Abstract
Antibody therapy is a validated treatment approach for several malignancies. All currently clinically applied therapeutic antibodies (Abs) are of the IgG isotype. However, not all patients respond to this therapy and relapses can occur. IgA represents an alternative isotype for antibody therapy that engages FcαRI expressing myeloid effector cells, such as neutrophils and monocytes. IgA Abs have been shown to effectively kill tumor cells both in vitro and in vivo. However, due to the short half-life of IgA Abs in mice, daily injections are required to reach an effect comparable to IgG Abs. The relatively long half-life of IgG Abs and serum albumin arises from their capability of interacting with the neonatal Fc receptor (FcRn). As IgA Abs lack a binding site for FcRn, we generated IgA Abs with the variable regions of the Her2-specific Ab trastuzumab and attached an albumin-binding domain (ABD) to the heavy or light chain (HCABD/LCABD) to extend their serum half-life. These modified Abs were able to bind albumin from different species in vitro. Furthermore, tumor cell lysis of IgA-Her2-LCABD Abs in vitro was similar to unmodified IgA-Her2 Abs. Pharmacokinetic studies in mice revealed that the serum exposure and half-life of the modified IgA-Her2 Abs was extended. In a xenograft mouse model, the modified IgA1 Abs exhibited a slightly, but significantly, improved anti-tumor response compared to the unmodified Ab. In conclusion, empowering IgA Abs with albumin-binding capacity results in in vitro and in vivo functional Abs with an enhanced exposure and prolonged half-life.
Abbreviations
| Ab | = | antibody |
| ABD | = | albumin-binding domain |
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| ASGPR | = | asialoglycoprotein receptor |
| CPM | = | counts per minute |
| FcR | = | Fc receptor |
| FcRn | = | neonatal Fc receptor |
| HC | = | heavy chain |
| HSA | = | human serum albumin |
| LC | = | light chain |
| PMN | = | polymorphonuclear cells |
Introduction
Her2 (Her2/neu; ErbB2) is a member of the epidermal growth factor receptor family and its over-expression in certain malignancies such as breast cancer is associated with a worse clinical prognosis.Citation1 Her2 is the target of the marketed IgG1 monoclonal antibodies (mAbs) trastuzumab (Herceptin®) and pertuzumab (Perjeta®), and several others under (pre-)clinical development.
Antibodies (Abs) can exhibit direct (Fab-mediated) and indirect (Fc-mediated) anti-tumor effects. Trastuzumab has been shown to induce cytostasis upon binding of the Fab arms to Her2 by inhibiting Her2 downstream signaling.Citation2 The dominant Fc-mediated effector mechanism employed by IgG1 Abs, including trastuzumab, is the engagement of Fcγ receptors (FcγR) expressed on immune effector cells such as natural killer (NK) cells, macrophages and neutrophils. In vitro studies suggested that NK cells have the highest cytotoxic capacity with human IgG1 Abs.Citation3,4
Despite demonstrated clinical effects, IgG mAb therapy (often in conjunction with other (chemo)therapeutics) rarely results in a complete cure. Partial responses are attributed to several factors: (a) exhaustion of cellular effector mechanisms,Citation5 (b) interaction with the non-signaling FcγRIIIb,Citation6 (c) co-engagement of activating FcγR and the inhibitory FcγRIIb on monocytes resulting in inhibitory signalingCitation7 and (d) polymorphisms in FcγR such as 131 H/R in FcγRIIa and 158 V/F in FcγRIIIa, which have been associated with worse clinical outcome upon IgG1 mAb treatment.Citation8,9
Due to the limitations of IgG anti-tumor mAbs, IgA Abs have been investigated as an alternative isotype. IgA in the polymeric form is predominant at the mucosal sites, whereas the monomeric form is mainly found in serum. In humans, monomeric IgA exists as 2 subclasses: IgA1 and IgA2. For IgA2, 3 allotypes have been described: IgA2(m1), IgA2(m2) and IgA2(n). The major structural difference between IgA1 and IgA2 lies within the hinge region, which is 13 amino acids longer in IgA1. The serine/proline/threonine rich hinge region of IgA1 Abs makes them more susceptible to proteolytic cleavage by IgA1 proteases produced by pathogenic bacteria.Citation10 Furthermore, the glycosylation pattern differs between both subclasses; 5 O-linked glycans and 2 N-linked glycans are attached to the heavy chain of IgA1 Abs, whereas IgA2 Abs carry 4–5 N-linked glycans, but no O-glycans.
IgA Abs interact with innate immune effector cells, such as polymorphonuclear cells (PMNs), monocytes, macrophages, granulocytes and Kupffer cells, by binding to the myeloid FcαRI (CD89) expressed on their surface. For FcαRI, no polymorphisms affecting IgA binding have been identified yet. Activation of immune effector cells via FcαRI binding results in destruction of invading pathogens by processes such as oxidative burst, cytokine release and phagocytosis.Citation11 PMNs are the most abundant effector cells in human blood, and they have been demonstrated to readily infiltrate tumor tissue.Citation12 It has recently been shown that IgA Abs targeting EGFR induce cytotoxicity in vitro with human leukocytes, in particular with isolated PMNs.Citation13,14 Additionally, human monocyte-mediated cytotoxicity by IgA Abs is comparable to IgG1 Abs.Citation13 An IgG/IgA hybrid Ab molecule, carrying an FcγR and FcαRI recognition site, had superior phagocytic capacity with human macrophages compared to IgG1 Abs.Citation15 In vivo efficacy of IgA anti-tumor Abs has been demonstrated using human FcαRI transgenic (Tg) mice.Citation14,16 However, to reach an effective Ab concentration in vivo in a long-term tumor model, daily injections of IgA Abs were required to compensate for the short serum half-life of human IgA in mice (~15 hours).Citation14
The short serum half-life of IgA Abs is partially caused by the rapid clearance via the asialoglycoprotein receptor (ASGPR) recognizing terminal galactose residues.Citation17 Blockage of the ASGPR with a specific ligand,Citation14 improved terminal sialylation of IgACitation18 and engineered IgA Abs with fewer N-glycosylation sites (data not shown) resulted in an extension of the in vivo half-life.
