Abstract
Immune checkpoint inhibitors are drugs that interfere with tumor escape responses. Some members of this class are already approved, and expected to be blockbusters in the future. Many companies have developed patent activities in this field. This article focuses on the patent landscape, and discusses key players and cases related to immune checkpoint inhibitors.
Introduction
The term “cancer immunotherapy” encompasses several strategies that support the body's immune system in fighting cancer, inter alia, immune checkpoint inhibitors (ICI), antibodies or fusion proteins evoking antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), bispecific antibodies or fusion proteins that bind tumor antigens and attract T cells, natural killer (NK) cells, dendritic cells and modified T cells with chimeric T cell receptors (CAR-T).
The immune system is characterized, inter alia, by particular checkpoints that tame the system and avoid the development of auto-immune responses, e.g., upon binding of ligands to particular receptors present on the surface of immune cells.
These checkpoints are called „immune checkpoints,” and they do provide a way to protect a given entity from being attacked by the immune system. Tumors have discovered this principle, and exploit it, e.g., by expressing respective ligands. This strategy is called „tumor immune escape.”
ICI are drugs that interrupt this strategy, e.g., by antagonistically blocking the respective receptors or binding their ligands, thus re-establishing the immune system's capacity to attack a tumor.
The ICI drug class is composed mostly of antibodies. Together with antibody-drug conjugates and bi- or tri-specific antibodies, they form the second generation of antibody-based therapeutics, after the first generation, which consisted of, literally, “naked” antibodies. Some of these antibodies reached blockbuster status between 2000 and 2010, such as adalimumab (anti-TNF) (Humira®) and infliximab (anti-TNF) (Remicade®, Remsima®, Inflectra®), rituximab (anti-CD20) (Rituxan®, MabThera®), bevacizumab (anti-VEGF-A) (Avastin®), trastuzumab (anti-HER-2/neu) (Herceptin®), or palivizumab (anti-RSV) (Synagis®).Citation1
According to a study published by the research company Decision Resources Group in June 2015, the cancer immunotherapy market will achieve sales of more than 13 bn USD in 2023, which sales will be dominated by ICI. Where there are commercial options, there is also intellectual property (IP). Not surprisingly, many companies have therefore developed patent activities in this field. In an interview with the online journal Life Sciences report,Citation2 Stephen Dunn, who manages the counseling firm LifeTech Capital, said that the patent landscape in immuno-oncology is “a bit murky at the present time,” while developments on the legal side have the “possibility of impacting stocks in the future.” At the same time, he modified this forecast by stating that Wall Street would not be “overly concerned right now, as patent cases take years to get resolved, if ever.”
In any case, the role of IP in the field of ICI requires a thorough understanding of the respective landscape, which is fundamental for those who want to play a role in this market. Such understanding helps in the development of strategies to protect results from being counterfeited, anticipate possible conflicts to either avoid or exploit them, secure market access and market position or produce income by royalty payments. On this motivation, this article will focus on the patent landscape related to ICI, to make it a little bit less “murky.”
Key IP Players
The IP landscape in ICI has many players. A patent search in the FamPat database provided by database provider Questel for patent familiesCitation3 which finds the terms “antibodies” and any of “CTLA-4,” “PD-1,” “PD-L1,” “LAG 3,” “TIM3” and “OX40” in their claims revealed a quite heterogeneous pattern (). Note that the full search algorithm is more sophisticated than the mere terms disclosed herein. In the upper line, the total number of patent families are shown, while assignees in bold type are discussed in this article in more detail. Note that not all patents found in the underlying search protect, in strictu sensu, antibodies against the respective targets, while some of the patents from the list may already be expired, or withdrawn. Further, the assignee information provided by the respective databases is not always reliable because patent applications can be transferred or assigned to more than one company. During the search it became obvious that a number of patent families is assigned, jointly, to Dana-Farber Cancer Institute, Brigham and Women's Hospital, Emory University, the Broad Institute of MIT and Harvard University. For reasons of simplicity, these applicants were treated as one. Further, patent families assigned to a company that was later acquired by a second company were counted as assigned to the latter.
Table 1. number of patent families assigned to applicants which recite antibodies and any of CTLA-4, PD-1, PD-L1, LAG 3, TIM3 and OX40 in their claims
A more detailed breakup of the 3 ICI targets to which approved, or almost-approved, antibodies exist (CTLA-4, PD-1, and PD-L1), plus key IP rights protecting these antibodies, is shown in , and will furthermore be discussed in the following. Figures 1–3 show the suggested mechanisms of action of antibodies directed agaist these targets.
Table 2. Key players in anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies, and their key patent estates
Example 1: Anti-CTLA-4 antibodies
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), also known as CD152, is a protein receptor on the surface of T cells. When bound to CD80 (B7–1) and CD86 (B7–2) present on the surface of antigen presenting cells, CTLA-4 deactivates the T cells. Blocking CTLA-4 by means of an antagonistic antibody interferes with this mechanism and thus preserves the activity of the T cells.
Currently, 2 anti-CTLA-4 antibodies have received US Food and Drug Administration (FDA) approval, namely Bristol-Myers Squibb's (BMS) ipilimumab (Yervoy®), and Pfizer's tremelimumab. Both drugs have an intertwined past.
The murine predecessor of ipilimumab was developed by Jim Allison and coworkers in the late 1990s at University of California (UC) Berkeley,Citation4 after discovering that CTLA-4 had an inhibitory effect on the immune system. In a collaboration with Medarex, where Nils Lonberg had established a technology to generate mice that carry the human immune repertoire,Citation5 the team developed a human version of their antibody, which was then licensed to Medarex, and named MDX-010. In August 1999, Medarex received an exclusive sublicense from Gilead, which gave Medarex access to the CTLA-4-patents of the Allison patent estate, owned by UC Berkeley (see ). The original licensor was NeXstar Pharmaceuticals, which later merged with Gilead.
In September 2004, Medarex and Pfizer announced a collaboration on therapeutic antibodies. Pfizer had, at that time, their own anti-CTLA antibody program, CP-675,206 (later named tremelimumab), which had been developed by AbgenixCitation6 using their transgenic mouse platform. Based thereon, Pfizer established their own patent portfolio (the „Hanson patents,” see ).
The agreement between Medarex and Pfizer also involved cross-licenses of patents relating to their respective anti-CTLA-4 antibodies (“cross-license agreement”).Citation7 While, generally, the cross licenses were royalty free, non-exclusive and provided the right to sublicense, a separate license agreement existed which related to the UC Berkeley's Allison patents („Allison license agreement”),Citation8 for which Medarex was eligible to obtain milestones and royalty payments for sales of any Pfizer anti-CTLA-4 antibodies. shows some of the patents that are subject of the 2 license agreements. Both companies agreed, however, to independently pursue their clinical anti-CTLA-4 programs, and retained the commercial rights to their separate anti-CTLA-4 products.
Soon thereafter, in January 2005, Medarex signed a collaboration agreement with BMS to develop MDX-010, then called ipilimumab, and in September 2009, BMS took over all shares, thus making Medarex a fully owned subsidiary.
In April 2008 Pfizer discontinued clinical trials on tremelimumab for advanced melanoma for lack of improvement over chemotherapy. This news also affected Medarex's stock prices significantly. Medarex soon thereafter issued a press release in which they explained that ipilimumab has significant differences relative to tremelimumab.
