Abstract
Rheumatoid arthritis is an autoimmune disease characterized by joint inflammation that affects approximately 1% of the general population. Itolizumab, a monoclonal antibody specific for the human CD6 molecule mainly expressed on T lymphocytes, has been shown to inhibit proliferation of T cells and proinflammatory cytokine production in psoriasis patients. We have now assessed the immunological effect of itolizumab in combination with methotrexate in rheumatoid arthritis by analyzing clinical samples taken from 30 patients enrolled in a clinical trial. T and B cell subpopulations were measured at different time points of the study. Plasma cytokine levels and anti-idiotypic antibody response to itolizumab were also evaluated. The combined treatment of itolizumab and methotrexate led to a reduction in the frequency of T cell subpopulations, and plasma levels of proinflammatory cytokines showed a significant decrease up to at least 12 weeks after treatment ended. No anti-idiotypic antibody response was detected. These results support the relevance of the CD6 molecule as a therapeutic target for the treatment of this disease.
Abbreviations
| ACR | = | American College of Rheumatology |
| ALC | = | Absolute lymphocyte counts |
| ALCAM | = | activated leucocyte-cell adhesion molecule |
| ELISA | = | Enzyme-linked immunosorbent assay |
| IFN | = | interferon |
| IL | = | interleukin |
| MTX | = | methotrexate |
| PBMC | = | peripheral blood mononuclear cells |
| RA | = | Rheumatoid arthritis |
| TNF | = | tumor necrosis factor |
| WBC | = | white blood cells |
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown etiology. The main characteristic of the disease is joint inflammation. Citation1,2 It has a slow progressive course leading to a more severe stage at which cartilage and bone destruction are observed. RA occurs in ∼ 1% of the overall population, and it is more common in women than in men. Citation3
Methotrexate (MTX) is currently recommended as first-line therapy for RA, Citation4,5 but it often fails to provide long-term disease control due to toxicity. Therefore, new therapeutic strategies have been developed, in particular biological agents against specific targets of the disease. These drugs have been proved in RA patients who are inadequate responders to MTX, Citation6-8 but they have not reached a good clinical response according to the criteria of the American College of Rheumatology (ACR), as only 10-40% of treated patients achieved ACR70 score (70% disease improvement). Citation9
T cells play a critical role in the immunopathogenesis of RA, Citation10,11 leading to inflammation and joint destruction. Citation12 Previous reports have shown high numbers of activated CD4+ T cells in the inflamed joint. Citation13,14 Once activated, T lymphocytes stimulate macrophages and fibroblasts to secrete proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1 and IL-6, as well as IL-17 and interferon (IFN)-γ, Citation12,15 which constitute the key soluble factors in the inflammatory process of RA. However, no differences have been observed in IL-10 levels when RA patients are compared with healthy individuals. In contrast, the levels of the Th2 cytokine IL-4 have been found to be significantly reduced in RA patients. Citation16
CD6 is a highly glycosylated membrane protein predominantly expressed on T lymphocytes. Its third membrane proximal domain contains the binding site for its receptor, the activated leucocyte-cell adhesion molecule (ALCAM), Citation17,18 which is expressed on antigen-presenting cells. CD6 plays a role in cell proliferation, adhesion, differentiation and survival, Citation19-21 and its relevance in autoimmunity has been previously shown. Citation22,23 The interaction between ALCAM and the CD6 molecule is one of the costimulatory pathways of T cell activation. Citation24,25 An anti-CD6 monoclonal antibody (mAb) that prevented renal and bone marrow graft rejection Citation26,27 was evaluated in multiple sclerosis patients, and a significant reduction in peripheral T cell counts was found. Citation28 More recently, the finding of CD6 as a susceptibility gene in multiple sclerosis, Citation29,30 as well as the overexpression of ALCAM and the presence of CD6+ T and B cells in salivary glands of patients with Sjögren syndrome, Citation31 support the role of CD6 in pathological autoimmunity and reinforces its relevance for targeted therapy.
Itolizumab is a humanized mAb that targets human CD6+ lymphocytes. This antibody recognizes the membrane-distal domain 1 of CD6 and was not able to inhibit soluble ALCAM binding to CD6-expressing HEK-293 cells. Citation32 Moreover, itolizumab did not cause T cell depletion when used as monotherapy in RA patients. Citation33 In experiments in vitro with peripheral blood mononuclear cells (PBMC) from healthy donors, itolizumab reduced IFN-γ, IL-6 and TNF production. Citation34 Recently, we found a significant decrease in T cell proliferation and reduction in circulating proinflammatory cytokine levels in psoriasis patients treated with itolizumab. Citation35
For the study presented here, clinical samples taken from 30 RA patients participating in a clinical trial to evaluate the safety and efficacy of itolizumab in combination with MTX were analyzed for lymphocyte counts and cytokine levels. Safety and efficacy data from this clinical study are not included in this report. Our findings point to the mechanism by which itolizumab reduces the proinflammatory response in RA patients.
Results
Itolizumab treatment in combination with methotrexate reduced absolute lymphocyte counts
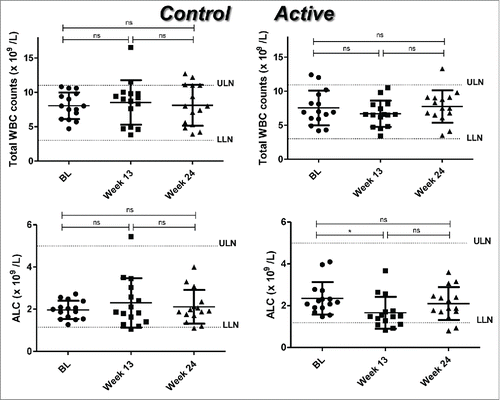
Because the CD6 molecule is an important mediator of lymphocyte activation, we determined whether white blood cells (WBC) and absolute lymphocyte counts (ALC) were modified with itolizumab or MTX treatment. We did not find significant differences (p > 0.05) in WBC between the groups. Conversely, ALC decreased significantly at week 13 with respect to BL (p = 0.012) in the active group (combined treatment). This significant reduction seemed to be transient because the differences were no longer significant (p = 0.410) 12 weeks after treatment ending (week 24). In the control group (MTX alone), no significant differences in ALC were found during the treatment (). Also ALC values for individual patients are shown in Suppl. .