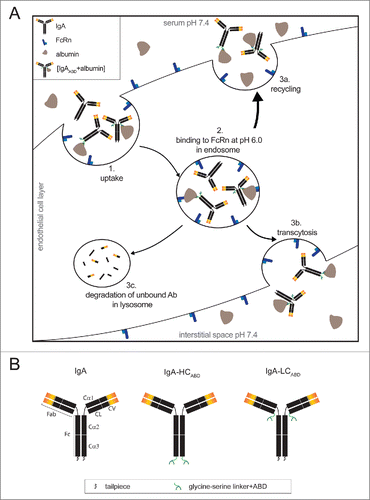
FcRn is an important receptor for placental transport of maternal IgG to the fetus,Citation19,20 enhanced phagocytosis,Citation21 and recycling or transcytosis of IgG.Citation22-25 This pH-dependent recycling pathway mediates the serum half-life of human IgG1 Abs (up to 21 d in humans,Citation26 ~9 d in miceCitation27,28) and albumin (19 d in humans,Citation29 ~2 d in mice).Citation30,31 However, it is not engaged by IgA Abs because they lack the FcRn binding site. FcRn can be indirectly targeted, e.g., by fusion of an albumin-binding domain (ABD) to the protein of interest. ABDs are small, 3-helical protein subunits expressed by various gram-positive bacteria. The most extensively studied ABD is derived from protein G of certain Streptococcus strains. Mutational studies resulted in the identification of an ABD with femtomolar affinity for human serum albumin (HSA).Citation32 ABD affinity, rather than valency, contributed to the extension of the in vivo half-life of the investigated protein.Citation33 To our knowledge, this approach has only been used for small molecules such as single-chain diabodiesCitation34 and affibody molecules.Citation35,36
Here, we aimed at targeting the FcRn recycling pathway to extend the serum half-life of full size IgA-Her2 Abs by attaching an ABD. The ABD was attached to either the heavy chain (HCABD) or light chain (LCABD) of the IgA Ab to assess whether the attachment site has an effect on FcαRI binding and subsequent Ab efficacy. We showed that ABD modified IgA Abs exhibit an increased in vivo half-life and significantly enhanced serum exposure. In vitro conjugate formation and cytotoxicity remained unaffected for the IgA-LCABD Abs upon albumin binding. However, albumin binding by IgA-HCABD Abs impaired the Fc-mediated engagement of FcαRI expressing cells. Finally, in a xenograft tumor model we showed that modified IgA1 Abs performed slightly, but significantly, better than unmodified IgA1 Abs.
Results
Generation of albumin binding IgA-Her2 antibodies
We generated Her2-targeting IgA1 and IgA2 Abs using the variable region of trastuzumab. IgA1-Her2 and IgA2-Her2 Abs mediated in vitro antibody-dependent cell-mediated cytotoxicity (ADCC) with both human PMNs and mouse effector cells and exerted anti-tumor effects in a xenograft tumor model (Fig. S1). However, in line with previous studies, serum half-life of IgA Abs was shorter than of trastuzumab, thus requiring daily injections to compensate for this. Therefore, we next generated IgA-Her2 Abs with an ABD to extend serum half-life by engaging the FcRn recycling pathway (). This was done by attaching a 5 kDa ABD derived from streptococcal protein G at the C-terminal end of either the HC or the LC (). For the attachment to the HC, the tailpiece in the Cα4 domain was substituted with the ABD. All Abs were produced in HEK293F cells and subsequently purified by anti-kappa affinity chromatography and size-exclusion chromatography (SEC; data not shown). Our final panel consisted of 2 unmodified (IgA1 and IgA2) and 4 modified (IgA1-HCABD, IgA2-HCABD, IgA1-LCABD and IgA2-LCABD) IgA-Her2 Abs. Their purity and integrity was assessed by SDS-PAGE (Fig. S2).
Figure 1. (A) Schematic illustration of neonatal Fc receptor mediated half-life extension. Abs and albumin are internalized by endothelial cells through pinocytosis (1). Upon the decrease of the pH from 7.4 to 6.0 in the endosome, albumin-bound IgA (IgAABD+albumin) associates with membrane-bound FcRn, whereas unmodified IgA Abs remain unbound (2). IgAABD+albumin complexes subsequently recycle back into the serum (3a) or undergo transcytosis and are released into the interstitial space (3b). Unmodified IgA Abs are degraded in the lysosome (3c). (B) Schematic illustration of design of albumin binding IgA-Her2 antibodies.
Glycosylation pattern analysis of albumin binding IgA-Her2 antibodies
Matrix-assisted laser desorption/ionization (MALDI)-time of flight (TOF)-mass spectrometry (MS) of enzymatically released and sialic acid-derivatized N-glycans was used to determine the main glycosylation properties of all used IgA antibodiesCitation37 (Fig. S3 and Table S2). In general, both IgA1 and IgA2 contain N-glycan structures ranging from diantennary to tetraantennary. Sialylation is mainly of the α2,3-linked variant, and ~30% of the galactoses are occupied by these sialic acids (Table S1). Attachment of the ABD to the LC shifts IgA1 toward an overall higher degree of sialylation, while IgA2 remains relatively the same. Attaching the ABD to the HC, on the contrary, leads in both IgAs to the loss of most of the larger structures (triantennary and tetraantennary species). Fucosylation is found almost exclusively at the core of the N-glycans, as determined by MS/MS, and is more abundant in the IgA2 samples than in the IgA1 samples (and in IgA1 mostly on the triantennary and tetraantennary structures).
In vitro characterization of albumin binding IgA-Her2 antibodies
First, we analyzed our panel of unmodified and modified Abs for their capability to bind to immobilized albumin from different species (). As expected, only modified IgA Abs recognized human, monkey, mouse and rat albumin, whereas bovine serum albumin (BSA) remained unbound (data not shown). The attached ABD had a high binding capacity to albumin derived from mouse, rat and monkey at serum dilutions as high as 1:100,000, corresponding to ~270–330 ng/mL albumin in rodentsCitation38 and 400–500 ng/mL albumin in monkeys.Citation39,40 However, the best binding capacity was observed for HSA as the detection signal only started to decrease at concentrations lower than 40 ng/mL, which is approximately 1×106 times less than found in human serum.
Figure 2. ABD modified IgAHer2 antibodies bind to albumin and simultaneously bind Her2 and FcαRI. (A) Solid phase binding of unmodified IgA Abs and HCABD and LCABD modified IgA Abs (100 ng/mL) to albumin derived from different species (HSA: 40 ng/mL; mouse, monkey and rat serum: 100,000x diluted) measured by ELISA. (B) Antibody-albumin complex formation in solution was determined by pre-incubating unmodified and HCABD and LCABD modified IgA Abs (130 nM) in the absence or presence of human albumin (130 nM and 260 nM) and subsequent analyses by HP-SEC. Retention time of Abs in the absence of albumin is indicated by a dotted line. (C) Conjugate formation between Her2 (SKBR3) and FcαRI (Ba/F3-FcαRI-eYFP) expressing cells upon simultaneous binding to Abs (20 μg/mL) pre-incubated without (black bar) and with (gray bar) 1 mg/mL HSA (mean ± SEM).