In January 2010, Pfizer announced a collaboration with Swiss biopharma specialist Debiopharm to start a new clinical program for advanced melanoma. In their announcement, they declared that analysis of the earlier trial had identified a biomarker that would be used for the new trial. As of July 2015, 18 ongoing clinical studies were registered on the website of the US. National Library of Medicine (clinicaltrials.gov), but details of the study with Debiopharm or the alleged biomarker were not available.
According to a report provided by business intelligence provider HealthAce in May 2010,Citation9 BMS explained that ipilimumab would be superior over tremelimumab because it would be of a different antibody subtype (see ), while the then ongoing trial NCT00324155Citation10 would comprise a combination with chemotherapy (dacarbazine), and would also provide a higher dosage (3 mg/kg triweekly). Further, BMS announced that, if the new trials conducted with Debiopharm would deliver a suitable biomarker, that marker would also be suitable for ipilimumab. In March 2011, ipilimumab obtained FDA approval for unresectable or metastatic melanoma.
In October 2011, Astra Zeneca announced that they had inlicensed tremelimumab from Pfizer, including the assumption of global rights to development and commercialization. Pfizer retained the rights to use tremelimumab with specified types of combination therapies.
In February 2015, results of a new tremelimumab trial on advanced melanoma (NCT00257205) were reported, according to which tremelimumab did not provide an advantage in survival compared to first-line standard-of-care chemotherapy.Citation11 Further, subset analysis of demographic factors and 2 biomarkers did not reveal a factor that would be predictive for increased efficacy.
As regards Pfizers's Hanson portfolio, European Patent EP1141028 has claims that define tremelimumab by its sequence, while corresponding US patent US6682736 has sequence claims too, but also broader claims to (i) a general anti-CTLA-4 antibody with particular binding properties, plus (ii) to an anti-CTLA-4 antibody that competes for binding to CTLA-4 with tremelimumab (see ). BMS's ipilimumab could possibly fall under the scope of the 2 latter claims, provided it has similar binding properties or binds the same epitope as tremelimumab, but it is questionable whether these claims are enforceable or not, in view of the fact that BMS's Allison patent estate (priority date of July 25, 1995) predates Pfizer's Hansen patent estate (Dec 23, 1998). Further, the Hanson patent estate is the subject of the cross-license agreement between Pfizer and Medarex discussed above, privileges of which also apply for BMS.
In the same report issued during May 2010, BMS also declared that, if tremelimumab makes it to the market eventually, Pfizer would have to pay royalties, presumably based on the UC Berkeley portfolio defined in the Allison license agreement (see ). US5811097 from the portfolio has claims directed to a method of tumor treatment with an anti-CTLA4 antibody (see ). It appears, however, that, due to this license agreement, BMS cannot use their Allison patent estate to block Pfizer's tremelimumab. Further, this payment obligation will fall soon because these patents expire by 2015 (see ). At the same time, BMS cannot use their Korman patent estate (see ), because the respective patents are either restricted to an IgG1 isotype (US7605238, note that tremelimumab is an IgG2) or to particular ipilimumab sequences (US6984720, EP1212422).
The anti-CTLA-4 patent landscape could therefore be a quite peaceful one, at least as long as BMS and Pfizer divide the market among themselves.
While, in the relationship between BMS and Pfizer, the Allison patent estate is considered to be the one with the earliest priority (July 25, 1995, see ), it needs to be said that earlier patent applications related to CTLA-4 exist. The underlying works were accomplished, inter alia, by Jeffrey Bluestone of UC California and Peter Linsley of the Benaroya Research Institute. Some of these studies resulted in the Linsley patent portfolio, which protects BMS's CTLA-4-Fc fusion peptide abatacept (see below). Linsley and colleagues had also made antibodies against CTLA-4, but it appears that they believed that anti-CTLA4 antibodies and anti-CD28 antibodies would synergistically costimulate proliferation of T lymphocytes.Citation12
Example 2: PD-1 and its ligands
Programmed cell death protein 1 (PD-1, also known as CD279) is a cell surface receptor from the immunoglobulin superfamily that is expressed on T cells and pro-B cells. PD-1 acts as an immune checkpoint, which upon binding of one of its ligands, PD-L1 or PD-L2, inhibits the activation of T cells. The mechanism of action relies on promoting apoptosis in antigen-specific T cells in lymph nodes while the apoptosis in regulatory T cells is reduced. Some tumors will upregulate PD-1 ligand expressionCitation13 to thus paralyze the body's immune defense. Anti-PD-1 antibodies interfere with ligand binding, and thus inhibit the deactivation of T cells.
PD-1 was discovered by Kyoto University scientist Tasuku Honjo in the 1990s.Citation14 However, the role of PD-1 was not immediately clear, although Professor Honjo deduced, from PD-1 knockout mice who developed lupus-like symptoms, that PD-1 has a negative regulatory effect on the immune system.
In the late 1990s, Gordon Freeman of Dana-Farber Cancer Institute and Arlene Sharpe of Harvard Medical School identified ligands to PD-1, one of which they called B7–4.Citation15 Taken together, the 2 are named inventors in about 33 patent families having the terms “PD-1,” “PD1” “programmed cell death” or “B7” in their claims.
Harvard and Dana-Farber out-licensed 11 patents from this portfolio non-exclusively to BMS, Merck & Co. (Merck), Roche, Novartis, Boehringer Ingelheim, Amplimmune, and MerckSerono ().Citation16 This open policy has tremendously spurred research on antibodies against PD-1 and its ligands, and today 34 candidates are in clinical studies (22 of which are in combination with other agents), as reported by business intelligence provider DelveInsight.Citation17
Table 3. Patents with Freeman et al as inventors outlicensed by Harvard and Dana-Farber to BMS, Merck, Roche, Novartis, Boehringer Ingelheim, Amplimmune, and MerckSerono
In parallel to Freeman and Sharpe, Lieping Chen of Mayo Clinic independently discovered PD-L1, which he called B7-H1.Citation18 Chen showed that B7-H1 has a costimulatory effect on T cells. Said effect could be inhibited by means of a suitable anti-B7-H1 antibody. This finding was somewhat contrary to what has been found by Freeman and his coworkers, who speculate that said effect might go back to an alternative receptor for PD-L1 that is not PD-1.Citation19 Interestingly, it appears that Freeman's B7–4 has now been renamed PD-L1,Citation20 and is thus identical to Chen's B7-H1. Chen also described B7-DC, which is today known as programmed cell death 1 ligand 2 (PD-L2).Citation21
An overview of Dr. Chen's patent estate is shown in . Some patents claim a method for inhibiting the above mentioned co-stimulatory effect of B7-H1 on T cells by use of an anti-B7-H1 antibody. The patent estate comprises 2 US patents (US8460927 and US8981063), which broadly claim antibodies that bind specifically to B7-H1. Both patents claim a priority of November 30, 1999, i.e., they are outranked in priority by the earliest patents of Freeman et al. that also claim antibodies against B7–4 (e.g., US7635757, which has a priority of Aug 23, 1999, see ).