Figure 1. Total white blood cell (WBC) counts and absolute lymphocyte counts (ALC) in RA patients treated with placebo plus MTX (Control) or with the combination of itolizumab plus MTX (Active). Individual values and means ± SD are represented. The graph shows the normal laboratory reference ranges. LLN: lower limit of normal, ULN: upper limit of normal, BL: baseline. * p < 0.05; ns: non-significant. Wilcoxon signed ranks test was used to compare between different time points in each group of the study.
Itolizumab treatment in combination with methotrexate reduced peripheral counts of T cells but not of B cells
Taking into account the reduction observed in the ALC in the active group, we investigated the behavior of T and B cell populations. To address this point, the frequency of CD3+ T cells and CD19+ B lymphocytes at the different time points were measured by flow cytometry.
A significant reduction in CD3+ T cell counts was observed at week 13 in the active group compared with BL (p = 0.001). This decrease was found only during the treatment period. At week 24, this population recovered to BL values (p = 0.256) (). A significant increase in the CD19+ B cell population was found at week 24 with respect to week 13 in both groups (control, p = 0.046; active, p = 0.035). No differences were observed (p > 0.05) when comparing BL either with week 13 or with week 24 in active group, but in control group although there was no difference (p > 0.05) between week 13 and BL, a significant increase at week 24 compared to BL was observed (p = 0.013) ().
Figure 2 (see previous page). Profile of T and B cells in RA patients treated with placebo plus MTX (Control) or with the combination of itolizumab plus MTX (Active). CD3+ (A), CD19+ (B), CD4+ (C) and CD8+ (D) lymphocytes were evaluated by FACS. Individual values and means ± SD are represented. The graph shows the normal laboratory reference ranges. LLN: lower limit of normal, ULN: upper limit of normal, BL: baseline. * p < 0.05; ** p < 0.01; ns: non-significant. Wilcoxon signed ranks test was used to compare between different time points in each group of the study.
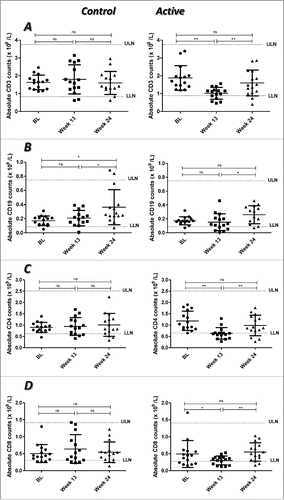
To better characterize the T cell subpopulation that changed during the combined treatment of itolizumab and MTX, the absolute numbers of CD4+ and CD8+ T cells were counted. We found a transient but significant decrease in both subpopulations (CD4+, p = 0.001; CD8+, p = 0.041) at week 13 compared with BL. At week 24 both subpopulations were completely recovered, reaching the BL values (p = 0.222 and p = 0.451, respectively) with significant differences regarding week 13 (p = 0.009 and p = 0.005, respectively) ( and D, respectively).
In the control group, in line with the results of ALC, no significant differences in T cell numbers were observed between the different time points (). There were no variations (p > 0.05) in the counts of CD3+ (), CD4+ () or CD8+ () T cells.
Itolizumab therapy in combination with MTX reduced proinflammatory cytokine levels
Based on our previous findings of the decrease of circulating proinflammatory cytokines in psoriasis patients treated with itolizumab, Citation35 some of these cytokines were evaluated in the samples of RA patients treated with either itolizumab and MTX or MTX alone. In particular, plasma levels of IL-6, TNF, IFN-γ, IL-4 and IL-10 were assessed by ELISA.
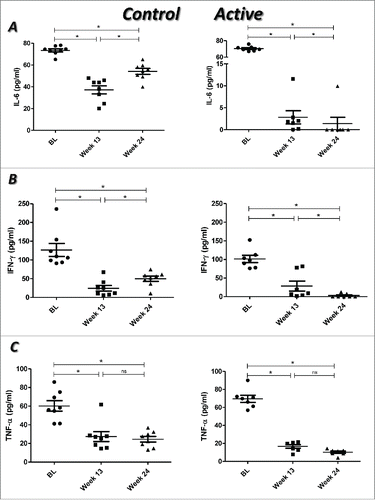
For IL-6 and IFN-γ, a significant decrease at week 13 compared with BL was verified in the control group (IL-6, p = 0.012; IFN-γ, p = 0.012). However, at week 24, there was a significant increase in the levels of both cytokines with respect to week 13 (p = 0.017 and p = 0.036, respectively). In the active group, a significant decrease at week 13 was found in comparison with BL (IL6, p = 0.018; IFN-γ, p = 0.018). Interestingly, in this group, the levels of these cytokines were also significantly reduced at week 24 with respect to week 13 (IL-6, p = 0.028; IFN-γ, p = 0.018) (). In the case of TNF, a significant decrease at week 13 compared with BL (control, p = 0.012; active, p = 0.018), but not with week 24 (control, p = 0.484; active, p = 0.091), was found for both groups ().
