, AD1.1.10, 2 µg/mL) in complete medium was overlaid and allowed to bind at room temperature under foil for 30 minutes. Media and reagents were removed from the wells and cells were fixed with 1% paraformaldehyde in PBS at room temperature for 10 minutes under foil. Cells were washed with PBS/Tween to permeabilize them and DAPI solution (500 ng/ml in PBS/0.05% Tween-20) was added to each well and allowed to incubate at room temperature for 10 minutes to visualize nuclei. Cells were washed with PBS and overlaid with 200 µL PBS per well for scanning. The plate was imaged with a 20x objective using a Turboscan system built on to an Olympus IX50 and captured using an Exi Aqua monochrome camera fitted with DAPI and Cy5 filter sets.
Collapse Assay
HEK293 cells transfected with PlexinB1 were seeded at 4000 cells per well onto PDL (1hr) + Laminin (2hrs) (5ug/ml each) coated coverglass chamber slides and allowed to adhere overnight. For collapse, cells were treated with medium containing 2 μg/ml of marmoset SEMA4D +/− 20 μg/ml of VX15/2503 for 15 minutes (VX15/2503 was pre-bound to SEMA4D for 1 hour on ice prior to addition to cells). Cells were then fixed with 4% paraformaldehyde for 10 minutes at RT and washed with PBS. Actin cytoskeleton and nuclei were stained using a 1:40 dilution of phalloidin-Alexa488 and 500ng/ml of DAPI in PBS+0.1% Triton-X100, respectively, for 30 minutes, followed by washing in PBS. Cells were imaged at 60x magnification in Z stack and deconvoluted using ImagePro.
CDC Assay
A human B cell line, Daudi (ATCC, VA), which is CD20 and SEMA4D positive, was used as a target in a non-radioactive CDC assay. Pooled human serum was used as a source of complement. Targets were incubated with antibodies at 37°C for 3 hours. Alamar blue (Invitrogen) was added at a 1:2 dilution and cells were incubated overnight at 37°C. The plate was read on a fluorimeter at an excitation / emission of 530/590 nM and % lysis was calculated using the formula: % CDC = 100 × [(Relative Fluorescence Unit (RFU) of target cells plus complement)-(RFU Experimental value) / (RFU of target cells plus complement)].
ADCC Assay
The aCella-TOX assay (Cell Technology, CA) was used to measure ADCC. The release of GAPDH from dying cells leads to ATP production, which is then coupled to the luciferase methodology, producing light. Targets were Daudi cells and freshly isolated human PBMCs were used as effectors. Antibodies and target cells were incubated at 37°C for 15 minutes to allow opsonization and effectors were added for an overnight incubation before being read employing a luminometer. % ADCC = 100×[(release from target cells in the presence of mAb)-(spontaneous release from targets alone)-(spontaneous release from effectors alone)] / (max release from target cells).
In Vivo Half Antibody
SCID mice were injected i.v. with various combinations of antibodies to determine if half-antibodies were formed. Mice were injected with 300 μg VX15/2503 and anti-C35 (Vaccinex, irrelevant human IgG4 antibody), which both have the serine to proline mutation at position 241 (S241P mutation, numbered according to Kabat numbering system), or with combinations of these same antibodies that did not have the S241P mutations. Mice were also injected with each of the 4 mAbs alone to ensure each mAb could be detected via ELISA. Serum was collected at 2, 24, 48, and 72 hours and frozen for analysis in one of 3 ELISAs; SEMA4D-specific, C35-specific, or bispecific. For the SEMA4D or C35 specific ELISAs, recombinant soluble human C35 or recombinant soluble marmoset SEMA4D was coated on microtiter plates in bicarbonate buffer (pH9.5) at a final concentration of 1 μg/ml. After blocking, titrated serum samples were added for 1 hour and detected using an HRP-conjugated goat anti-human IgG Fc (Jackson ImmunoResearch, PA). For the bispecific ELISA, recombinant soluble marmoset SEMA4D was coated on microtiter plates in bicarbonate buffer (pH 9.5) at a final concentration of 1μg/ml. After blocking, titrated serum samples were added for 1 hour and detected with biotinylated recombinant C35 followed by streptavidin-HRP (Jackson, 016-030-084). All ELISAs were developed for 15 min using 3,3′,5,5′-Tetramethylbenzidine (TMB; BioFX Laboratories, Owings Mills, MD) and read spectrophotometrically at 450/570 nm.