Table 4. Patents and patent applications related to B7 ligands naming Dr. Lieping Chen as inventor
Anti-PD-1 Antibodies
The US-based company Medarex began to establish an anti-PD-1 program in the late 1990s. After acquiring Medarex, BMS joined forces with Japan's Ono Pharmaceutical, who had a research collaboration with Tasuku Honjo of Kyoto University, the discoverer of PD-1.
While Ono's dowry to this collaboration included the Honjo patent estate (see ), which broadly claims a method of tumor treatment by administration of an anti-PD-1 antibody, Medarex and Ono jointly contributed to the Korman patent estate (see ). US8779105, which is from that family, claims a sequence-wise specified antibody against PD-1, plus further antibodies that cross-compete therewith for binding to PD-1.
It needs to be said, in this context, that patent claims reciting antibodies that compete for binding a particular epitope with a reference antibody (“competes with” claims) bear legal uncertainties. Like all patent claims, “competes with” claims also have to pass the test for novelty. Compared to a patent claim that specifies an antibody by its sequence (thus, a „true” sequence claim), the likelihood that a “competes with” claim is anticipated by a prior art antibody is relatively high.
It appears that patent authorities examine such claims with increasing scrutiny on the basis that their scope is indefinite or expresses a mere desideratum (i.e., a wish). The European Patent Office (EPO) might consider such claim feature an “unusual parameter,” where no meaningful comparison with the prior art can be made, and with help of which lack of novelty may be disguised. For this reason, the EPO stipulates that the onus of proof that an unusual parameter is a genuine distinctive feature vis-à-vis the prior art lies with the applicant.Citation22
BMS and Ono ran into such situation with their European counterpart from the Korman patent estate, EP2161336. This patent has an independent claim 1 that protects nivolumab by its complementarity-determining region (CDR) sequences, plus another independent claim 3 that relates to antibodies that cross-compete for binding to PD-1 with nivolumab.
In the pending opposition proceedings, Ono deleted claim 3 to comply with a novelty objection against raised by the opponents (see ). It would appear that Ono opted for this step to avoid a revocation of the patent by the EPO for lack of patentability of said claim 3. Such revocation could have been considered a negative prejudice with respect to Ono's corresponding US patent US8779105, which only has “competes with” claims, like claim 3 of EP2161336, but no “true” sequence claims.
Unfortunately, Ono's withdrawal of claim 3 of EP2161336 has also avoided an ex officio clarification of the benchmark that “competes with” claims have to pass under the European Patent Convention (EPC), Such clarification would have served the interest of legal certainty.
Under their joint program, BMS and Ono developed the anti-PD-1 antibody nivolumab (Opdivo®), which was first approved in Japan in December 2014 for melanoma.
The BMS and Ono approval was predated by the approval of Merck, who had their own anti-PD-1 program already. Pembrolizumab was developed by Carven and colleagues at Organon, the healthcare section of Akzo Nobel, Organon was acquired by Schering-Plough in November 2007. Schering-Plough was then taken over by Merck in November 2009. In September 2014, Merck received FDA approval for pembrolizumab (Keytruda ®) for advanced melanoma, i.e., 3 months before BMS.
Interestingly, Organon had in May 2007 already signed a deal with Medarex to develop human antibodies using Medarex's transgenic mouse technology. Because pembrolizumab is a humanized antibody that has been created by CDR grafting, the Medarex cooperation does not appear to have had any impact thereon.
On September 4, 2014, BMS, together with Ono, filed suit against Merck for patent infringement at the Delaware District Court.Citation23 The claimants alleged that the marketing of pembrolizumab would infringe Ono's US Patent US8728474 (see ), which is from the Honjo estate and has a broad claim language that merely claims a method for tumor treatment by means of an anti-PD-1 monoclonal antibody. It thus appears that these claims would cover pembrolizumab. Interestingly, the claimant did not seek for an injunction, but so far only demanded damages, albeit on a willful infringement basis. Later, in July 2015, BMS filed a further suit based in just granted US Patent US9073994, also from the Honjo estate (see ). Both cases are still pending. Further details of the lawsuit below are discussed below.
Merck's own patent portfolio consists essentially of the Carven patents (see ). These patents have a later filing date and are restricted to the pembrolizumab sequences. They are thus narrower than BMS's Honjo and Korman patents. It therefore appears that Merck cannot use them against nivolumab.
Anti-PD-L1 Antibodies
The rationale of anti-PD-L1 antibodies is closely related to that of anti-PD-1 antibodies, with the difference that not the receptor is blocked, but its ligand. Anti-PD-L1 antibodies are thus meant to neutralize PD-L1 ligands, which are secreted by the tumor to pacify the anti-tumor activity of the surrounding immune system.
The clinically most advanced anti-PD-L1 antibody is Genentech's atezolizumab, which received breakthrough therapy designation by the FDA for bladder cancer in May 2014,Citation24 and for non-small cell lung cancer (NSCLC) in February 2015.Citation25 Such status gives Genentech a preferred treatment in the approval process for atezolizumab.
Atezolizumab's key patent specifies the antibody by its hypervariable heavy chain sequences (see ). The patents or patent applications protecting 3 competing anti-PD-L1-antibodies, durvalumab (AstraZeneca), avelumab (MerckSerono) and BMS-936559 (BMS) have claims of similar type (), however, AstraZeneca's US8779108, MerckSerono's US2014341917 (still pending) and BMS's US7943743 also claim antibodies that compete with the one specified by its sequence for binding to the same epitope of PD-L1.
As discussed, such claims are significantly broader than „true” sequence claims, which only cover the very specific antibody, and bear legal uncertainties for both competitors and assignees. Note however, that MerckSerono's US2014341917 is not granted yet ().
If the 4 anti-PD-L1 patents and patent applications discussed above had only „true” sequence claims, no overlap in scope of protection would exist because each patent would only protect the sequence of its particular antibody. However, it appears that AstraZeneca's US8779108, MerckSerono's US2014341917 (should the latter be granted) and BMS's US7943743 can give rise to future IP conflicts, namely in case that one of the competing antibodies falls under the scope of the respective claims encompassing antibodies that compete for binding with the one that is actually specified.
In any case, none of the 4 patent estates has claims that broadly refer to any type of anti-PD-L1 antibodies as such, or methods of using anti-PD-L1 antibodies, as, e.g., BMS has them in their Allison patents for anti-CTLA-4 antibodies ().
The reason for this relatively narrow scope in the claims might lie in Dr. Freeman's outlicensed IP portfolio (see ). This portfolio encompasses 2 US patents with a priority of August 23, 1999, the first of which (US7635757) claims antibodies against B7–4 (equivalent to PD-L1), with no sequence restriction as regards the antibody, while the second (US7101550) claims immune stimulation by administration of an antibody against B7–4. These patents outrank the above patent estates by priority, and anticipate the broad generic concept of anti-PD-L1 antibodies, leaving sequence claims, and “competes with” claims, as remaining, yet narrower, options.
Interestingly, Dr. Freeman's portfolio does not comprise a patent application of similar broadness for anti-PD-1 antibodies. The only patent from said portfolio related to anti-PD-1 antibodies (US6808710, see ) claims immune downmodulation by administration of an anti-PD-1 antibody. It thus appears that, other than in PD-1, where BMS and Merck are already involved in a patent litigation (with BMS's/Ono's US8728474 not being restricted to a given sequence, and thus encompassing also Merck's candidate), PD-L1 is the less conflict-prone target because the players have narrower patents only, although 3 of them (AstraZeneca's US8779108, MerckSerono's pending US2014341917 and BMS's US7943743) bear residual risks for their competitors.