Figure 3. Proinflammatory cytokine profile in RA patients treated with placebo plus MTX (Control) or with the combination of itolizumab plus MTX (Active). Plasma concentrations of IL-6 (A), IFN-γ (B) and TNF (C) were assessed by ELISA. Individual values and means ± SD are represented. BL: baseline. * p < 0.05; ns: non-significant. Wilcoxon signed ranks test was used to compare between different time points in each group of the study.
In both groups, a significant increase in the levels of IL-4 was detected at week 13 compared with the BL values (control, p = 0.012; active, p = 0.018). Conversely, a significant decrease was seen at week 24 as compared with week 13 (control, p = 0.012; active, p = 0.028). In the case of the control group, this decrease was also significant in comparison with BL (p = 0.012). In the active group, the levels of IL-4 at week 24 were similar to BL values ().
Figure 4. Levels of IL-4 (A) and IL-10 (B) in RA patients treated with placebo plus MTX (Control) or with the combination of itolizumab plus MTX (Active). Plasma concentrations of these cytokines were determined by ELISA. Individual values and means ± SD are represented. BL: baseline. * p < 0.05; ns: non-significant. Wilcoxon signed ranks test was used to compare between different time points in each group of the study.
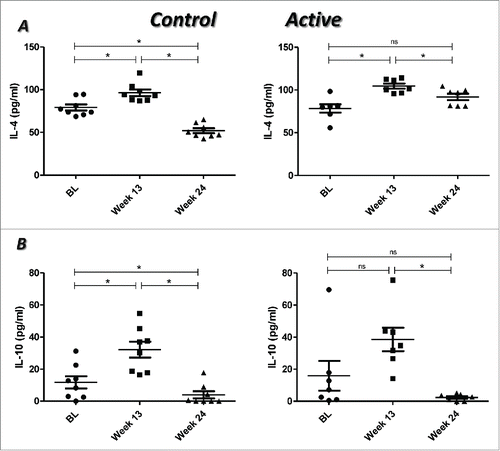
An increase in the levels of IL-10 was seen at week 13 in comparison with BL, which was significant in the control group (p = 0.012). However, in both groups a significant decrease was observed at week 24 with respect to week 13 (control, p = 0.012; active, p = 0.018) ().
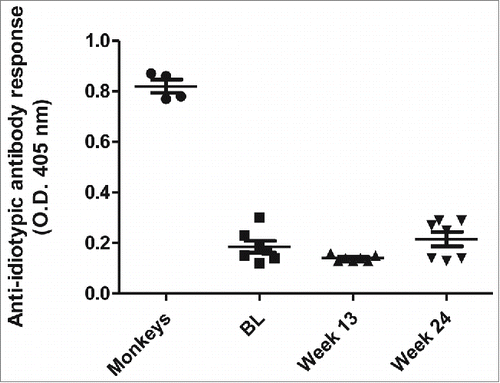
Itolizumab administration did not induce a measurable anti-idiotypic antibody response
Low immunogenicity of itolizumab has been demonstrated in previous studies,Citation33,35 including one in RA patients but only after 6 weeks of treatment. Citation33 Here, we assessed the generation of anti-idiotypic antibodies against itolizumab after 12 weeks of treatment in the active group (itolizumab plus MTX). No anti-idiotypic antibody response was detected in any of the evaluated patients ().
Figure 5. Anti-idiotypic antibody response to itolizumab in RA patients treated with the combination of itolizumab plus MTX. The presence of anti-idiotypic antibodies in plasma from patients was determined by ELISA. Plates were coated with itolizumab F(ab´)2 and samples were assayed at 1:100 dilution. Sera from monkeys immunized with itolizumab were used as positive control. Individual values and means ± SD are represented.
Discussion
RA is characterized by an inflammatory process leading to destruction of cartilage and bone. Many cells have been associated with the onset and maintenance of this autoimmune disease, but T cells have demonstrated a central role, mainly the IFN-γ-producing subpopulation. Citation14 The proinflammatory cytokine TNF also plays a pivotal role in regulating the inflammation associated to the disease. Therefore, biological drugs targeting TNF Citation36 or blocking T cell signaling Citation37 have shown clinical benefits in RA patients; however, some patients experience side effects or develop resistance to these drugs.
Anti-CD6 therapy has emerged as a new therapeutic strategy for the treatment of autoimmune disorders. Citation25,38,39 Itolizumab, an anti-CD6 mAb, has demonstrated clinical benefits,Citation33,40 as well as modulation of T cell proliferation and decrease of proinflammatory cytokine levels in psoriasis patients. Citation35
Studies have shown that itolizumab is not a T cell-depleting antibody when used as monotherapy both in Cuban RA Citation33 and psoriasis (unpublished data) patients. A small and transient decrease in ALC was, however, reported in an Indian study with psoriasis patients, Citation40 although no increase in infections was observed. In the present study, a transient decrease in the amount of T cells was also observed in RA patients.
In an Israeli study, in which PBMC from active RA patients were treated with MTX for 24 h, a 30% decrease in activated CD4+ T cells was observed. Citation41 Moreover, in another study where the effect of MTX in RA patients was evaluated, a decrease in the Th17 subpopulation was seen after 12 weeks of treatment. Citation42
As the majority of the biological drugs approved for RA treatment focus on inflammatory cytokines, changes in the immune cell profile are insufficiently studied when these drugs are combined with MTX. In one study Citation43 in which RA patients were treated during 12 weeks with MTX plus etanercept, a TNF inhibitor comprising the TNF receptor fused to the Fc portion of human IgG1, Citation44 a decrease in Th1 and Th17 cells was observed. Citation43 In another study Citation45 evaluating the effect of abatacept, a T cell activation-blocker consisting of the extracellular domain of CTLA-4 fused to the Fc portion of human IgG1, Citation46 a significant decrease in IL-17- and IFN-γ-producing CD8+ T cells was observed after 6 months of treatment, while after 12 months IL-17- producing CD4+ T cells also decreased. Citation45
In our study, in those patients treated only with MTX, ALC remained stable during the whole study. In the group administered itolizumab, the decrease in ALC, and in particular in the CD3+ T cell population, was probably due to the combined action of the antibody on CD6+ lymphocytes and the immunosuppressing effect of MTX. It is worth noting that this decrease in ALC was transient and for most of the patients did not go under normal limits, which confirms that itolizumab is not a depleting antibody.