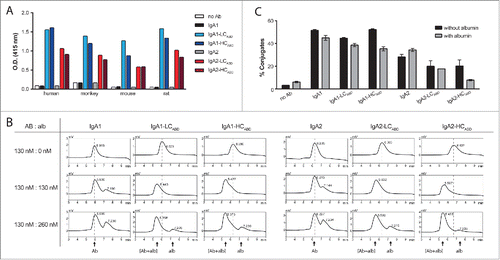
Next, we assessed albumin binding capacity in solution by pre-incubating the panel of IgA Abs in the absence or presence of albumin at various molar ratios and subsequent analysis on HP-SEC (). Albumin pre-incubation resulted in a shift of the Ab peak (from retention time ∼6 min to ∼5.5 min) for only the modified IgA Abs. This indicated that both the HCABD and LCABD IgA Abs had bound albumin in solution. When antibody and albumin were mixed in a 1:1 molar ratio, a single peak corresponding to a 1:1 complex was observed. By contrast, with a 1:2 ratio of antibody to albumin, a second peak next to the antibody-albumin complex peak was detected, which corresponds to free albumin. This suggests that only one of the 2 ABDs present in the modified IgA Abs binds albumin.
Additionally, to study whether albumin binding interferes with the simultaneous recognition of Her2 and the FcαRI, we set up a conjugation assay with SKBR3 cells and Ba/F3-FcaRI-eYFP cells (). IgA1-based Abs induced higher percentages of conjugates than IgA2-based Abs. For IgA-HCABD Abs of both subclasses, conjugate formation was impaired in the presence of albumin.
ABD attachment increases the serum exposure and half-life of IgA-Her2 antibodies
Human IgA Abs do not bind to FcRn, and therefore have a roughly 14 times shorter serum half-life compared to human IgG in mice. First, to assess whether the attachment of an ABD to a full-size IgA Ab results in an extended serum half-life, we injected 200 μg Ab intravenously into SCID mice and followed the serum Ab concentrations over time (). The overall serum exposure () of IgA1-HCABD and IgA1-LCABD was increased to a similar extent (4.7- and 5.2-fold, respectively) compared to unmodified IgA1. IgA2-LCABD Abs showed a 4.8-fold higher serum exposure compared to unmodified IgA2, which was further pronounced for IgA2-HCABD Abs (9.6-fold). Whereas the best terminal half-life (t1/2) extension was reached with the LC-modified IgA Abs, serum exposure was the least improved for the IgA2-LCABD Ab ().
Figure 3. Pharmacokinetic profiles of un-/modified IgA-Her2 antibodies. SCID mice were injected intravenously (A, B) or subcutaneously (C–F) with 200 μg un-/modified IgA1 (A, C, E) and IgA2 (B, D, F) Abs. Ab concentrations in the serum (A–D) and peritoneal cavity (E, F) were determined by ELISA (mean ± SEM).
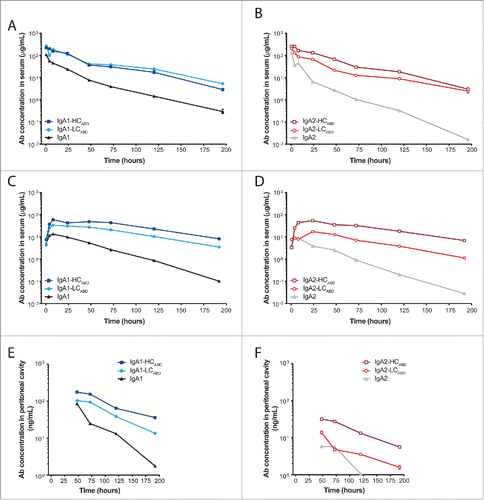
Table 1. Exposure (AUC) and terminal half-life (t1/2) of un-/modified IgA Abs.
Next, we performed a multi-compartment pharmacokinetics (PK) study to determine distribution and clearance properties of the Abs, which were required for the set-up of our in vivo tumor models. Mice were injected with 200 μg Ab subcutaneously and the Ab concentrations in both the serum () and peritoneal cavity () were monitored. All IgA Abs reached their maximum serum levels within 24 h, with serum levels for the unmodified IgA Abs remaining lower than for the modified IgA Abs. In this experimental setting, both IgA-LCABD Abs reached a comparable increase in serum exposure (IgA-LCABD 5.3-fold; IgA2-LCABD 4.5-fold). The IgA-HCABD Abs also reached a similar serum exposure, but compared to the matched unmodified subclass, the serum exposure of IgA2-HCABD Abs was increased 17.9-fold compared to 10.1-fold for IgA1-HCABD Abs. t1/2 was enhanced for all modified IgA Abs to a comparable extend after subcutaneous injection (). All Abs, except IgA2, were detectable in the peritoneal cavity until at least day 8, although at levels 2 orders of magnitude lower than in serum (). IgA1 Abs were transported to the peritoneal cavity more efficiently compared to IgA2 Abs. Like in the serum, concentrations of the IgA-HCABD Abs in the peritoneal cavity were the highest, probably reflecting the higher serum exposure of these Abs.
Tumor cell killing ex vivo is comparable between unmodified and IgA-LCABD antibodies
IgA Abs induce cytotoxicity by the interaction of their Fc tail with FcαRI expressed on effector cells. To determine if albumin binding to modified IgA Abs influences tumor cell lysis, we performed a human PMN ADCC assay (). We pre-incubated the panel of IgA Abs either in the absence or presence of albumin before adding them to the freshly isolated PMNs and SKBR3 cells. In the absence of albumin, all IgA1 and IgA2 Abs induced similar tumor cell lysis (). The unmodified and IgA-LCABD Abs remained unaffected in their lytic capacity after pre-incubation with albumin; however, IgA-HCABD Abs induced a lower maximal tumor cell lysis.
Figure 4. Cytotoxic potential of un-/modified IgA-Her2 antibodies. Specific lysis of SKBR3 cells by IgA (A, D), IgA-LCABD (B, E) and IgA-HCABD (C, F) Abs determined in 4 h ADCC assays with human PMNs (E:T = 40:1) (mean ± SEM). Un-/modified IgA1 Abs (A–C) or IgA2 Abs (D–F) were pre-incubated without (solid line) or with (dotted line) human albumin. Results are representative of 4 separate assays.
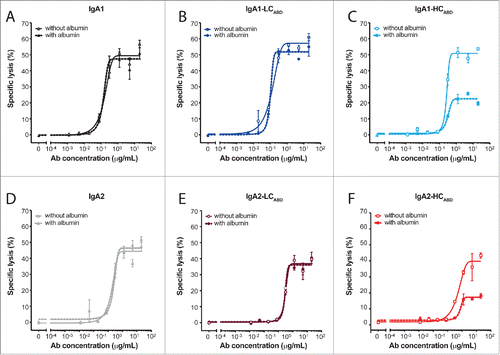
Table 2. Maximal lytic capacity of un-/modified IgA Abs in human PMN ADCC assay.