Antibody Mimetics
It appears that many patents that protect ICI refer, in their claim language, explicitly to antibodies. This may leave room for companies that do not develop antibodies in strictu sensu, but antibody mimetics, i.e., designed ankyrin repeats (DARPins),Citation26 Adnectins, Affibodies, Anticalins, or engineered Kunitz-type inhibitors,Citation27 to name a few.
These molecules lack some functions of typical IgG-formatted antibodies, like Fc-mediated effector functions. To be active as an immune checkpoint inhibitor, these functions are, however, not always required or even desirable, as is the case in 3 of the 4 anti-PD-L1 antibodies shown in in which the Fc domain has been engineered to avoid ADCC (atezolizumab, durvalumab) or is naturally low in ADCC (BMS-936559). Interestingly, however, MerckSerono claims that their anti-PD-L1 antibody, avelumab, is not engineered to avoid ADCC and is thus capable of mediating lysis of tumor cells, as well as blocking PD-1/PD-L1 interactions.Citation28
Biomarkers and Companion Diagnostics
The efficacy of ICI varies between different tumor types, and between different patients having the same tumor.Citation29 Further, only a subset of patients treated with ICI experience sustainable control of their disease.Citation30 A better understanding of the reasons behind these differences, and insufficiencies, would significantly increase the therapeutic value of this new treatment modality.
One step in this direction would be biomarkers that help to identify patients who are most likely to respond to a given treatment, and least likely to experience side effects. While for some targeted therapies such companion diagnostics have already been developed (see, e.g., the anti-HER-2/neu antibody trastuzumab, where suitable tests define the HER-2 expression status, and thus predict therapy success in the treatment of breast cancer),Citation31 in ICI the search for suitable biomarkers that reliably predict the efficacy of a therapy for a given patient is still ongoing.Citation32,33
Nonetheless, many companies have already staked their claims in this field in terms of patent applications. Patents that protect the use of a particular biomarker, or a kit for detecting the same, can have tremendous commercial value, namely in case that, under the respective marketing authorization, a test for said biomarker is mandatory before an ICI can be prescribed, or its costs reimbursed, respectively.
In the above-mentioned case of trastuzumab, 10 tests for HER-2 expression status from 6 different suppliers (Ventana, Abbott, Biogenex, LifeTechnologies, Leica Biosystems, and DAKO) have been approved by the FDA.Citation34 While use of one of these tests is mandatory,Citation35 it becomes obvious that no supplier managed to monopolize this market, e.g., by obtaining broad enough patent protection. Nonetheless, there is still room for dispute, as, e.g., Dako and Abbott Molecular Inc. sued each other in 2005 for invalidity, or infringement, of UC California's patents US6596479 and US5447841, licensed to Abbott.Citation36
With respect to anti-CTLA-4 therapy, numerous biomarkers have been suggested, namely, frequency of ICOS+ CD4 T cells, upregulation of HLA-DR/CD45RO, increase in tumor-infiltrating lymphocytes, forkhead box P3-positive and indoleamine 2,3-dioxygenase-positive tumors, presence of antibodies to cancer antigen NY-ESO-1, NY-ESO-1-specific T cell reactivity, high levels of IFN-γ-related genes and decrease in genes associated with cellular proliferation and melanoma-specific antigens.Citation37
Other authorsCitation38 report that absolute lymphocyte count, and baseline absolute eosinophil count and relative eosinophil count have also been associated with improved survival in these patients.
Compared to anti-CTLA-4 therapy, anti-PD-1 therapy is a relatively new field. Therefore, not surprisingly, the search for suitable biomarkers is not as advanced as in the anti-CTLA-4 area. While expression of PD-L1 seems to play a role in the responsiveness of a tumor to anti-PD-1 therapy, the results obtained are ambiguous,Citation39 which may be due to variations in methodology, cancer type, primary vs. metastatic status, and treatment history, while, furthermore, PD-L1 expression has been shown to be dynamic, and associated with tumor-intrinsic and tumor-extrinsic factors.Citation40
In October 2014, Merck's anti-PD-1 antibody pembrolizumab received a breakthrough therapy designation from FDA for the treatment of patients with EGFR mutation-negative, and ALK rearrangement-negative NSCLC whose disease has progressed on or following platinum-based chemotherapy. While the author could not retrieve a respective patent application assigned to Merck, it appears that Caris Life Sciences of Irving, TX, has established a patent estate, comprising, inter alia, international patent application WO2015116868A1 (Gatalica et al.) with priority of January 29, 2014, that might cover this embodiment (see ).
Table 5. Selected patents and patent applications related to biomarkers for anti-PD-1 and anti-CTLA-4 therapy
However, PD-L1 appears not be a binary indicator of potential durable benefit because, to date, no study has revealed a 0% response rate in patients with PD-L1-negative tumors either. Citation41 Despite this unclear situation, many patent applications exist already which relate to biomarkers or companion diagnostics for anti-CTLA-4 or anti-PD-1 therapies. shows some selected patents and patent applications related to biomarkers for anti-PD-1 and anti-CTLA-4 therapy.
In this context, it is important to understand that, to meet the inventive step/non-obviousness criterion of a patent application, the requirements as to experimental evidence are regularly lower than in scientific literature, let alone than to obtain regulatory approval, where substantiation of therapeutic efficacy is required.Citation42 To convince a patent examiner that a new compound has a particular effect, data obtained with relatively small sample sizes are usually sufficient, and statistical verification is usually not required. Further, in most cases non-clinical assay data are deemed sufficient.Citation43 This means that patents can be obtained for a companion diagnostic that will never make it to the marketplace.
Further, when drafting patent applications related to biomarkers, care should be taken to account for particular requirements in the respective patent legislations. Under the EPC, claims related to a method of diagnosis are not patent eligible (Art 53 (c) EPC). In decision G1/04, the Enlarged Board of Appeal of the EPO has defined the criteria that make such methods of diagnosis exempt from patent protection. In particular, the patent claim must include the steps of: 1) examination involving the collection of data, 2) comparison of the data with standard values, 3) the finding of any significant deviation (i.e. a symptom), and 4) the attribution of the deviation to a particular clinical picture.
Methods that lack one of these steps do not qualify as diagnostic methods in the meaning of Art 53 (c) EPC. Thus, by omitting one of the 4 steps in the patent claim, this statutory exemption can be bypassed, provided the lacking feature is not essential to the method (which would result in an objection regarding lack of clarity or insufficient enablement). Other options to obtain protection for respective methods provide the claiming of a method of prediction of a therapy success, or prognosis of disease progression, both of which are not diagnosis in strictu sensu.
Further, under the EPC, use restrictions have no limiting effect on compound claims (with the exception of purpose-bound therapeutic claims, which are privileged under Art 54 (4) EPC). This means that a claim reciting “Biomarker X for use in the prediction of success of an anti-CTLA-4 treatment of cancer” would not be deemed novel in case the biomarker was already prior art. Suitable claim formats would be “Use of biomarker X for the prediction of success of an anti-CTLA-4 treatment of cancer,” or “Kit comprising labeled antibody X or nucleic acid probe Y,” provided the antibody or the probe is novel.