B cells are important mediators of RA pathogenesis, Citation47 and in fact anti-CD20 rituximab has demonstrated therapeutic efficacy. Citation48 In another study, although treatment of RA patients with MTX reduced activation of B lymphocytes, the numbers of circulating cells were not affected. Citation49 In patients with juvenile idiopathic arthritis, the most common rheumatic disease in younger people, treatment with MTX caused a significant decrease in CD19+ B cells in comparison with administration of etanercept. Citation50 In our study, the increase in CD19+ B cells at the end of the treatment did not hamper the positive clinical outcome (unpublished data).
Several cytokines have been implicated in RA pathogenesis, perpetuating inflammation and contributing to cartilage destruction and bone remodeling. Proinflammatory cytokines (e.g., TNF, IL-6, IL-1, IFN-γ) are abundant in the RA profile, but anti-inflammatory and regulatory cytokines (e.g.,, IL-4, IL-10, IL-13) have also been related to the disease. Citation51 Most of the biological drugs approved for RA treatment have proinflammatory cytokines as targets, although these agents cannot be used over long time periods. Citation52
It has been demonstrated that MTX induces IL-6 secretion in human cell lines. Citation53 However, when PBMC from RA patients were stimulated with phytohemagglutinin and treated with MTX, an increase in Th2 cytokines and a decrease in Th1 cytokines was observed. Citation16 In RA patients treated with MTX, a significant decrease in IL-6, IFN-γ Citation54 and TNF Citation54,55 levels has also been seen. The same effect was obtained in the cases of IL-6 and TNF in patients treated with a combination of MTX with either etanercept Citation43 or abatacept. Citation56 In the first study, IFN-γ levels did not change, Citation43 while the second combination was able to diminish synovial inflammatory gene expression, including IFN-γ. Citation57
In the present study, we used a mAb targeting CD6, which is expressed mainly on T cells and is related with differentiation, survival and activation of these lymphocytes. Citation20,21 A significant decrease in the levels of the proinflammatory cytokines IL-6, TNF and IFN-γ was found in patients treated with anti-CD6 itolizumab in combination with MTX even when measured 12 weeks after treatment ended. In contrast, MTX alone showed this effect only during the course of administration.
The Th2 cytokine IL-4 inhibits activation of synovial cells and suppresses IFN-γ-producing T cells. Citation58 IL-10 inhibits IL-6 and TNF production by macrophages and IFN-γ by T cells. Citation59 In PBMC stimulated with mitogens and treated with MTX, an increase in IL-4 and IL-10 gene expression was found and correlated with serum levels of these cytokines. Citation16 In RA patients treated with MTX alone or in combination with etanercept, no significant changes were observed in serum concentration of IL-4 and IL-10. Citation43 In our study, itolizumab did not have a clear effect on IL-10, as was previously demonstrated in psoriasis patients receiving the antibody as monotherapy. Citation35 Regarding IL-4, its levels were higher in the group receiving the antibody, and this effect was maintained during the follow up.
The development of anti-idiotypic antibodies in patients leads to hypersensitivity reactions and the ineffectiveness of the treatment due to clearance of the therapeutic molecule. Citation60,61 This response can occur even when patients are treated with fully human mAbs. Citation62-64 Anti-drug antibodies to the 5 anti-TNF agents approved to treat RA patients have been measured. In the case of adalimumab, a fully human antibody, immunogenicity has been linked with a lack of clinical response. Citation65 In a Korean study, the development of these antibodies was significantly associated with treatment failure in RA and ankylosing spondylitis patients. Citation66 Itolizumab treatment, however, did not induce an anti-idiotypic antibody response in RA patients. These results are consistent with our previous studies. Citation33,35
RA is a heterogeneous disorder where T cells contribute to disease development and chronicity. Here, we focused on the effect of the anti-CD6 mAb itolizumab on these cells. This study demonstrates, for the first time, that itolizumab in combination with MTX inhibits the secretion of proinflammatory cytokines in RA patients. Therefore, itolizumab may be a useful treatment for this illness based on the modulation of T cell activity. These results also reinforce the relevance of CD6 as a target for autoimmune disease therapy.
Patients and Methods
Study design
The study was a 24-week, Phase 2, multicenter, double blind, randomized, double-arm, and placebo-controlled trial in patients with moderate to severe active RA conducted in Cuba. Itolizumab and placebo were given intravenously weekly over 12 weeks. MTX was administered orally to all patients in a constant dose ranging from 7 to 25 mg.
A total of 80 patients were included in the clinical trial and 30 patients from this study were selected for immunological evaluation, based on availability of samples. A half of these patients were included in the first arm and were administered with placebo solution plus MTX (placebo + MTX arm, control group); while the other half was included in the second arm and received itolizumab at 0.4 mg/kg in combination with MTX (itolizumab + MTX arm, active group). Patients were followed up for 12 weeks after treatment ending (Suppl. ).