Enhanced serum exposure and half-life by modified IgA antibodies improve anti-tumor effect in vivo
To study whether the improved half-life of the modified IgA Abs results in better tumor cell killing in a therapeutic setting in vivo, we tested the Abs in a multi-compartment tumor model. First, we performed a pilot experiment with A431-luc2-Her2 cells injected intraperitoneally and after 7 d treated tumor-bearing SCID mice once with various concentrations of trastuzumab injected subcutaneously. From this we considered 10 μg Ab to be a suboptimal dose for trastuzumab (Fig. S4A and B). The final treatment regimen was determined by modeling IgA2-HCABD, which had the best PK values (), to find optimal exposure time without accumulation upon repeated treatment (Fig. S4C). Eventually, we injected wild type (WT) and FcαRI Tg tumor-bearing SCID mice with 50 μg IgA Abs subcutaneously once a week, for 5 weeks, starting one week after tumor inoculation. Tumor outgrowth was monitored by serial bioluminescent imaging (BLI). No Fab-mediated anti-tumor effect could be observed, as the tumor outgrowth in Ab treated WT mice was comparable to the phosphate-buffered saline (PBS)-treated control group (). In Tg mice, IgA1 treatment () resulted in an anti-tumor response, which was similarly improved by both modified IgA1 Abs. The increase in tumor volume over time (from day 6 to day 56) of the unmodified IgA1 Ab was significantly higher than for the modified IgA1 Abs (384 vs. 167 CPM/cm2 per day for IgA1-HCABD, P = 0.0057; 384 vs. 188 CPM/cm2 per day for IgA1-LCABD, P = 0.0106). For IgA2, the effect of the ABD attachment was less pronounced. As shown in , only the IgA2-LCABD Ab appeared effective in therapy until a week after the last treatment (day 41). Until day 41, a significantly lower increase in tumor volume over time was achieved with IgA2-LCABD Abs compared to the unmodified IgA2 Ab (460 vs. 1124 CPM/cm2 per day, P = 0.0003).
Figure 5. (See previous page) Therapeutic efficacy of un-/modified IgA-Her2 antibodies in a multi-compartment xenograft model. FcαRI Tg or WT SCID mice were injected intraperitoneally with 105 A431-luc2-Her2 cells. On day 6 tumor growth was measured by BLI and mice were randomized. Starting on day 7, mice were treated once weekly (arrow) with 50 μg un-/modified IgA Abs or PBS. Tumor outgrowth was measured one day before treatment by BLI. Tumor volume (CPM/cm2) over time for each measurement (5–10 mice/group) for WT SCID mice (A,B) and FcαRI Tg SCID mice (C,D) for (A,C) IgA1, IgA1-HCABD, IgA-1LCABD and PBS or (B,D) IgA2, IgA2-HCABD, IgA2-LCABD and PBS (mean ± SEM). (E) Representative BLI images of FcαRI Tg SCID mice treated with IgA Abs and WT SCID mice treated with PBS 6 d after first treatment (day 13), 6 d after last treatment (day 41) and 15 d after last treatment (day 56). (F) Tumor volume (CPM/cm2) of FcαRI Tg SCID mice on day 41 (mean ± SEM). (G) Ab concentrations in peritoneal cavity and serum of mice on day 36 (mean ± SEM; n.d. not detectable).
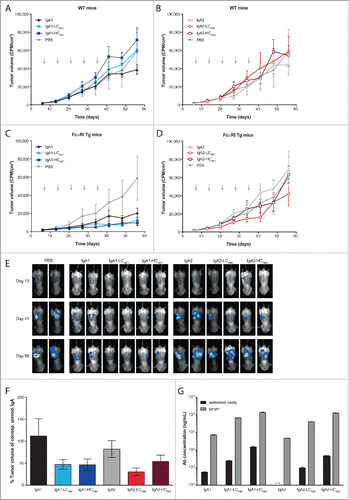
To investigate whether the differences between the IgA1 and IgA2 modified Abs could be explained by differences in their transport to the tumor site, we examined Ab distribution into the peritoneal cavity on day 36, one day after the last treatment. As shown in , IgA1 Abs reached the peritoneal cavity more effectively, confirming what we have seen before (). Ab levels found in the serum reflected Ab concentrations in the peritoneal cavity. Furthermore, for each Ab, serum Ab titers one day after the first injection (day 8) and last injection (day 36) were comparable, demonstrating that the dosing regimen indeed did not cause accumulation of the Abs neither in serum nor in the peritoneal cavity (Fig. S5A and B). Both unmodified IgA Abs were mostly cleared or undetectable in serum one day before the last injection (day 34) (Fig. S5C). However, the modified Abs were still detectable in the serum at this time point.
In conclusion, ABD addition to full-size IgA Abs significantly increased their serum exposure. This resulted in an increased Ab concentration in the peritoneal cavity, and provided modified IgA1 Abs and IgA2-LCABD Abs with significantly better therapeutic potential than unmodified parental Abs of the same subclass.
Discussion
Monomeric IgA Abs represent promising therapeutic agents for the treatment of cancer.Citation13,14,16,41 However, in vivo, daily injections are required to compensate for their short half-life in mice.Citation14 In this study, we generated and evaluated albumin binding IgA-Her2 Abs regarding in vitro and in vivo functional activity and half-life extension in mice.
ABD modified IgA Abs bound to albumin derived from different species immobilized on a surface and to HSA in solution. The attachment of the ABD had no effect on the lytic capacity of human PMNs in the absence of albumin. However, ABD attachment to the HC resulted in an impaired ADCC efficacy in vitro after pre-incubation of the Abs with albumin. This is possibly due to interference of the bound albumin with FcαRI binding, which occurs at the interface of Cα2 and Cα3. As both IgA subclasses were equally affected, neither their structural differences nor the additional N-linked glycosylation sites in IgA2 are likely to play a role. This is in line with previously published literature stating that FcαRI binding by IgA remains unaffected by the antibodies' glycosylation state.Citation42 The partial reduction of the ADCC capacity of IgA-HCABD Abs compared to the unmodified IgA Abs suggests that the binding of one albumin, as determined by HP-SEC, does not interfere with both FcαRI recognition sites on the IgA Fc tail.
In our PK studies, all IgA Abs carrying an ABD exhibited an augmented serum exposure compared to the unmodified IgA Abs, suggesting that albumin binding and subsequent recycling by FcRn occurred in vivo. The attachment of an ABD to small molecules was demonstrated previously to result in the extension of their half-lives in mice. The involvement of FcRn mediated recycling was confirmed by performing PK studies in FcRn knockout mice.Citation43 However, the reduced renal clearance due to their increased molecular weight (>60 kDa) contributed further to the extension of the half-life, a mechanism that is irrelevant for our full-size IgA Abs.