In the United States, diagnostic method claims in general, and biomarker claims in particular, are subject to extreme scrutiny by the United States Patent and Trademark Office (USPTO) after the new guidance which came into effect with 2 Supreme Court landmark decisions, Myriad and Prometheus.Citation44 According to these 2 decisions, generally speaking, products of nature, including isolated genes, are deemed non-patent-eligible. This new policy led to a de-facto moratorium of allowances of patent applications related to diagnostic methods and biomarkers. Citation45 shows selected patents and patent applications that claim biomarkers or companion diagnostics that their applicants deem predictive with respect to either anti-CTLA-4 or anti-PD-1 therapy.
Combination Therapies
Very early it became clear that the efficacy of ICI is likely to be increased when used in combination with other drugs.Citation46 ICI combinations with other drugs are not necessarily reflected in the IP landscape in terms of explicit combination patents because very often a patent application devoted to a given ICI also claims, or discloses, with routine language, optional combinations thereof with other standard cancer drugs, even if such combination has not been experimentally tested.
One example is Ono's US patent US8728474 (see ), claim 1 of which is directed to the treatment of a tumor by administering an anti-PD-1 monoclonal antibody, while claim 15 recites an optional combination thereof with an anti-CTLA-4 antibody. The combination of claims 1 and 15 thus encompasses, in particular, combinations of the 2. This patent therefore covers all combinations of either BMS's nivolumab or Merck's pembrolizumab with either BMS's ipilimumab or Pfizer's tremelimumab.
One rationale behind a combination therapy of an ICI with other drugs is that ICI therapy may require some time to take effect because the immune system has to be activated while the tumor is still growing. A combination involving, e.g., 1) a small molecular chemotherapeutic, or a targeted antibody, and 2) an ICI, may thus provide a short-term effect, in which the tumor growth is halted by the first component, and a long-term effect in which the immune system eliminates the tumor in response to the ICI therapy. Strictly speaking, such concept does not exactly rely on what patent professionals often call a “synergistic effect,” which is a code word when inventive step/obviousness issues of a given drug combination are discussed. The clue of this concept seems merely to bridge the time gap until the immunotherapy becomes effective. See the theoretical Kaplan-Meier curves in for an explanation.
Figure 1. CTLA-4 is a T-cell specific inhibitory receptor which has higher affinity to B7-2, displayed on antigen presenting cells (APC), than the T-cell specific co-stimulatory receptor CD28, and suppresses CD28-mediated T cell activation. Blocking CTLA4 with an anti-CTLA-4 antibody, like ipilimumab or tremelimumab, allows CD28 to bind B7-2, and thus stimulate T cell activity. CTLA-4 is upregulated during the early activation phase in the lymph nodes.
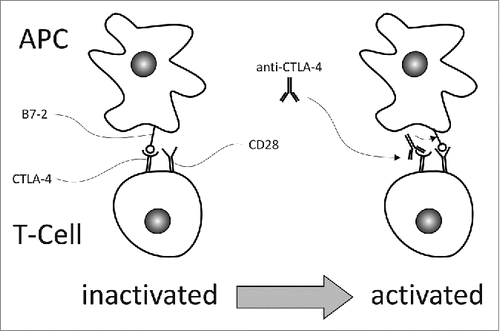
Figure 2. Actively binding B7-2 with a CTLA-4-Ig fusion protein, like abatacept, interrupts the interplay between CD28 and B7-2 and thus suppresses CD28-mediated T cell activation. This approach is applied in the treatment of autoimmune diseases.
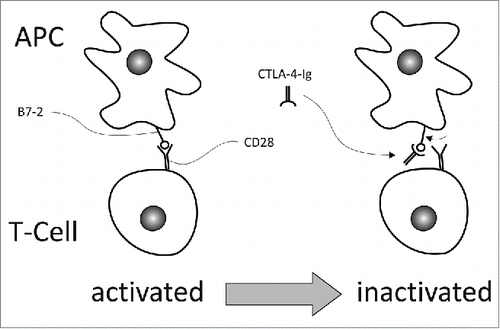
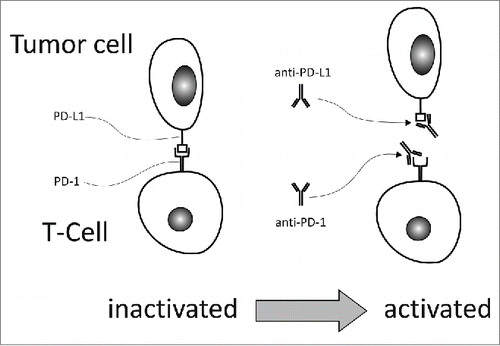
Figure 3. PD-1 is expressed on activated T-cells, while its ligand, PD-L1 is usually expressed on epithelial and endothelial cells. Binding to PD-1 deactivates the T-cell and thus protects the cells bearing PD-L1. Tumor cells do also express PD-L1, in order to escape the T-cell mediated immune response. Anti-PD-1 antibodies, like nivolumab or pembrolizumab, and anti-PD-L1 antibodies, like atezolizumab, interrupt the interplay between PD-1 and PD-L1 and thus inhibit T-cell deactivation. PD-1 modulates T cell function mainly during the effector phase in peripheral tissues including tumors.
A second rationale of ICI combinations with other drugs is where the combination develops a true synergistic effect in terms of efficacy.Citation47 In the eyes of the EPO, synergy requires that “the functional interaction between the features achieves a combined technical effect which is different from, e.g., greater than, the sum of the technical effects of the individual features” (combination vs. juxtaposition).Citation48 If sufficiently made plausible by an applicant, such synergistic effect is almost certainly a ticket for patent allowance.
However, even with one of the key arguments for inventive step/non-obviousness missing, patents can still be awarded for such type of combination therapy, namely when a true benefit is actually achieved, in particular if this effect was not entirely foreseeable from the prior art relating to the solo products, or to combinations thereof different to the actual combination. In practice, applicants should argue that the benefit shown was not foreseeable because, given the many disappointments and failures in the development of anti-cancer drugs, the increase in efficacy, or the decrease in side effects, could not be anticipated. Even if the effect of the 2 components lacks any synergistic effect, and is thus merely cumulative, applicants can argue that a novel combination of 2 drugs always bears the risk to develop a toxicity, or both compounds can reciprocally inhibit one another, thus rendering even the mere cumulative effect unforeseeable.
It needs to be said, however, that for many of the drug combinations in discussion that include an immune checkpoint inhibitor, a scientific rationale for study has not been found yet. Furthermore, it is difficult to decide where to look for hints of efficacy in view of so many potential combinations.Citation49
One typical type of combination patents is assigned to small biotechnology companies. The above problem of finding a suitable pair in the haystack of drug candidates does not necessarily apply here because these companies sometimes seek to protect a combination of their key candidates with one or more ICI. In other words, the motivation to combine 2 drugs with one another is not always a scientific one, as it can sometimes simply reflect the mere pragmatic approach to combine what they have with what is supposed to be the next blockbuster. To successfully prosecute a respective patent to allowance, however, a benefit of such combination still needs to be demonstrated. This, of course, may also be helpful for subsequent approval and successful marketing. shows some examples for such type of patents.