Cytokine Release
Fresh human PBMCs from 19 healthy donors were precultured in 1.5 mL medium for 2 days at 1x107 cells/ml in a 24-well flat-bottom suspension culture plate. Cells were washed, diluted to 1 × 106 cells/ml and 0.2 ml of cells cultured with 4 μg/ml of antibody for 24 hours in a 96-well flat-bottom tissue culture plate. OKT3 (anti-human CD3, eBioscience) and ANC28 (agonist anti-human CD28, Calbiochem) were used as positive control antibodies. A human IgG4 isotype control (Vaccinex antibody 2269, anti-C35) was used as a negative control in addition to a no antibody control. PBMC supernatants were analyzed in duplicate for the presence of IL-1β, IL-6, TNFα, and IFNγ by Cytometric Bead Array (BD Biosciences, CA) using a BD FACS Canto cytometer. Analysis of cytokine levels was completed using FCAP analysis software (BD).
Epitope Mapping
The characterization of the epitope of human SEMA4D recognized by VX15/2503 was performed at Pepscan (Lelystad, Netherlands) utilizing CLIPS (Chemical LInkage of Peptides onto Scaffolds) epitope mapping technology.Citation42 CLIPS mapping presents peptides in spatially constrained orientations, thus helping better predict conformational epitopes compared to simple linear mapping.
Internalization assays
Flow cytometry: To measure VX15/2503 internalization after cellular SEMA4D binding, PBMCs were incubated with Alexa 488 fluorochrome-labeled VX15/2503 or human IgG4 isotype control antibody (mAb 2269) at 37°C for up to 24 hours. The cells were washed to remove labeled antibody and incubated at 4°C in the presence or absence of an anti-Alexa 488 quenching antibody. Samples were subsequently stained with anti-human CD3-Alexa 647 to label CD3+ T cells and analyzed via flow cytometry. The quenching antibody blocks any fluorescent signal from the cell surface, so any signal remaining after quenching represents internalized antigen.Citation37 A comparison was made between the GMFI values from the quenched and unquenched samples; this allowed calculation of the percent SEMA4D internalized using GMFI values from the Alexa 488 channel. The percent internalization data represent the mean internalization from 3 different human donors.
Microscopy: For microscopy, PBMC were isolated and stained as above, then fixed with 0.5% paraformaldehyde and washed with PBS. Cells were spun down to concentrate, resuspended in 50 μl of PBS with 0.05% Tween-20 and 300 ng/ml of DAPI for 10 minutes. Cells were then dropped on a slide with an equal volume of ProLong Gold mounting medium to preserve fluorescence and stabilize cells. Fluorescence was captured using an Exi Aqua camera with a 60 × 1.35 NA oil objective in Z stack, and deconvolution was performed using nearest neighbor with Image-Pro Plus. For illustration, images were pseudocolored to represent DAPI (blue), CD3 (red), and VX15/2503 (green).
Collagen-Induced Arthritis Model
This evaluation was performed at a contract research organization (CRO), Washington Biotechnology (Baltimore, MD), using male DBA1/J mice in a treatment (verses prophylactic) model of murine CIA. The disease was induced by subcutaneous injection with an emulsion containing 100 μg bovine type 2 collagen and 100 μg heat-inactivated Mycobacterium tuberculosis. On Day 21, mice were boosted with an injection of 100 μg bovine type 2 collagen emulsified with IFA. The first therapeutic intraperitoneal injection with mAb 67-2 and the positive control (Enbrel®, subcutaneous) occurred on Day 31. The CRO was blinded to the specific treatment groups. Animals were dosed twice weekly, at 30 mg/kg, scored for signs of arthritis 3x / week, and the study was terminated on Day 42. Selected formalin preserved limbs were decalcified in 5% formic acid for 2-3 days, tissues were trimmed, processed for paraffin embedding, sectioned at 8 μm and stained with toluidine blue (T blue). Paws were embedded and sectioned in the frontal plane. When scoring paws or ankles from mice with lesions of type II collagen arthritis, severity of changes as well as number of individual joints affected must be considered. When only 1–3 joints of the paws or ankles out of a possibility of numerous metacarpal/metatarsal/digit or tarsal/tibiotarsal joints were affected, an arbitrary assignment of a maximum score of 1, 2 or 3 for parameters below was given depending on severity of changes. If more than 3 joints were involved, the criteria below were applied to the most severely affected/majority of joints. The joints were scored for severity of arthritis using 4 parameters; inflammation, pannus, cartilage damage and bone damage; on a scale of 0 (normal) to 5 (severe effects) using pre-study defined histologic criteria.