So far, few PK studies directly comparing IgA1 and IgA2 Abs have been performed in mice. We detected less IgA2 than IgA1 in the serum of mice after intravenous and subcutaneous injections. However, glycosylation analysis of the antibodies has shown that sialylation is higher for IgA2 than for IgA1. In addition, antennarity is higher for IgA1 than for IgA2, leading to more exposed galactoses. This is counter-intuitive to literature, as it has been shown that clearance of plasma IgA1 Abs is less affected by the clearance via the ASGPR than of IgA2 Abs,Citation44 possibly due to the higher degree of terminal sialylation of N-linked glycans of monomeric serum IgA1.Citation45-49 Indeed, when the glycosylation pattern of IgA2 Abs was modified, either by increasing the degree of terminal sialylation,Citation18 or by reducing the number of glycosylation sites (data not shown), an extended in vivo half-life in mice was achieved. However, comparing these half-life data obtained in different studies is complicated since distinct mechanisms were altered and different recombinant protein production systems were used. It is known that expression cell lines differ in their expression of sugar-modifying enzymes such as sialyltransferases.Citation50 Furthermore, we found that IgA2-HCABD Abs had a higher serum exposure compared to IgA2 and IgA2-LCABD Abs, possibly due to the replacement of the tailpiece with the ABD, resulting in the removal of an N-linked glycosylation site at Asn 459 and subsequent loss of most tri- and tetraantennary glycan species. Addition of the ABD causes albumin binding but also glycosylation changes, and the contribution of either property to the different kinetics remains to be elucidated.
Our modified IgA Abs did not reach the same half-life and exposure as IgG1 Abs. This, however, was not unexpected as the half-life of human IgG1 in mice is suggested to be overestimated due to a 10–Citation51 to 85–Citation52 fold higher affinity of murine FcRn for human IgG1 compared to murine IgG1. Using values from literature, we estimated a roughly 14-fold difference in half-life of human IgG1 compared to human IgA in mice. However, albumin in mice has a half-life of ~2 days. With our LC-modified IgA Abs, we reached a similar terminal half-life. Thus, to enhance the in vivo half-life of human IgA Abs in mice to facilitate pre-clinical testing, a combination of both strategies is likely needed: (a) glyco-engineering toward full sialylation to avoid quick hepatic clearance, and (b) FcRn engagement to guarantee successful recycling.
Mechanisms contributing to the clearance of IgA Abs are similar between humans and mice. Based on studies in non-human primates, the catabolism of IgA Abs in humans has been suggested to be predominantly mediated by hepatic uptake via the ASGPR. In humans, a 3.5-fold difference in serum half-life of human monomeric IgA1 and IgA2 Abs (5–9 days and 4–5 dCitation53) compared to IgG1 Abs (21 daysCitation26) was found. To extrapolate our findings to the human context, we anticipate that mainly glyco-engineering of IgA Abs is required to achieve an effective half-life. However, next to recycling and phagocytosis, the FcRn plays a role in transcytosis of IgG over epithelial cell layers. Thus, for improved transcytosis of IgA Abs to reach tumor tissues, targeting FcRn might be helpful. However, the binding of an albumin to a full-size Ab significantly increases molecular weight, thereby restricting tissue penetration. As an alternative to the indirect targeting of FcRn, small peptides directly interacting with FcRn have been developed.Citation54-56
So far, no studies with ABD fusion molecules have been performed in humans. Possible immunogenicity of the ABD because of its bacterial origin is conceivable, but might be minimal due to its small size. Additionally, ABDs are part of the normal bacterial flora and thus have evolved to escape immune response. Furthermore, a successful deimmunization of an ABD has been reported.Citation57
Due to the extension of the in vivo half-life and enhancement of Ab exposure upon ABD attachment, we were able to design an in vivo therapeutic study with less frequent Ab injections compared to a previous study with IgA Abs.Citation14 We detected more IgA1 than IgA2 in the peritoneal cavity of tumor-bearing mice, possibly explaining the better anti-tumor response with IgA1 Abs. Ab concentrations in serum for each modification were lower for IgA2 Abs than for IgA1 Abs, but not as pronounced as in the peritoneal cavity. These findings suggest that albumin binding to IgA might trigger FcRn-mediated transcytosis, and that this process is somehow more effective for IgA1 Abs than IgA2 Abs. Furthermore, although IgA1-HCABD had ~2-fold higher Ab concentrations in the peritoneal cavity compared to IgA1-LCABD, both Abs performed similarly with respect to the therapeutic activity. Thus, the higher exposure reached with IgA1-HCABD Abs in the peritoneal cavity may result in the best possible anti-tumor response, whereas with the IgA1-LCABD Ab probably no full tumor opsonization was achieved due to suboptimal dosing. Among the IgA2 Abs, tumor growth was significantly impaired until day 41 with the LC modified variant. The response and Ab titers with IgA2-LCABD in the peritoneal cavity were comparable to the unmodified IgA1 Ab, suggesting that the multi-compartment xenograft model used is likely to be suboptimal for the study of the in vivo efficacy of modified IgA2 Abs.
In conclusion, ABD-modified IgA Abs showed in vitro and in vivo anti-tumor effects, further strengthening the concept of therapeutic IgA Abs. As demonstrated here, the major in vivo limitation of their short half-life can be successfully addressed by Ab engineering strategies. For future strategies it would be advised to use albumin-deficient mice for ABD-modified IgA therapeutics, as injected HSA has a half-life of even 20 days,Citation58 therefore fewer injections of IgA will be needed.
Material and Methods
Cell lines
A431 cells (ATCC) were lentivirally transduced with a luciferase-GFP construct and then retrovirally transduced with Her2 to generate A431-luc2-Her2 cells. A subclone with good in vivo outgrowth and stable Her2 expression was further used. A431-luc2-Her2 cells and SKBR3 cells (ATCC) were cultured in RPMI 1640+HEPES+glutamine (Invitrogen) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies) at 37°C/5% CO2. Ba/F3-FcαRI-eYFP cells were generated by co-electroporation of pEGFP-N1 containing the FcαRI(wt)-YFP sequence (lacking the internal eGFP) and pSG5-CMV-Hygro (0.28V; capacitance 960 μFD) into Ba/F3 cells. Stable cell lines were cultured in RPMI 1640 Glutamax + HEPES (Invitrogen) supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 250 μg/ml G418 (Invitrogen) and murine IL-3 (kindly provided by Paul Coffer, UMC Utrecht). HEK FreeStyleTM293F cells (Invitrogen) were cultured in GIBCO FreeStyleTM 293 expression medium (Invitrogen) at 37°C/8% CO2 on an orbital shaker (125 rpm).
Mice
Human FcαRI Tg mice were generated at the UMC UtrechtCitation59 and were backcrossed to an immunodeficient SCID background (Charles River) and maintained as hemizygous. Transgene-negative littermates were used as controls. All experiments were approved by the Animal Ethical Committee of the UMC Utrecht.