Table 6. Patents protecting combinations comprising at least one ICI
These patents can serve as an entry ticket to enter into a research and development deal with large pharmaceutical companies, considering the high costs for the ICI already approved, plus the competition to enroll patients for clinical trials.Citation50 However, large pharmaceutical companies have also discovered the potential of drug combinations that include ICI. Typically, the respective projects involve the combination of 2 or more proprietary molecules, comprising an ICI and one other agent, e.g., either another immune checkpoint inhibitor, a conventional antibody or a small molecule. Likewise typically, the second component can already be off-patent.
Other types of drug combinations comprising an immune checkpoint inhibitor are codeveloped by 2 or more large pharmaceutical companies. It has been postulated that this would be a typical indicator that the immune checkpoint inhibitor drug development pathway is already quite crowded.Citation51 This development is not necessarily reflected in the IP landscape because, in such deals, each player contributes his particular candidate, which is already subject of a patent that also discloses or claims, optionally, the other component of the combination.
An overview of ongoing and discontinued projects exploring combinations of ICI with other drugs is shown in .
Commercial Conflicts or Litigation
Patents provide the right to sue a third party that the patent proprietor deems to illegitimately use the protected invention. The court's role in such dispute is to clarify whether such accusation is justified or not, and, in case it is, oblige the infringer to pay damages and/or stop the infringement. Because of the commercial potential of ICI, and the considerable expenses for ICI-related research and development, it is not surprising that sooner or later conflicts that escalated to court arose.
BMS vs Merck (PD-1)
As mentioned above, on September 4, 2014, BMS, together with Ono, filed suit against Merck in the Delaware District Court for patent infringement.Citation52 The claimants alleged that the marketing of pembrolizumab would infringe Ono's US Patent US8728474 (), which is from the Honjo estate.
According to a prima facie analysis, it appears that Merck's pembrolizumab indeed falls under the scope of Ono's patent, as it broadly claims a method for treatment of a tumor comprising administration of an anti-PD-1 monoclonal antibody to a patient. The court trial has been scheduled for November 2016.
On July 7, 2015, BMS and Ono filed a second suit against Merck at the same court,Citation53 this time based Ono's US patent US9073994 (see ), which is also from the Honjo estate and was granted earlier that day. The patent claims methods of treating metastatic melanoma using an anti-PD-1 antibody. The claimants alleged that pembrolizumab infringes said claims, too.
In June 2011, Merck had already filed an opposition against Ono's European counterpart of US8728474, EP1537878. In its decision, the Opposition Division came to the conclusion that the claims were novel over a prior at reference cited by Merck, WO0114557, which is assigned to the Dana-Farber Cancer Institute. The corresponding US patent US6808710 is part of the portfolio Dana-Farber nonexclusively out-licensed to different pharma companies (see supra), but claims immune downmodulation by administration of an anti-PD-1 antibody (see ).
The Opposition Division found that WO0114557 did only disclose a relationship between anti-PD-1 antibodies and autoimmune diseases, but lacked a direct and unambiguous disclosure of a relationship between anti-PD-1 antibodies and cancer. Accordingly, the Opposition Division rejected the opposition in June 2014, and left the European patent maintained in unamended form. In February 2015, Merck filed an appeal against this decision, which is now pending under appeal No T1994/14.
Earlier, in April 2014, Merck had already filed an opposition against another European patent assigned to Ono, EP2161336, which is from the Korman estate (see ). Claim 1 claims nivolumab by its CDR sequences, while claim 3 refers to monoclonal antibodies that cross-compete therewith, thus covering not only nivolumab, but also Merck's pembrolizumab. Ono's corresponding US patent US8779105 has claims of similar broadness (see ). Co-opponents in the opposition are Novartis, 4-Antibody and Janssen.
In February 2015, Ono filed a new main request in which claim 3 and some dependent claims were deleted, thus no longer embracing pembrolizumab. Ono has pre-emptively deleted these claims, i.e., without waiting for the preliminary opinion by the Opposition Division. The Opponents had, inter alia, attacked these claims for lack of novelty, in view of the earlier patent application WO2004056875 assigned to Wyeth, which claims a priority of December 23, 2002, and is entitled “Nucleic acids encoding antibodies against PD-1.” This application ranks in priority after most of the seminal PD-1 patents that had been outlicensed by Freeman et al., which were filed in August 1999 (see .), but has been published before the priority date of the Korman patents, which is May 2005. A number of anti-PD-1 antibodies are disclosed in this application by their sequences, and the opponents alleged that some of them would cross–compete with 5C4, which is nivolumab, thus anticipating the subject matter of claim 3.
In May 2014, Merck sued Ono in the UK for invalidity of the UK parts of both European patents. One month later, Ono and BMS countered by filing an action for a declaratory judgment that the EP1537878 patent (Honjo) would be infringed in the UK by the marketing of pembrolizumab. Merck replied by filing a request for declaration of non-infringement with respect to the EP2161336 patent (Korman). Because Ono had already amended the claims thereof in the co-pending European opposition proceedings, they requested that the UK court only considers said amended said of claims. This motion is likely to dissolve any infringement issues relating to EP2161336, while issues relating to EP1537878 are still on the agenda.Citation54 The UK court hearing will address issues of both validity and infringement of both patents. Further, Merck sued Ono in Australia for revocation of AU2011203119, which is the Australian counterpart to the Honjo patents.Citation55
In the 2 US trials at the Delaware court, BMS and Ono further alleged that in the opposition against EP1537878, Merck's representative had admitted that Merck was aware of the corresponding US patent, and thus knew that pembrolizumab would fall under said patent. In fact, Merck's representative in the European opposition proceedings had justified late introduction of a prior art document with the fact that Ono had already disclosed the same document in the prosecution of one of the 2 corresponding US patents.Citation56
The claimants have used this argument to establish that Merck willfully infringed their US patent, which, under certain circumstances, may qualify them to demand tripled damages for past and future infringements. The claimants further asked for a reimbursement of their attorney's fees and other expenses under 35 USC. § 285, i.e., on the grounds that this be an “exceptional case,” which, according to a US Supreme Court ruling,Citation57 requires that it “stands out from others with respect to the substantive strength of a party's litigating position or the unreasonable manner in which the case was litigated.”
Interestingly, BMS and Ono did not request an injunction yet, which they would theoretically be eligible for under 35 USC. § 283, provided the court confirmed a patent infringement. Generally speaking, obtaining an injunction may be difficult before a US court in case public interest is affected, which is oftentimes acknowledged when healthcare issues are concerned.Citation58
Taking the predicted annual sales figures of pembrolizumab of 5 bn USD in 2020 as predicted by business intelligence provider EvaluatePharma,Citation59 multiplied by royalties of 10% (which appears the upper ceiling to calculate damages in pharma patents), and further considering tripling thereof because of alleged willfulness, the damages Merck would have to pay in case they were found liable for infringement could become quite substantial.
Other than in Europe, where Merck has filed oppositions against EP1537878 (Honjo) and EP2161336 (Korman), Merck has not instituted an Inter Partes Review (IPR) against US9073994 (Honjo). According to 35 USC. § 315 (b), the term to do so expired one year after the date on which Merck was served with Ono's complaint, i.e., September 4, 2015. This passive conduct could be interpreted as a first sign that the parties may want to settle. In view of the fact that numerous competitors have an anti-PD-1 antibody in their pipeline that likely falls under the scope of the Honjo patent, it might be in the interest of both BMS/Ono and Merck that the latter remains in force, to avoid that further anti-PD-1 antibodies flood the market.