Patients included in this study had clinical diagnosis of active RA according to the criteria of the American College of Rheumatology (ACR), with at least 8 tender or swollen joints as inclusion criterion (Suppl. Table 1). Demographic characteristics of the patients included in this study are shown in supplementary table 1.
The study protocol, including collection and analysis of the biological samples, was approved by the ethic review board of each hospital. All patients provided written informed consent. The trial was conducted in accordance to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. It is registered at the Cuban Registry of Clinical Trials (Safety and therapeutic effect study of T1h (anti-CD6) as monotherapy and combined with methotrexate in patients with rheumatoid arthritis: http://registroclinico.sld.cu/trials/RPCEC00000035-En
PBMC and plasma samples
Blood samples were taken at 3 time points: before the first administration of itolizumab or placebo (baseline, BL); one week after the end of treatment (week 13); and at the end of the study (week 24). The study design is illustrated in Suppl. .
To obtain PBMC and plasma, blood samples were collected in tubes with ethylenediaminetetraacetic acid (Becton Dickinson, Franklin Lakes, NJ). Plasma was frozen at −80°C until use. For flow cytometer experiments, red blood cells were lysed and after centrifugation for 5 min at 1800 rpm at 4°C the pellet with the PBMC was collected, washed with phosphate-buffered saline (PBS) and analyzed immediately.
Antibodies and reagents
Anti-human CD3-R-phycoerythrin (PE) cyanine 5 (UCH-T1 clone), CD4-fluorescein isothiocyanate (FITC) (RPA-T4 clone), CD8-FITC (LT8 clone) and CD19-FITC (LT19 clone) were obtained from AbD Serotec (Kidlington, Oxford, UK). Itolizumab was produced at the Center of Molecular Immunology (Havana, Cuba).
Flow cytometry assay
Surface markers on PBMC were detected by incubation with the appropriate mAbs, followed by fixation in 4% paraformaldehyde. Samples were analyzed in a Gallios flow cytometer (Beckman Coulter, Brea, CA) by collecting a minimum of 10000 events and analyzing the dot plot graphs (Kaluza Software1.2, Beckman Coulter).
Cytokines in plasma
Cytokines were measured using different sandwich ELISA kits, according to the respective manufacturer's instructions. Cytokines measured were IFN-γ, IL-4 (Mabtech AB, Stockholm, Sweden), IL-6, TNF (R&D Systems) and IL-10 (BD Biosciences, Franklin Lakes, NJ). Samples and standards were analyzed in duplicated.
Anti-idiotypic antibody response to itolizumab
The anti-idiotypic antibody response to itolizumab was measured by ELISA as previously described. Citation35 Itolizumab F(ab´)2 was used to coat ELISA plates. Sera from Cercopitecus aethiops monkeys containing anti-idiotypic antibodies against itolizumab Citation67 were used as positive control. Absorbance values of at least 2-fold the signal with the pre-immune sera were considered positive for anti-idiotypic antibodies.
Statistical analysis
Data were analyzed using the SPSS IBM 16.0 software for Windows. The Wilcoxon Signed Ranks Test for matched pairs was used to compare patient's samples. A bilateral level of p < 0.05 was used to determine statistical significance. Data are expressed as mean ± standard deviation (SD).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors´ Contributions
Conception and design of the study: Lazaro E. Aira, Patricia Hernández, Zaima Mazorra, Carmen Viada.
Clinical investigators (recruited and treated patients): Dinorah Prada, Araceli Chico, Jorge A. Gómez.
Acquisition of data: Lazaro E. Aira, Patricia Hernández, Zuyén González, Karla Fuentes, Carmen Viada.
Analysis and interpretation of data: Lazaro E. Aira, Patricia Hernández, Zaima Mazorra.
Writing and/or revision of the manuscript: Lazaro E. Aira, Patricia Hernández, Zaima Mazorra.
Final approval: Lazaro E. Aira, Patricia Hernández, Zaima Mazorra.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
KMAB_A_1105416_supplemental_material.pdf
Download PDF (196.5 KB)Acknowledgments
This study was conducted by the Center of Molecular Immunology. We thank Dr. Alejandro López-Requena for the valuable assistance in the reviewing of this manuscript. We also thank to all patients and their families, and the staffs of all medical institutions involved in this study.