Antibody production and purification
The variable region of the HC and LC for the generation of IgA-Her2 Abs is derived from trastuzumab (Accession number DB00072). Sequences coding for the albumin binding domain (ABD-H33) attached to the HC or the kappa LC by a glycine-serine linker (9GS) were synthesized by Shinegene (Shanghai, China). Antibody sequences were cloned into Lonza expression vectors with the following backbones: pEE14.4-kappaLC, pEE14.4-IgA1 and pEE14.4-IgA2(m1) (vector sequences available upon request). All IgA-Her2 Abs were produced by transient transfection of HEK293F cells with the HC coding plasmid, the LC coding plasmid and pAdvantage (Accession Number U47294; Promega) using 293fectin transfection reagent (Invitrogen) following the manufacturer's instructions. Ab containing supernatants were harvested 4 d after transfection. Abs were purified as described before.Citation13 Briefly, Abs secreted into the supernatant were isolated by anti-kappa affinity chromatography (HiTrap KappaSelect, GE-Healthcare) and bound protein was eluted using 0.1 M glycine pH 2.5 (VWR International) and directly neutralized with 1 M Tris-HCl pH 8.8 (Roche Diagnostics). Elution fractions were subsequently subjected to SEC (HiPrep 26/60 Sephacryl S-300 High Resolution, GE-Healthcare). Ab concentrations were determined using the following formula: OD values at 280 nm/correction factor 1.4. The production yield of the unmodified and modified Abs varied between 30 to 70 μg antibody/mL; the ABD-modified Abs tended to be in the lower part of the range (30 to 50 μg antibody/mL).
Antibody concentration determination in serum samples
Maxisorp NUNC plates (Sanbio) were coated overnight at 4°C with 0.5 μg/mL goat IgG anti-human kappa (Southern Biotech) in PBS followed by a blocking step with 1% BSA (Roche Diagnostics) in 0.05% Tween 20 (Immunologic) in PBS. Samples and standards were diluted in 1% BSA/0.05% Tween 20 in PBS and incubated on the microtiter plate for 1.5 h at room temperature. Horseradish peroxidase (HRP)-labeled goat IgG anti-human IgA (1:2,000; Southern Biotech) was used for 1 h at room temperature for detection. Plates were developed using ABTS substrate (Roche Diagnostics) and read on a Multiscan RC (Thermolab systems) at 415 nm.
Antibody binding to albumin
HSA (Albuman, Sanquin) or serum derived from different species (SCID mouse, Wistar rat, cynomolgus monkey, kindly provided by the Animal Facility Utrecht and Synthon Biopharmaceuticals BV) was coated on Maxisorp NUNC plates (Sanbio) overnight at 4°C followed by a blocking step with 1% BSA in 0.05% Tween 20 in PBS. 100 ng/mL IgA Abs diluted in 1% BSA in 0.05% Tween 20 in PBS were added to the wells and incubated for 1.5 h at room temperature. HRP-labeled anti-human IgA (1:2,000; Southern Biotech) was used for 1 h at room temperature for detection. Plates were developed for 10 min using ABTS substrate (Roche Diagnostics) and read on a Multiscan RC (Thermolab systems) at 415 nm.
Conjugate assay
SKBR3 cells labeled with CellTrace violet (Life Technologies) were incubated for 45 min on ice with 20 μg/mL Abs pre-incubated without or with 1 mg/mL HSA for 1 h at room temperature. After extensive washing with cold fluorescence-activated cell sorting (FACS) buffer, Ba/F3-FcαRI-eYFP cells were added in a 1:1 ratio and the cell mix was incubated for another hour on ice. Cells were fixed with 1% paraformaldehyde (Klinipath) in PBS and subsequently analyzed on a FACS CantoII (BD Biosciences). % conjugates was calculated as (events double positive cells / events (double positive cells + CellTrace violet+ tumor cells))*100%.
HP-SEC
130 nM IgA Abs were pre-incubated at various molar ratios with HSA diluted in PBS for at least 1 h at room temperature. Formation of albumin-antibody complexes was analyzed by HPLC size-exclusion chromatography (HP-SEC) (Yarra™ 3u SEC-2000 column; Phenomenex) with 100 mM sodium phosphate/150 mM NaCl pH 6.8 as running buffer and a flow rate of 0.35 mL/min.
Human PMN ADCC
ADCC was measured using a chromium-release assay as previously described.Citation41 Briefly, SKBR3 cells labeled with 100 μCi 51chromium (per 106 cells) were added to Abs pre-incubated for 1 h in PBS or HSA (final concentration in assay 1 mg/mL) in round-bottom microtiter plates (Corning Inc.). PMNs were isolated from blood from healthy donors (MiniDonorDienst UMC Utrecht) by ficoll-histopaque density gradient and added with an effector-to-target (E:T) ratio of 40:1 in a final assay volume of 200 μL/well. The cells were incubated for 4 h at 37°C/5% CO2. Subsequently, the supernatant was transferred to a Deepwell LumaPlate (Perkin Elmer) and counted in a liquid scintillation counter (MicroBeta; Perkin Elmer). Lysis was calculated using the following formula: % lysis = ((counts of sample - minimal release)/(maximum release − minimum release))*100. Culture medium was used to determine minimal release and 3% TritonX-100 (Roche Diagnostics) to determine maximum release.
PK study
FcαRI Tg or WT SCID mice were injected intravenously or subcutaneously with 200 μg IgA Abs (3 mice/group). Blood was collected by tail vein cut or cheek pouch from alternating mice at indicated time points. Blood samples were processed and serum stored at −20°C. Peritoneal lavages were performed with 1 mL PBS at indicated time points. The human IgA Ab concentrations in the sera and peritoneal lavage samples were determined by ELISA. Serum exposure and t1/2 was calculated with WinNonlin 6.3 (Certara, Princeton, USA).
Multi-compartment xenograft model
FcαRI Tg/WT male SCID mice were injected intraperitoneally with 1 × 105 A431-luc2-Her2 cells in 100 μL PBS on day 0. On day 6, tumor size was assessed by BLI (PhotonImager, Biospace Lab) and the mice were randomized into different treatment groups (5–10 mice/group). 50 μg Ab/mouse was injected subcutaneously weekly (day 7, 14, 21, 28, 35) and tumor outgrowth was followed by serial BLI. Image analysis was performed using M3Vision software (Biospace Lab). To assess the PK of the Abs, 2 additional tumor-bearing mice per Ab type were used. Blood samples were obtained a day after the first treatment (day 8) and a day before (day 34) and after the last (day 36) treatment (1 mouse/time point). Peritoneal lavage samples in 2 mL PBS were obtained 24 h after the first injection and 1 day after the last injection (1 mouse/time point). Ab concentrations in peritoneal lavages and serum samples were determined as described above.
Statistical analysis
Data were graphed and analyzed using GraphPad Prism 6.0. (Graph Pad Software, CA, USA). In the xenograft model, linear regression analysis was performed to estimate tumor outgrowth over time in each treatment group and the slopes were compared using an F-test. A P value <0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
KMAB_A_1106658_supplemental_material.zip
Download Zip (5.7 MB)Acknowledgments
We thank the personnel of the animal facility of UMC Utrecht for excellent animal care, Dr. Stefan Lohse and Dr. Jan G. J. van de Winkel for discussion, Julia Drylewicz for the useful advice on the statistical analysis and the Minidonors from the UMC Utrecht. This work was supported by Synthon Biopharmaceuticals BV, the Netherlands. K.R.R. was supported by the European Union (Seventh Framework Program HighGlycan project, grant number 278535).