In their SEC Form 10-K of February 27, 2015,Citation60 Merck emphasized that they maintain to believe that both the 2 US patents as well as the 2 European patents are invalid. Because this is a multi-faceted dispute, it remains exciting to see how the different cases evolve, not only because the outcome of this dispute will direct cash flow between the 2 parties.
Further, because both BMS's nivolumab and Merck's pembrolizumab are still in the starting phase, the outcome of this dispute will significantly contribute to the future market positions of both drugs, even for the time after expiry of the Ono patent and its family members.
Repligen vs BMS (CTLA-4)
This case does not relate to an ICI, but to BMS's biologic abatacept (Orencia®), rendering this molecule slightly off-topic (yet hopefully still interesting). Abatacept is a fusion protein consisting of the Fc region of an IgG1 fused to the extracellular domain of CTLA-4. Quite opposite to anti-CTLA-4 antibodies like ipilimumab and tremelimumab, which activate T cells, abatacept inhibits T-cell activation (see ), and is used for treating patients with rheumatoid arthritis who have had an inadequate response to one or more disease-modifying antirheumatic drugs (DMARDs), like adalimumab, etanercept, infliximab, golimumab or methotrexate.
BMS has established a patent portfolio protecting abatacept (the Linsley patent estate, see ) with a priority of June 27, 1991. In August 2002, Repligen and the University of Michigan sued BMS at the Michigan Eastern District Court on the allegation that one key inventor, Craig Thompson, who was a university scientist then, was not named as inventor by BMS, thus depriving the university and their partner, Repligen, from legitimate rights to the said patents. The court found that Repligen had not provided sufficient evidence for this allegation, and dismissed the claim in September 2003.Citation61 Repligen appealed the decision to the Court of Appeal of the Federal Circuit (CAFC) in October 2003, but to no avail.Citation62
Table 7. Patents that played a role in the dispute between BMS and Repligen regarding abatacept
In January 2006, Repligen and the University of Michigan sued BMS again in the Eastern Texas District Court,Citation63 this time for infringement of their patent US6685941 (). This patent, which has apriority of November 23, 1988, and is thus older than the Linsley patent estate, claims methods of treating patients having, inter alia, rheumatoid arthritis, by administering to the patient CTLA-4Ig, which is a fusion protein of CTLA-4 and an antibody.
The patent was granted in February 2004 already, but BMS had received FDA approval for abatacept for the treatment of rheumatoid arthritis only in December 2005. Sales figures rose quickly to $441 million in 2008,Citation64 and reached blockbuster status (i.e., over $1 billion)Citation65 in 2012.Citation66
In a so-called “Markman hearing” in September 2007, the court construed the claims of the US6685941 patent in a way that was favorable for Repligen. Thereupon, in April 2008, both parties settled. Under the agreement reached, BMS had to make an initial payment of 5 mn USD, plus an escalating license fee of between 1.8 and 4% for sales until December 21, 2013. In 2014, Repligen reported that the total cash revenues they received from BMS have exceeded 41 mn USD.Citation67
Conclusion
The class of immune checkpoint inhibitors encompasses candidate drugs that will likely reach blockbuster status in the near future. Not surprisingly, many players have tried to stake their claims in this field by filing respective patent applications. Such activity created, and continues to create, a maze of third-party patents that pose considerable risks for both newcomers and established companies. At the same time, it holds tremendous promise in case it results in a meaningful patent strategy that is ultimately successful. Companies who plan to establish an immune checkpoint inhibitor program should seek qualified legal counsel to account for this situation.
Disclaimer
The information provided herein reflect the personal views and considerations of the author. They do not represent legal counsel and should not be attributed to Michalski · Hüttermann and Partner Patent Attorneys or to any of its clients. Patent numbers and patent lifetimes have been verified with utmost care, but no liability is taken for their correctness.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Dimitrov DS, Marks JD. Therapeutic antibodies: current state and future trends–is a paradigm change coming soon? Methods Mol Biol 2009; 525:1-27, xiii; PMID:19252861; http://dx.doi.org/10.1007/978-1-59745-554-1_1
- The Life Sciences Report: Immuno-Oncology Promises Continued Wealth and Health: LifeTech Capital's Stephen Dunn. http://www.thelifesciencesreport.com/pub/na/immuno-oncology-promises-continued-wealth-and-health-lifetech-capitals-stephen-dunn, accessed August 20, 2015.
- Lambert N. Announcing FamPat, A New International Patent Database from Questel-Orbit. Information Today. February 14, 2005. For the search algorithms used in this study contact the author 2005.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271(5256):1734-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8596936; PMID:8596936; http://dx.doi.org/10.1126/science.271.5256.1734
- Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol 2005; 23(9):1117-25; PMID:16151405; http://dx.doi.org/10.1038/nbt1135
- Hanson DA, Canniff PC, Primiano MJ. Preclinical in vitro characterization of anti-CTLA4 therapeutic antibody CP-675,206. Proc Am Assoc Cancer Res 2004; 45:877. Abstract 3802.
- published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/874255/000119312504188088/dex993.htm), accessed August 20, 2015.
- published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/874255/000119312504188088/dex992.htm), accessed August 20, 2015.
- Oncology Intelligence Report, May 19, 2010. http://oncology.healthace.com/051910/Oncology_Report_051910.pdf, accessed August 20, 2015.
- Registry number provided by ClinicalTrials.gov. In PubMed, registry numbers are included in the Secondary Source ID (SI) field, and searchable using the [si] tag.
- Ribas A. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with tremelimumab (CP-675,206). The Oncologist 2008; 13 Suppl 4(Supplement 4):10-5; PMID:19001146; http://dx.doi.org/10.1634/theoncologist.13-S4-10
- Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med 1992; 176(6):1595-604. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2119471&tool=pmcentrez&rendertype=abstract; PMID:1334116; http://dx.doi.org/10.1084/jem.176.6.1595
- Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004; 101(49):17174-9; PMID:15569934; http://dx.doi.org/10.1073/pnas.0406351101
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007; 19(7):813-24; PMID:17606980; http://dx.doi.org/10.1093/intimm/dxm057
- Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PloS One 2013; 8(10):e77780; PMID:24204962; http://dx.doi.org/10.1371/journal.pone.0077780
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Armand P. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N Engl J Med 2014; 372(4):311-9 141206100011003; http://doi.org/10.1056/NEJMoa1411087
- PD-1 and PD-L1 inhibitors - Competitive Landscape, Pipeline and Market Analysis, 2015. Apr 1, 2015, DelveInsight.
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5(12):1365-9; PMID:10581077; http://dx.doi.org/10.1038/70932
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192(7):1027-34. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2193311&tool=pmcentrez&rendertype=abstract; PMID:11015443; http://dx.doi.org/10.1084/jem.192.7.1027
- Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PloS One 2013; 8(10), e77780; PMID:24204962; http://dx.doi.org/10.1371/journal.pone.0077780
- Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med 2003; 197(12):1721-30; PMID:12810690; http://dx.doi.org/10.1084/jem.20022089
- European Patent Office (EPO): Guidelines for examination, F-IV-4.1.1.