References
- van de Sande MG, de Hair MJ, van der Leij C, Klarenbeek PL, Bos WH, Smith MD, Maas M, de Vries N, van Schaardenburg D, Dijkmans BA, et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann Rheum Dis 2011; 70:772-7; PMID:21177292; http://dx.doi.org/10.1136/ard.2010.139527
- Sangha O. Epidemiology of rheumatic diseases. Rheumatology (Oxford) 2000; 39 Suppl 2:3-12; PMID:11276800; http://dx.doi.org/10.1093/rheumatology/39.suppl_2.3
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001; 358:903-11; PMID:11567728; http://dx.doi.org/10.1016/S0140-6736(01)06075-5
- Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, Gorter S, Knevel R, Nam J, Schoels M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010; 69:964-75; PMID:20444750; http://dx.doi.org/10.1136/ard.2009.126532
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008; 59:762-84; PMID:18512708; http://dx.doi.org/10.1002/art.23721
- Keystone E, Heijde D, Mason D, Jr., Landewe R, Vollenhoven RV, Combe B, Emery P, Strand V, Mease P, Desai C, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008; 58:3319-29; PMID:18975346; http://dx.doi.org/10.1002/art.23964
- Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, Pazdur J, Bae SC, Palmer W, Zrubek J, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis 2009; 68:789-96; PMID:19066176; http://dx.doi.org/10.1136/ard.2008.099010
- van Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, Zickert A, Theander J, Thorner A, Hellstrom H, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009; 374:459-66; PMID:19665644; http://dx.doi.org/10.1016/S0140-6736(09)60944-2
- Carter PH, Zhao Q. Clinically validated approaches to the treatment of autoimmune diseases. Expert Opin Investig Drugs 2010; 19:195-213; PMID:20050823; http://dx.doi.org/10.1517/13543780903418452
- Panayi GS, Corrigall VM, Pitzalis C. Pathogenesis of rheumatoid arthritis. The role of T cells and other beasts. Rheum Dis Clin North Am 2001; 27:317-34; PMID:11396095; http://dx.doi.org/10.1016/S0889-857X(05)70204-0
- Guzmán-Moreno R. Artritis Reumatoide. Bases inmunológicas para la terapia biológica. Rev Colom Reumatol 2003; 10:119-34; PMID:Can't
- Goronzy JJ, Weyand CM. T-cell regulation in rheumatoid arthritis. Curr Opin Rheumatol 2004; 16:212-7; PMID:15103247; http://dx.doi.org/10.1097/00002281-200405000-00008
- Snir O, Backlund J, Bostrom J, Andersson I, Kihlberg J, Buckner JH, Klareskog L, Holmdahl R, Malmstrom V. Multifunctional T cell reactivity with native and glycosylated type II collagen in rheumatoid arthritis. Arthritis Rheum 2012; 64:2482-8; PMID:22392632; http://dx.doi.org/10.1002/art.34459
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001; 344:907-16; PMID:11259725; http://dx.doi.org/10.1056/NEJM200103223441207
- McInnes IB. Leukotrienes, mast cells, and T cells. Arthritis Res Ther 2003; 5:288-9; PMID:14680504; http://dx.doi.org/10.1186/ar1017
- Constantin A, Loubet-Lescoulie P, Lambert N, Yassine-Diab B, Abbal M, Mazieres B, de Preval C, Cantagrel A. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum 1998; 41:48-57; PMID:9433869; http://dx.doi.org/10.1002/1529-0131(199801)41:1%3c48::AID-ART7%3e3.0.CO;2-K
- Bodian DL, Skonier JE, Bowen MA, Neubauer M, Siadak AW, Aruffo A, Bajorath J. Identification of residues in CD6 which are critical for ligand binding. Biochemistry 1997; 36:2637-41; PMID:9054570; http://dx.doi.org/10.1021/bi962560+
- Whitney GS, Starling GC, Bowen MA, Modrell B, Siadak AW, Aruffo A. The membrane-proximal scavenger receptor cysteine-rich domain of CD6 contains the activated leukocyte cell adhesion molecule binding site. J Biol Chem 1995; 270:18187-90; PMID:7543097; http://dx.doi.org/10.1074/jbc.270.31.18187
- Zimmerman AW, Joosten B, Torensma R, Parnes JR, van Leeuwen FN, Figdor CG. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 2006; 107:3212-20; PMID:16352806; http://dx.doi.org/10.1182/blood-2005-09-3881
- Ibanez A, Sarrias MR, Farnos M, Gimferrer I, Serra-Pages C, Vives J, Lozano F. Mitogen-activated protein kinase pathway activation by the CD6 lymphocyte surface receptor. J Immunol 2006; 177:1152-9; PMID:16818773; http://dx.doi.org/10.4049/jimmunol.177.2.1152
- Singer NG, Fox DA, Haqqi TM, Beretta L, Endres JS, Prohaska S, Parnes JR, Bromberg J, Sramkoski RM. CD6: expression during development, apoptosis and selection of human and mouse thymocytes. Int Immunol 2002; 14:585-97; PMID:12039910; http://dx.doi.org/10.1093/intimm/dxf025
- Le Dantec C, Alonso R, Fali T, Montero E, Devauchelle V, Saraux A, Pers JO, Renaudineau Y. Rationale for treating primary Sjogren's syndrome patients with an anti-CD6 monoclonal antibody (Itolizumab). Immunol Res 2013; 56:341-7; PMID:23576060; http://dx.doi.org/10.1007/s12026-013-8423-x
- Alonso-Ramirez R, Loisel S, Buors C, Pers JO, Montero E, Youinou P, Renaudineau Y. Rationale for Targeting CD6 as a Treatment for Autoimmune Diseases. Arthritis 2010; 2010:130646; PMID:22076177; http://dx.doi.org/10.1155/2010/130646
- Gimferrer I, Calvo M, Mittelbrunn M, Farnos M, Sarrias MR, Enrich C, Vives J, Sanchez-Madrid F, Lozano F. Relevance of CD6-mediated interactions in T cell activation and proliferation. J Immunol 2004; 173:2262-70; PMID:15294938; http://dx.doi.org/10.4049/jimmunol.173.4.2262