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235:177-82; PMID:3798106; http://dx.doi.org/10.1126/science.3798106
- Fiszman GL, Jasnis MA. Molecular mechanisms of trastuzumab resistance in HER2 overexpressing breast cancer. Int J Breast Cancer 2011; 2011:352182; PMID:22295219; http://dx.doi.org/10.4061/2011/352182
- Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med 2008; 6:25, 5876-6-25; PMID:18485193; http://dx.doi.org/10.1186/1479-5876-6-25
- Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol 2011; 2011:379123; PMID:21660134; http://dx.doi.org/10.1155/2011/379123
- Beurskens FJ, Lindorfer MA, Farooqui M, Beum PV, Engelberts P, Mackus WJ, Parren PW, Wiestner A, Taylor RP. Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol 2012; 188:3532-41; PMID:22368276; http://dx.doi.org/10.4049/jimmunol.1103693
- Derer S, Glorius P, Schlaeth M, Lohse S, Klausz K, Muchhal U, Desjarlais JR, Humpe A, Valerius T, Peipp M. Increasing FcγRIIa affinity of an FcγRIII-optimized anti-EGFR antibody restores neutrophil-mediated cytotoxicity. mAbs 2014; 6:409-21; PMID:24492248; http://dx.doi.org/10.4161/mabs.27457
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:10742152; http://dx.doi.org/10.1038/74704
- Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 2007; 25:3712-8; PMID:17704420; http://dx.doi.org/10.1200/JCO.2006.08.8021
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26:1789-96; PMID:18347005; http://dx.doi.org/10.1200/JCO.2007.14.8957
- Senior BW, Woof JM. The influences of hinge length and composition on the susceptibility of human IgA to cleavage by diverse bacterial IgA1 proteases. J Immunol 2005; 174:7792-9; PMID:15944283; http://dx.doi.org/10.4049/jimmunol.174.12.7792
- Morton HC, van Egmond M, van de Winkel JG. Structure and function of human IgA fc receptors (fc α R). Crit Rev Immunol 1996; 16:423-40; PMID:8954257
- Gregory AD, Houghton AM. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res 2011; 71:2411-6; PMID:21427354; http://dx.doi.org/10.1158/0008-5472.CAN-10-2583
- Lohse S, Brunke C, Derer S, Peipp M, Boross P, Kellner C, Beyer T, Dechant M, van der Winkel JG, Leusen JH, et al. Characterization of a mutated IgA2 antibody of the m(1) allotype against the epidermal growth factor receptor for the recruitment of monocytes and macrophages. J Biol Chem 2012; 287:25139-50; PMID:22679018; http://dx.doi.org/10.1074/jbc.M112.353060
- Boross P, Lohse S, Nederend M, Jansen JH, van Tetering G, Dechant M, Peipp M, Royle L, Liew LP, Boon L, et al. IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med 2013; 5:1213-26; PMID:23918228; http://dx.doi.org/10.1002/emmm.201201929
- Kelton W, Mehta N, Charab W, Lee J, Lee CH, Kojima T, Kang TH, Georgiou G. IgGA: A “cross-isotype” engineered human fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol 2014; 21:1603-9; PMID:25500223; http://dx.doi.org/10.1016/j.chembiol.2014.10.017
- Pascal V, Laffleur B, Debin A, Cuvillier A, van Egmond M, Drocourt D, Imbertie L, Pangault C, Tarte K, Tiraby G, et al. Anti-CD20 IgA can protect mice against lymphoma development: Evaluation of the direct impact of IgA and cytotoxic effector recruitment on CD20 target cells. Haematologica 2012; 97:1686-94; PMID:22689689; http://dx.doi.org/10.3324/haematol.2011.061408
- Stockert RJ, Kressner MS, Collins JC, Sternlieb I, Morell AG. IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci U S A 1982; 79:6229-31; PMID:6292896; http://dx.doi.org/10.1073/pnas.79.20.6229
- Rouwendal GJA, van der Lee MM, Meyer S, Reiding KR, Schouten J, de Roo G, Egging DF, Leusen JHW, Boross P, Wuhrer M, et al. A comparison of anti-HER2 IgA and IgG1 in vivo efficacy is facilitated by high N-glycan sialylation of the IgA. mAbs 2016; http://dx.doi.org/10.1080/19420862.2015.1102812
- Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I-like fc receptor cloned from human placenta: Possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med 1994; 180:2377-81; PMID:7964511; http://dx.doi.org/10.1084/jem.180.6.2377
- Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, Ghetie V, Ward ES. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol 2001; 13:993-1002; PMID:11470769; http://dx.doi.org/10.1093/intimm/13.8.993
- Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M, van de Winkel JG. FcRn: An IgG receptor on phagocytes with a novel role in phagocytosis. Blood 2006; 108:3573-9; PMID:16849638; http://dx.doi.org/10.1182/blood-2006-05-024539
- Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest 1999; 104:903-11; PMID:10510331; http://dx.doi.org/10.1172/JCI6968
- Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, et al. MHC class I-related neonatal fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol 2001; 166:3266-76; PMID:11207281; http://dx.doi.org/10.4049/jimmunol.166.5.3266
- Ghetie V, Ward ES. Transcytosis and catabolism of antibody. Immunol Res 2002; 25:97-113; PMID:11999172; http://dx.doi.org/10.1385/IR:25:2:097
- Goebl NA, Babbey CM, Datta-Mannan A, Witcher DR, Wroblewski VJ, Dunn KW. Neonatal fc receptor mediates internalization of fc in transfected human endothelial cells. Mol Biol Cell 2008; 19:5490-505; PMID:18843053; http://dx.doi.org/10.1091/mbc.E07-02-0101
- Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest 1970; 49:673-80; PMID:5443170; http://dx.doi.org/10.1172/JCI106279
- Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 1988; 18:313-6; PMID:3350037; http://dx.doi.org/10.1002/eji.1830180221
- Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci U S A 2009; 106:2788-93; PMID:19188594; http://dx.doi.org/10.1073/pnas.0810796106
- Peters T Jr. Serum albumin. Adv Protein Chem 1985; 37:161-245; PMID:3904348; http://dx.doi.org/10.1016/S0065-3233(08)60065-0
- Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack β 2-microglobulin: Possible protective role of FcRn. Immunology 1996; 89:573-8; PMID:9014824; http://dx.doi.org/10.1046/j.1365-2567.1996.d01-775.x
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in β 2-microglobulin-deficient mice. Eur J Immunol 1996; 26:690-6; PMID:8605939; http://dx.doi.org/10.1002/eji.1830260327
- Jonsson A, Dogan J, Herne N, Abrahmsen L, Nygren PA. Engineering of a femtomolar affinity binding protein to human serum albumin. Protein Eng Des Sel 2008; 21:515-27; PMID:18499681; http://dx.doi.org/10.1093/protein/gzn028
- Hopp J, Hornig N, Zettlitz KA, Schwarz A, Fuss N, Muller D, Kontermann RE. The effects of affinity and valency of an albumin-binding domain (ABD) on the half-life of a single-chain diabody-ABD fusion protein. Protein Eng Des Sel 2010; 23:827-34; PMID:20817756; http://dx.doi.org/10.1093/protein/gzq058
- Stork R, Muller D, Kontermann RE. A novel tri-functional antibody fusion protein with improved pharmacokinetic properties generated by fusing a bispecific single-chain diabody with an albumin-binding domain from streptococcal protein G. Protein Eng Des Sel 2007; 20:569-76; PMID:17982179; http://dx.doi.org/10.1093/protein/gzm061
- Andersen JT, Pehrson R, Tolmachev V, Daba MB, Abrahmsen L, Ekblad C. Extending half-life by indirect targeting of the neonatal fc receptor (FcRn) using a minimal albumin binding domain. J Biol Chem 2011; 286:5234-41; PMID:21138843; http://dx.doi.org/10.1074/jbc.M110.164848
- Malm M, Bass T, Gudmundsdotter L, Lord M, Frejd FY, Stahl S, Lofblom J. Engineering of a bispecific affibody molecule towards HER2 and HER3 by addition of an albumin-binding domain allows for affinity purification and in vivo half-life extension. Biotechnol J 2014; 9:1215-22; PMID:24678002; http://dx.doi.org/10.1002/biot.201400009
- Reiding KR, Blank D, Kuijper DM, Deelder AM, Wuhrer M. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal Chem 2014; 86:5784-93; PMID:24831253; http://dx.doi.org/10.1021/ac500335t
- Peters T Jr. All about Albumin: Biochemistry, Genetics and Medical Applications. San Diego, CA, USA: Elsevier Academic Press, 1996.