- BMS & Ono v. Merck & Co., case 1:14-cv-01131, complaint filed (D. Del. 2014).
- Roche press release, July 13, 2015, http://www.roche.com/media/store/releases/med-cor-2015-07-13.htm.
- Roche Press release, February 2, 2015, http://www.roche.com/media/store/releases/med-cor-2015-02-02.htm.
- Stumpp MT, Binz HK, Amstutz P. DARPins: a new generation of protein therapeutics. Drug Discov Today 2008; 13(15–16):695-701; PMID:18621567; http://dx.doi.org/10.1016/j.drudis.2008.04.013
- Gebauer M, Skerra A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr Opin Chem Biol 2009; 13(3), 245-55; PMID:19501012; http://dx.doi.org/10.1016/j.cbpa.2009.04.627
- Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res 2015; 3(10):1148-57; PMID:26014098; http://doi.org/10.1158/2326-6066.CIR-15-0059
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in cancer Therapy. J Clin Oncol: Official Journal of the American Society of Clinical Oncology 2015; 33(17):1974-82; http://dx.doi.org/10.1200/JCO.2014.59.4358
- Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer 2014; 111(12):2214-9; PMID:25211661; http://dx.doi.org/10.1038/bjc.2014.348
- Prescribing information of Trastuzumab, Revised Version 4/2015, item 5.6.
- Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Sharma P. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 2013; 1(4):229-34; PMID:24777852; http://dx.doi.org/10.1158/2326-6066.CIR-13-0020
- Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer 2014; 111(12):2214-9; PMID:25211661; http://dx.doi.org/10.1038/bjc.2014.348
- Website of the FDA (http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm, accessed August 20, 2015).
- Prescribing information of Trastuzumab, Revised Version 4/2015, item 5.6.
- University of California v. Dakocytomation California, Inc., Nos. 06-1334, -1452, 07-1202 (Fed. Cir. 2008).
- Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 2013; 13:5. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3559193&tool=pmcentrez&rendertype=abstract; PMID:23390376
- Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer 2014; 111(12):2214-9; PMID:25211661; http://dx.doi.org/10.1038/bjc.2014.348
- Grosso J, Horak CE, Inzunza D, Cardona DM, Simon JS, Gupta AK, Sankar V, Park JS, Kollia G, Taube JM. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol 2013; 2013:31 ([abstract 3016]).
- Callahan MK, Horak CE, Curran MA, Hollman T, Schaer DA, Yuan J, Lesokhin AM, Kitano S, Hong Q, Ariyan CE. Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. J Clin Oncol 2013; 2013:31 ([abstract 3003]).
- Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer 2014; 111(12):2214-9; PMID:25211661; http://dx.doi.org/10.1038/bjc.2014.348
- Directive 2001/83/EC (medicinal products for human use): Article 26.
- EPO Technical Board decision T0018/09, 2009, EPO Board of appeal decisions database T0018/09.
- Association for Molecular Pathology case vs. Myriad Genetics Inc., case No. Twelve-398 (2013) and Mayo Collaborative Services vs. Prometheus Laboratories, case No 10-1150 2012.
- Harrison C. Isolated DNA patent ban creates muddy waters for biomarkers and natural products. Nat Rev Drug Discov 2013; 12(8):570-1; PMID:23903213; http://dx.doi.org/10.1038/nrd4084
- Sheridan C. Immune-checkpoint inhibitors march on, now in combinations. Nat Biotechnol 2014; 32(4):297-9; PMID:24714455; http://dx.doi.org/10.1038/nbt0414-297
- Kim T, Amaria RN, Spencer C, Reuben A, Cooper ZA, Wargo JA. Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma. Cancer Biol Med 2014; 11(4):237-46. Retrieved from http://www.cancerbiomed.org/index.php/cocr/article/view/821/868; PMID:25610709
- European Patent Office (EPO): Guidelines for Examination, G VII, 7.
- Kim T, Amaria RN, Spencer C, Reuben A, Cooper ZA, Wargo JA. Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma. Cancer Biol Med 2014; 11(4):237-46. Retrieved from http://www.cancerbiomed.org/index.php/cocr/article/view/821/868; PMID:25610709
- The Life Sciences Report: Immuno-Oncology Promises Continued Wealth and Health: LifeTech Capital's Stephen Dunn. http://www.thelifesciencesreport.com/pub/na/immuno-oncology-promises-continued-wealth-and-health-lifetech-capitals-stephen-dunn, accessed August 20, 2015.
- The Life Sciences Report: Immuno-Oncology Promises Continued Wealth and Health: LifeTech Capital's Stephen Dunn. http://www.thelifesciencesreport.com/pub/na/immuno-oncology-promises-continued-wealth-and-health-lifetech-capitals-stephen-dunn, accessed August 20, 2015.
- BMS & Ono v. Merck & Co., case 1:14-cv-01131, complaint filed (D. Del. 2014).
- BMS & Ono v. Merck & Co., case 1:15-cv-00572, complaint filed (D. Del. 2015).
- 2014 Merck&Co 10-K SEC form, published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/310158/000031015815000005/mrk1231201410k.htm), accessed August 20, 2015.
- 2014 Merck&Co 10-K SEC form published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/310158/000031015815000005/mrk1231201410k.htm), accessed August 20, 2015.
- See the minutes of the oral proceedings of Sept 05, 2014, page 2, under https://register.epo.org/application?number=EP03741154&lng=en&tab=doclist.
- Octane Fitness, LLC v. Icon Health & Fitness, Inc., case no 12–1184 2014.
- Johnson & Johnson Vision Care v. Ciba Vision Corp, case 712 F. Supp. 2d 1285 (M.D. Fla. 2010), z4 Technologies Inc. v. Microsoft Corp, case 34 F. Supp. 2d 437 (E.D. Tex. 2006), WBIP LLC v. Kohler Co, case CA 11-10374-NMG (D. Mass. Aug. Twelve, 2013).
- World Preview 2015, Outlook to 2020. 8th edition, June 2015. Pharma market report provided by EvaluatePharma.
- 2014 Merck&Co 10-K SEC form published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/310158/000031015815000005/mrk1231201410k.htm) accessed August 20, 2015.
- University of Mich. & Repligen v. BMS, 301 F. Supp. 2d 633 (E.D. Mich. 2003).
- Regents Univ of Mich v Bristol-Myers Squibb, case Number 04-1015 (Fed. Cir. 2004).
- Repligen et al. v. Bristol-Myers Squibb, case No. Two:06-CV-004-TJW (E.D. Tex.).
- 2008 BMS 10-K SEC form, published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/14272/000119312509033538/d10k.htm), accessed August 20, 2015.
- European Commission, Pharmaceutical Sector Inquiry, Preliminary Report (DG Competition Staff Working Paper), 28 November 2008, page 17 (http://ec.europa.eu/competition/sectors/pharmaceuticals/inquiry/preliminary_report.pdf).
- 2013 BMS 10-K SEC form, published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/14272/000001427215000055/bmy-20141231´10xk.htm), accessed August 20, 2015.
- 2009 Repligen 10-K SEC form, published on the website of the US. Securities and Exchange Commission (http://www.sec.gov/Archives/edgar/data/730272/000119312515095569/d854858d10k.htm), accessed August 20, 2015.