- Hassan NJ, Barclay AN, Brown MH. Frontline: Optimal T cell activation requires the engagement of CD6 and CD166. Eur J Immunol 2004; 34:930-40; PMID:15048703; http://dx.doi.org/10.1002/eji.200424856
- Kirkman RL, Araujo JL, Busch GJ, Carpenter CB, Milford EL, Reinherz EL, Schlossman SF, Strom TB, Tilney NL. Treatment of acute renal allograft rejection with monoclonal anti-T12 antibody. Transplantation 1983; 36:620-6; PMID:6197792; http://dx.doi.org/10.1097/00007890-198336060-00005
- Reinherz EL, Geha R, Rappeport JM, Wilson M, Penta AC, Hussey RE, Fitzgerald KA, Daley JF, Levine H, Rosen FS, et al. Reconstitution after transplantation with T-lymphocyte-depleted HLA haplotype-mismatched bone marrow for severe combined immunodeficiency. Proc Natl Acad Sci U S A 1982; 79:6047-51; PMID:6764536; http://dx.doi.org/10.1073/pnas.79.19.6047
- Hafler DA, Fallis RJ, Dawson DM, Schlossman SF, Reinherz EL, Weiner HL. Immunologic responses of progressive multiple sclerosis patients treated with an anti-T-cell monoclonal antibody, anti-T12. Neurology 1986; 36:777-84; PMID:3486383; http://dx.doi.org/10.1212/WNL.36.6.777
- Swaminathan B, Matesanz F, Cavanillas ML, Alloza I, Otaegui D, Olascoaga J, Cenit MC, de las Heras V, Barcina MG, Arroyo R, et al. Validation of the CD6 and TNFRSF1A loci as risk factors for multiple sclerosis in Spain. J Neuroimmunol 2010; 223:100-3; PMID:20430450; http://dx.doi.org/10.1016/j.jneuroim.2010.03.020
- Johnson BA, Wang J, Taylor EM, Caillier SJ, Herbert J, Khan OA, Cross AH, De Jager PL, Gourraud PA, Cree BC, et al. Multiple sclerosis susceptibility alleles in African Americans. Genes Immun 2010; 11:343-50; PMID:19865102; http://dx.doi.org/10.1038/gene.2009.81
- Alonso R, Buors C, Le Dantec C, Hillion S, Pers JO, Saraux A, Montero E, Marianowski R, Loisel S, Devauchelle V, et al. Aberrant expression of CD6 on B-cell subsets from patients with Sjogren's syndrome. J Autoimmun 2010; 35:336-41; PMID:20810246; http://dx.doi.org/10.1016/j.jaut.2010.07.005
- Alonso R, Huerta V, de Leon J, Piedra P, Puchades Y, Guirola O, Chinea G, Montero E. Towards the definition of a chimpanzee and human conserved CD6 domain 1 epitope recognized by T1 monoclonal antibody. Hybridoma (Larchmt) 2008; 27:291-301; PMID:18707547; http://dx.doi.org/10.1089/hyb.2008.0007
- Rodriguez PC, Torres-Moya R, Reyes G, Molinero C, Prada D, Lopez AM, Hernandez IM, Hernandez MV, Martinez JP, Hernandez X, et al. A clinical exploratory study with itolizumab, an anti-CD6 monoclonal antibody, in patients with rheumatoid arthritis. Results Immunol 2012; 2:204-11; PMID:24371585; http://dx.doi.org/10.1016/j.rinim.2012.11.001
- Nair P, Melarkode R, Rajkumar D, Montero E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol 2010; 162:116-30; PMID:20726988; http://dx.doi.org/10.1111/j.1365-2249.2010.04235.x
- Aira LE, Lopez-Requena A, Fuentes D, Sanchez L, Perez T, Urquiza A, Bautista H, Falcon L, Hernandez P, Mazorra Z. Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab. MAbs 2014; 6:783-93; PMID:24594862; http://dx.doi.org/10.4161/mabs.28376
- Gabay C, Hasler P, Kyburz D, So A, Villiger P, von Kempis J, Walker U. Biological agents in monotherapy for the treatment of rheumatoid arthritis. Swiss Med Wkly 2014; 144:w13950; PMID:24723273
- Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, Gomez-Reino J, Grassi W, Haraoui B, Shergy W, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 2009; 68:1870-7; PMID:19124524; http://dx.doi.org/10.1136/ard.2008.101121
- Gangemi RM, Swack JA, Gaviria DM, Romain PL. Anti-T12, an anti-CD6 monoclonal antibody, can activate human T lymphocytes. J Immunol 1989; 143:2439-47; PMID:2794503
- Osorio LM, Garcia CA, Jondal M, Chow SC. The anti-CD6 mAb, IOR-T1, defined a new epitope on the human CD6 molecule that induces greater responsiveness in T cell receptor/CD3-mediated T cell proliferation. Cell Immunol 1994; 154:123-33; PMID:7509726; http://dx.doi.org/10.1006/cimm.1994.1062
- Krupashankar DS, Dogra S, Kura M, Saraswat A, Budamakuntla L, Sumathy TK, Shah R, Gopal MG, Narayana Rao T, Srinivas CR, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol 2014; 71:484-92; PMID:24703722; http://dx.doi.org/10.1016/j.jaad.2014.01.897
- Herman S, Zurgil N, Langevitz P, Ehrenfeld M, Deutsch M. The immunosuppressive effect of methotrexate in active rheumatoid arthritis patients vs. its stimulatory effect in nonactive patients, as indicated by cytometric measurements of CD4+ T cell subpopulations. Immunol Invest 2004; 33:351-62; PMID:15495793; http://dx.doi.org/10.1081/IMM-120039865
- Yue C, You X, Zhao L, Wang H, Tang F, Zhang F, Zhang X, He W. The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int 2010; 30:1553-7; PMID:19847432; http://dx.doi.org/10.1007/s00296-009-1179-x
- Lina C, Conghua W, Nan L, Ping Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol 2011; 31:596-605; PMID:21562776; http://dx.doi.org/10.1007/s10875-011-9542-6