- Wolfe-Coote S, ed. The Handbook of Experimental Animals: The Laboratory Primate. San Diego, CA, USA: Elsevier Academic Press; 2005.
- Wang H, Niu YY, Si W, Li YJ, Yan Y. Reference data of clinical chemistry, haematology and blood coagulation parameters in juvenile cynomolgus monkeys (macaca fascicularis). Veterinarni Medicina 2012; 57:233-8.
- Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JG, Valerius T. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol 2007; 179:2936-43; PMID:17709508; http://dx.doi.org/10.4049/jimmunol.179.5.2936
- Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB. Analysis of IgA1 N-glycosylation and its contribution to FcalphaRI binding. Biochemistry 2008; 47:11285-99; PMID:18826328; http://dx.doi.org/10.1021/bi801185b
- Stork R, Campigna E, Robert B, Muller D, Kontermann RE. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J Biol Chem 2009; 284:25612-9; PMID:19628871; http://dx.doi.org/10.1074/jbc.M109.027078
- Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (ig)A1 and IgA2 isotypes. J Exp Med 2000; 191:2171-82; PMID:10859341; http://dx.doi.org/10.1084/jem.191.12.2171
- Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem 1974; 249:7260-9; PMID:4436308
- Endo T, Mestecky J, Kulhavy R, Kobata A. Carbohydrate heterogeneity of human myeloma proteins of the IgA1 and IgA2 subclasses. Mol Immunol 1994; 31:1415-22; PMID:7823967; http://dx.doi.org/10.1016/0161-5890(94)90157-0
- Field MC, Amatayakul-Chantler S, Rademacher TW, Rudd PM, Dwek RA. Structural analysis of the N-glycans from human immunoglobulin A1: Comparison of normal human serum immunoglobulin A1 with that isolated from patients with rheumatoid arthritis. Biochem J 1994; 299 ( Pt 1):261-75; PMID:8166649; http://dx.doi.org/10.1042/bj2990261
- Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, fab, and fc regions and the role of N-glycosylation on fcalpha receptor interactions. J Biol Chem 1998; 273:2260-72; PMID:9442070; http://dx.doi.org/10.1074/jbc.273.4.2260
- Oortwijn BD, Roos A, Royle L, van Gijlswijk-Janssen DJ, Faber-Krol MC, Eijgenraam JW, Dwek RA, Daha MR, Rudd PM, van Kooten C. Differential glycosylation of polymeric and monomeric IgA: A possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol 2006; 17:3529-39; PMID:17050773; http://dx.doi.org/10.1681/ASN.2006040388
- Raju TS, Briggs JB, Borge SM, Jones AJ. Species-specific variation in glycosylation of IgG: Evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 2000; 10:477-86; PMID:10764836; http://dx.doi.org/10.1093/glycob/10.5.477
- Zhou J, Mateos F, Ober RJ, Ward ES. Conferring the binding properties of the mouse MHC class I-related receptor, FcRn, onto the human ortholog by sequential rounds of site-directed mutagenesis. J Mol Biol 2005; 345:1071-81; PMID:15644205; http://dx.doi.org/10.1016/j.jmb.2004.11.014
- Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem 2010; 285:4826-36; PMID:20018855; http://dx.doi.org/10.1074/jbc.M109.081828
- Morell A, Skvaril F, Noseda G, Barandun S. Metabolic properties of human IgA subclasses. Clin Exp Immunol 1973; 13:521-8; PMID:4717094
- Mezo AR, McDonnell KA, Hehir CA, Low SC, Palombella VJ, Stattel JM, Kamphaus GD, Fraley C, Zhang Y, Dumont JA, et al. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal fc receptor FcRn. Proc Natl Acad Sci U S A 2008; 105:2337-42; PMID:18272495; http://dx.doi.org/10.1073/pnas.0708960105
- Seijsing J, Lindborg M, Hoiden-Guthenberg I, Bonisch H, Guneriusson E, Frejd FY, Abrahmsen L, Ekblad C, Lofblom J, Uhlen M, et al. An engineered affibody molecule with pH-dependent binding to FcRn mediates extended circulatory half-life of a fusion protein. Proc Natl Acad Sci U S A 2014; 111:17110-5; PMID:25406323; http://dx.doi.org/10.1073/pnas.1417717111
- Sockolosky JT, Tiffany MR, Szoka FC. Engineering neonatal fc receptor-mediated recycling and transcytosis in recombinant proteins by short terminal peptide extensions. Proc Natl Acad Sci U S A 2012; 109:16095-100; PMID:22991460; http://dx.doi.org/10.1073/pnas.1208857109
- Frejd FY. Half-life extension by binding to albumin through an albumin binding domain. In: Kontermann R, ed. Therapeutic Proteins: Strategies to Modulate their Plasma Half-Lives. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2012:269.
- Roopenian DC, Low BE, Christianson GJ, Proetzel G, Sproule TJ, Wiles MV. Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs. mAbs 2015; 7:344-51; PMID:25654695; http://dx.doi.org/10.1080/19420862.2015.1008345
- van Egmond M, van Vuuren AJ, Morton HC, van Spriel AB, Shen L, Hofhuis FM, Saito T, Mayadas TN, Verbeek JS, van de Winkel JG. Human immunoglobulin A receptor (FcalphaRI, CD89) function in transgenic mice requires both FcR gamma chain and CR3 (CD11b/CD18). Blood 1999; 93:4387-94; PMID:10361137/