Disclosure of Potential Conflicts of Interest
Vaccinex, Inc.., a private Delaware corporation, has patent rights based on inventions described in this publication and is developing a humanized anti-SEMA4D antibody for clinical use. All authors are or were employees of Vaccinex, Inc..
References
- Giger RJ, Hollis ER, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2010 Jul; 2:a001867; PMID: 20519341
- Ch'ng ES, Kumanogoh A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol Cancer 2010; 9:251; PMID:20858260; http://dx.doi.org/10.1186/1476-4598-9-251
- Nkyimbeng-Takwi E, Chapoval SP. Biology and function of neuroimmune semaphorins 4A and 4D. Immunol Res 2011 Jan 4; 50:10-21; PMID: 21203905; http://dx.doi.org/10.1007/s12026-010-8201-y
- Zhang Y, Liu B, Ma Y, Jin B. Sema 4D/CD100-plexin B is a multifunctional counter-receptor. Cell Mol Immunol 2012 Dec 24; 10:97-8; PMID: 23262975; http://dx.doi.org/10.1038/cmi.2012.65
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol 2008 Jan; 9:17-23; PMID: 18087252; http://dx.doi.org/10.1038/ni1553
- Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci U.S.A 1996 Oct 15; 93:11780-5; PMID: 8876214; http://dx.doi.org/10.1073/pnas.93.21.11780
- Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cell Mol Immunol 2010 Mar; 7:83-8; PMID: 20118971; http://dx.doi.org/10.1038/cmi.2009.111
- Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U.S.A 2007 Jan 23; 104:1621-6; http://dx.doi.org/10.1073/pnas.0606344104
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci 2003 Oct 8; 23:9229-39
- Smith ES, Jonason AJ, Reilly C, Veeraraghavan J, Fisher T, Doherty M, Klimatcheva E, Mallow C, Cornelius C, Leonard JE, et al. SEMA4D compromises blood-brain barrier, activates microglia, and inhibits remyelination in neurodegenerative disease. Neurobiol Dis 2014; 73:254-68; http://dx.doi.org/10.1016/j.nbd.2014.10.008
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999 Oct 1; 99:71-80; PMID: 10520995; http://dx.doi.org/10.1016/S0092-8674(00)80063-X
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 2002 Sep; 4:720-4; PMID: 12198496; http://dx.doi.org/10.1038/ncb843
- Giraudon P, Vincent P, Vuaillat C, Verlaeten O, Cartier L, Marie-Cardine A, Mutin M, Bensussan A, Belin MF, Boumsell L. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J Immunol 2004 Jan 15; 172:1246-55; http://dx.doi.org/10.4049/jimmunol.172.2.1246
- Witherden DA, Watanabe M, Garijo O, Rieder SE, Sarkisyan G, Cronin SJ, Verdino P, Wilson IA, Kumanogoh A, Kikutani H, et al. The CD100 receptor interacts with Its plexin B2 ligand to regulate epidermal gammadelta T cell function. Immunity. 2012 Aug 14; 37:314-25; http://dx.doi.org/10.1016/j.immuni.2012.05.026
- Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000 Nov; 13:621-31; http://dx.doi.org/10.1016/S1074-7613(00)00062-5
- Kumanogoh A, Kikutani H. The CD100-CD72 interaction: a novel mechanism of immune regulation. Trends Immunol. 2001 Dec; 22:670-6; PMID: 11738997; http://dx.doi.org/10.1016/S1471-4906(01)02087-7
- Ishida I, Kumanogoh A, Suzuki K, Akahani S, Noda K, Kikutani H. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: implications for the regulation of immune and inflammatory responses. Int Immunol 2003 Aug; 15:1027-34; PMID: 12882840; http://dx.doi.org/10.1093/intimm/dxg098
- Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol 2001 Apr 1; 166:4348-54; PMID: 11254688; http://dx.doi.org/10.4049/jimmunol.166.7.4348
- Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol 2001 Apr 1; 166:4341-7; http://dx.doi.org/10.4049/jimmunol.166.7.4341
- Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000 Nov; 13:633-42; http://dx.doi.org/10.1016/S1074-7613(00)00063-7
- Leonard JE, Fisher TL, Winter LA, Cornelius CA, Reilly C, Smith ES, Zauderer M. Nonclinical safety evaluation of VX15/2503, a humanized IgG4 anti-SEMA4D antibody. Mol Cancer Ther 2015 Apr; 14:964-72; PMID: 25657333; http://dx.doi.org/10.1158/1535-7163.MCT-14-0924
- Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol 2010 May 30; 11:594-600; PMID: 20512151; http://dx.doi.org/10.1038/ni.1885
- Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U.S.A 2006 Jun 13; 103:9017-22; PMID: 16754882; http://dx.doi.org/10.1073/pnas.0508825103
- Campos M, DE Campos SG, Ribeiro GG, Eguchi FC, Silva SR, DE Oliveira CZ, DA Costa AM, Curcelli EC, Nunes MC, Penna V, et al. Ki-67 and CD100 immunohistochemical expression is associated with local recurrence and poor prognosis in soft tissue sarcomas, respectively. Oncol Lett 2013 May; 5:1527-35
- Sun Q, Zhou H, Binmadi NO, Basile JR. Hypoxia inducible factor-1-mediated regulation of Semaphorin 4D affects tumor growth and vascularity. J Biol Chem 2009 Sep 17; 284:32066-74; PMID: 19762474; http://dx.doi.org/10.1074/jbc.M109.057166
- Evans EE, Jonason AS, Jr., Bussler H, Torno S, Veeraraghavan J, Reilly C, Doherty MA, Seils J, Winter LA, Mallow C, et al. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunol Res 2015 Jan 22; 3:689-701; PMID: 25614511; http://dx.doi.org/10.1158/2326-6066.CIR-14-0171
- Kumanogoh A, Suzuki K, Ch'ng E, Watanabe C, Marukawa S, Takegahara N, Ishida I, Sato T, Habu S, Yoshida K, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J Immunol 2002 Aug 1; 169:1175-81; PMID: 12133937; http://dx.doi.org/10.4049/jimmunol.169.3.1175
- Okuno T, Nakatsuji Y, Moriya M, Takamatsu H, Nojima S, Takegahara N, Toyofuku T, Nakagawa Y, Kang S, Friedel RH, et al. Roles of sema4D-plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 2010 Feb 1; 184:1499-506; PMID: 20038643; http://dx.doi.org/10.4049/jimmunol.0903302
- Yang YH, Zhou H, Binmadi NO, Proia P, Basile JR. Plexin-B1 activates NF-kappaB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS ONE 2011; 6:e25826; PMID:22028792; http://dx.doi.org/10.1371/journal.pone.0025826
- Labrijn AF, Aalberse RC, Schuurman J. When binding is enough: nonactivating antibody formats. Curr Opin Immunol 2008 Aug; 20:479-85; http://dx.doi.org/10.1016/j.coi.2008.05.010
- Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, Thorpe SJ, Killestein J, Polman CH, Aalberse RC, Schuurman J, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 2009 Aug; 27:767-71; http://dx.doi.org/10.1038/nbt.1553
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006 Sep 7; 355:1018-28; http://dx.doi.org/10.1056/NEJMoa063842
- Romer PS, Berr S, Avota E, Na SY, Battaglia M, Ten B, I, Einsele H, Hunig T. Preculture of PBMC at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood 2011 Sep 19; 118:6772-82; PMID: 21931118; http://dx.doi.org/10.1182/blood-2010-12-319780
- Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol 2003 Oct; 10:843-8; http://dx.doi.org/10.1038/nsb977
- Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature 2010 Sep 26; 467:1118-22; PMID: 20877282; http://dx.doi.org/10.1038/nature09468
- Vugmeyster Y, Xu X, Theil FP, Khawli LA, Leach MW. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J Biol Chem 2012 Apr 26; 3:73-92; PMID: 22558487; http://dx.doi.org/10.4331/wjbc.v3.i4.73
- Beers SA, French RR, Chan HT, Lim SH, Jarrett TC, Vidal RM, Wijayaweera SS, Dixon SV, Kim H, Cox KL, et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood 2010 Jun 24; 115:5191-201; PMID: 20223920; http://dx.doi.org/10.1182/blood-2010-01-263533
- Southwell AL, Franciosi S, Villanueva EB, Xie Y, Winter LA, Veeraraghavan J, Jonason A, Felczak B, Zhang W, Kovalik V, et al. Anti-semaphorin 4D immunotherapy ameliorates neuropathology and some cognitive impairment in the YAC128 mouse model of Huntington disease. Neurobiol Dis 2015 Feb 3; 76:46-56; http://dx.doi.org/10.1016/j.nbd.2015.01.002
- Brown DM, Fisher TL, Wei C, Frelinger JG, Lord EM. Tumours can act as adjuvants for humoral immunity. Immunology 2001 Apr; 102:486-97; http://dx.doi.org/10.1046/j.1365-2567.2001.01213.x
- Fisher TL, Nocera M, Willis RA, Turner MJ, Abdul Alim CS, Brown DM, Bourne PA, di Sant'Agnese PA, Messing EM, Lord EM, et al. Generation of monoclonal antibodies specific for human kallikrein 2 (hK2) using hK2-expressing tumors. Prostate 2002 May 15; 51:153-65; PMID: 11967950; http://dx.doi.org/10.1002/pros.10071
- Lewis KB, Meengs B, Bondensgaard K, Chin L, Hughes SD, Kjaer B, Lund S, Wang L. Comparison of the ability of wild type and stabilized human IgG(4) to undergo Fab arm exchange with endogenous IgG(4)in vitro and in vivo. Mol Immunol 2009 Oct; 46:3488-94; PMID: 19683345; http://dx.doi.org/10.1016/j.molimm.2009.07.009
- Timmerman P, Puijk WC, Meloen RH. Functional reconstruction and synthetic mimicry of a conformational epitope using CLIPS technology. J Mol Recognit 2007 Sep; 20:283-99; http://dx.doi.org/10.1002/jmr.846
- Yoshida Y, Ogata A, Kang S, Ebina K, Shi K, Nojima S, Kimura T, Ito D, Morimoto K, Nishide M, et al. Semaphorin 4D contributes to rheumatoid arthritis by inducing inflammatory cytokine production: Pathogenic and therapeutic implications. Arthritis Rheumatol. 2015 Feb 23; 67:1481-90; PMID: 25707877; http://dx.doi.org/10.1002/art.39086
- Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012 Mar; 33:127-35; http://dx.doi.org/10.1016/j.it.2012.01.008
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 2011 Oct 23; 17:1473-80; PMID: 22019888; http://dx.doi.org/10.1038/nm.2489
- Okamoto K, Takayanagi H. Regulation of bone by the adaptive immune system in arthritis. Arthritis Res Ther 2011; 13:219; PMID:21635718; http://dx.doi.org/10.1186/ar3323
- Heider KH, Kiefer K, Zenz T, Volden M, Stilgenbauer S, Ostermann E, Baum A, Lamche H, Kupcu Z, Jacobi A, et al. A novel Fc-engineered monoclonal antibody to CD37 with enhanced ADCC and high proapoptotic activity for treatment of B-cell malignancies. Blood 2011 Oct 13; 118:4159-68; PMID: 21795744; http://dx.doi.org/10.1182/blood-2011-04-351932