- Marotte H, Cimaz R. Etanercept - TNF receptor and IgG1 Fc fusion protein: is it different from other TNF blockers? Expert Opin Biol Ther 2014; 14:569-72; PMID:24611432; http://dx.doi.org/10.1517/14712598.2014.896334
- Scarsi M, Zanotti C, Chiarini M, Imberti L, Piantoni S, Frassi M, Tincani A, Airo P. Reduction of peripheral blood T cells producing IFN-gamma and IL-17 after therapy with abatacept for rheumatoid arthritis. Clin Exp Rheumatol 2014; 32:204-10; PMID:24428959
- Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest 1999; 103:1243-52; PMID:10225967; http://dx.doi.org/10.1172/JCI5857
- Panayi GS. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology (Oxford) 2005; 44 Suppl 2:ii3-ii7; PMID:15851524
- Lopez-Olivo MA, Amezaga Urruela M, McGahan L, Pollono EN, Suarez-Almazor ME. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev 2015; 1:CD007356; PMID:25603545
- Coffey G, Betz A, Graf J, Stephens G, Hua Lin P, Imboden J, Sinha U. Methotrexate and a spleen tyrosine kinase inhibitor cooperate to inhibit responses to peripheral blood B cells in rheumatoid arthritis. Pharmacol Res Perspect 2013; 1:e00016; PMID:25505569; http://dx.doi.org/10.1002/prp2.16
- Glaesener S, Quach TD, Onken N, Weller-Heinemann F, Dressler F, Huppertz HI, Thon A, Meyer-Bahlburg A. Distinct effects of methotrexate and etanercept on the B cell compartment in patients with juvenile idiopathic arthritis. Arthritis Rheumatol 2014; 66:2590-2600; PMID:24909567; http://dx.doi.org/10.1002/art.38736
- Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm 2014; 2014:545493; PMID:24733962
- Dimitroulas T, Nikas SN, Trontzas P, Kitas GD. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun Rev 2013; 12:958-66; PMID:23542506; http://dx.doi.org/10.1016/j.autrev.2013.03.015
- Olsen NJ, Spurlock CF, 3rd, Aune TM. Methotrexate induces production of IL-1 and IL-6 in the monocytic cell line U937. Arthritis Res Ther 2014; 16:R17; PMID:24444433; http://dx.doi.org/10.1186/ar4444
- Crilly A, McInness IB, McDonald AG, Watson J, Capell HA, Madhok R. Interleukin 6 (IL-6) and soluble IL-2 receptor levels in patients with rheumatoid arthritis treated with low dose oral methotrexate. J Rheumatol 1995; 22:224-226; PMID:7738942
- Cronstein BN. Molecular therapeutics. Methotrexate and its mechanism of action. Arthritis Rheum 1996; 39:1951-60; PMID:8961899; http://dx.doi.org/10.1002/art.1780391203
- Weisman MH, Durez P, Hallegua D, Aranda R, Becker JC, Nuamah I, Vratsanos G, Zhou Y, Moreland LW. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol 2006; 33:2162-6; PMID:17014006
- Buch MH, Boyle DL, Rosengren S, Saleem B, Reece RJ, Rhodes LA, Radjenovic A, English A, Tang H, Vratsanos G, et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann Rheum Dis 2009; 68:1220-7; PMID:18772191; http://dx.doi.org/10.1136/ard.2008.091876
- Sarkar S, Cooney LA, White P, Dunlop DB, Endres J, Jorns JM, Wasco MJ, Fox DA. Regulation of pathogenic IL-17 responses in collagen-induced arthritis: roles of endogenous interferon-gamma and IL-4. Arthritis Res Ther 2009; 11:R158; PMID:19852819; http://dx.doi.org/10.1186/ar2838
- Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Urayama S, Kawabe Y, Aoyagi T, Maeda K, Nagataki S. Inhibitory effects of interleukin-10 on synovial cells of rheumatoid arthritis. Immunology 1997; 91:252-9; PMID:9227325; http://dx.doi.org/10.1046/j.1365-2567.1997.00244.x
- Jullien D. ; Anti-drug antibodies, auto-antibodies and biotherapy in psoriasis. Ann Dermatol Venereol 2012; 139 Suppl 2:S58-67; PMID:22541730; http://dx.doi.org/10.1016/S0151-9638(12)70112-6
- Matsumoto Y, Maeda T, Tsuboi R, Okubo Y. Anti-adalimumab and anti-infliximab antibodies developed in psoriasis vulgaris patients reduced the efficacy of biologics: report of two cases. J Dermatol 2013; 40:389-92; PMID:23414225; http://dx.doi.org/10.1111/1346-8138.12093
- Kneepkens EL, Wei JC, Nurmohamed MT, Yeo KJ, Chen CY, van der Horst-Bruinsma IE, van der Kleij D, Rispens T, Wolbink G, Krieckaert CL. Immunogenicity, adalimumab levels and clinical response in ankylosing spondylitis patients during 24 weeks of follow-up. Ann Rheum Dis 2013; 74:396-401; PMID:24326011; http://dx.doi.org/10.1136/annrheumdis-2013-204185
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665-74; PMID:18486739; http://dx.doi.org/10.1016/S0140-6736(08)60725-4
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675-1684; PMID:18486740; http://dx.doi.org/10.1016/S0140-6736(08)60726-6
- van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 2013; 9:164-72; PMID:23399692; http://dx.doi.org/10.1038/nrrheum.2013.4
- Jung SM, Kim HS, Kim HR, Kim NY, Lee JH, Kim J, Kwok SK, Park KS, Park SH, Kim HY, et al. Immunogenicity of anti-tumour necrosis factor therapy in Korean patients with rheumatoid arthritis and ankylosing spondylitis. Int Immunopharmacol 2014; 21:20-5; PMID:24752013; http://dx.doi.org/10.1016/j.intimp.2014.04.006
- Roque-Navarro L, Mateo C, Lombardero J, Mustelier G, Fernandez A, Sosa K, Morrison SL, Perez R. Humanization of predicted T-cell epitopes reduces the immunogenicity of chimeric antibodies: new evidence supporting a simple method. Hybrid Hybridomics 2003; 22:245-57; PMID:14511570; http://dx.doi.org/10.1089/153685903322328974
