Abstract
The quadroma antibody represents the first attempt to produce a bispecific heterodimeric IgG antibody by somatic fusion of 2 hybridoma cells each expressing monoclonal antibodies with distinctive specificities. However, because of random heavy and light chain pairing, the desired functional bispecific antibody represents only a small fraction of the protein produced. Subsequently, the knobs-into-holes (KiH) approach was developed to enforce correct heavy chain heterodimerization. Assuming equimolar expression of 4 unmodified chains comprising 2 heavy and 2 light chains, the statistical distribution of all paired combinations can be calculated. With equimolar expression as the goal, we transfected HEK cells with 1:1:1:1 plasmid ratios and analyzed the protein A affinity-purified antibodies from the quadroma and KiH approaches qualitatively and quantitatively with regard to the estimated relative amounts of the products using electrospray quadrupole time-of-flight mass spectrometry. Our results show that all expected species are formed, and that, within the methodological limits, the species distribution in the mixtures corresponds approximately to the statistical distribution.
Abbreviations
| Ang2 | = | angiopoietin-2 |
| bsAbs | = | bispecific antibodies |
| ESI | = | electrospray ionization |
| GuA HCl | = | guanidine hydrochloride |
| HEK | = | human embryonic kidney |
| HC | = | heavy chain |
| IgG1 | = | immunoglobulin G1 |
| ISCID | = | ion source collision induced dissociation |
| KiH | = | knobs-into-holes |
| LC | = | light chain |
| MS | = | mass spectrometry |
| TCEP | = | tris(2-carboxyethyl)phosphine |
| UHR | = | ultrahigh-resolution |
| VEGF-A | = | vascular endothelial growth factor A |
| QTOF | = | quadrupole time-of-flight |
Introduction
Since the development of recombinant antibody technology, researchers have been fascinated by the idea of bispecific antibodies (bsAbs). Not only are these antibodies able to bind to 2 different antigens such as 2 cell surface proteins or 2 ligands, they are also able to, for example, simultaneously bind to tumor proteins and cytotoxic T-cells, thus directing therapeutic agents to the intended target (for recent reviews, see Riethmüller,Citation1 Kontermann,Citation2 and Spiess et al.Citation3). Among the first attempts to produce such compounds, the so-called “Quadroma” approach has been used, where 2 antibody-secreting cell lines were combined by somatic fusion. The resulting hybrid cell line, by co-expression of light chain (LC) and heavy chain (HC) of the first and LC and HC of the second antibody, was then able to produce the intended bsAb, where one arm binds the first, one arm the second antigen.Citation4,5,6 It was, however, immediately clear that, due to the statistical association of the 2 HCs ("HC heterodimerization problem") as well as of heavy and light chains ("LC mispairing problem") the intended compound was only a minor component of a mixture of a variety of species, and it would be nearly impossible to develop a purification method to provide the compound on a scale necessary for therapeutic purposes. To address this problem, Lindhofer et al.Citation7 developed the rat/mouse bsAbs (Triomabs) with species-restricted HC and LC chain pairing and thereby reduced the mismatch variants to <10%.
The HC heterodimerization problem alone could be solved by modification of the 2 CH3 domains by the so-called “knobs-into-holes” (KiH) method.Citation8 Using this method, in the CH3 domain of one heavy chain, a “knob” is created by replacement of a small amino acid by a larger one (T366Y), whereas replacement of large amino acids by smaller ones (such as Y407T) in the opposite CH3 domain leads to a “hole.” In addition, the KiH technology includes a stabilizing disulfide bridges between the CH3 domains. Together, these 2 domains constitute a perfect heterodimer. A number of alternative approaches for the same purpose have been developed recently.Citation9,10,11,12,13,14 Upon application of one of these methods alone, however, there still remains a mixture of 4 compounds except for cases where the chain association issue is circumvented by a common LC approach.Citation15
The LC mispairing problem is more difficult to address because a total of 4 possible pairings of heavy and light chains have to be considered. Nevertheless, a variety of formats have been developed where the mispairing can be avoided, e.g. by use of linkers, in vitro assembly of KiH half antibodies,Citation16 or HC-LC interface engineering.Citation17,18,19 Our solution, the CrossMabs, uses KiH for the HC heterodimerization and a domain exchange of HC and LC domains in one arm of the antibody to avoid LC mispairing.Citation20 Two antibodies of this type, both directed simultaneously against angiopoietin-2 (Ang2) and vascular endothelial growth factor A (VEGF-A) are currently being clinically investigated in oncology.Citation21 and ophthalmology.Citation22 Since the LC-HC interaction is species-specific with the Triomab approach, one can use mouse HC and LC for one arm and rat LC and HC for the other to avoid mispairing; however, the resulting antibody may exhibit a high degree of immunogenicity. Catumaxomab (trade name Removab), which has been approved as a therapeutic drug, is produced on this basis using a sophisticated purification method.Citation23
Since Milstein and Cuello's original publication,Citation4 graphical representations of the prediction of 10 species in the quadroma approach based on statistical arguments (4 in the case of KiH) can be found in many publications about bsAbs (e.g., Suresh et al.Citation5,24 Moldenhauer,Citation6 Smith et al,Citation25 Tada et al.Citation26). However, to our knowledge, this theoretical prediction has never been experimentally verified. Here, we transfected HEK cells with plasmids in 1:1:1:1 ratios to co-express the 4 quadroma or KiH chains and analyzed the products by mass spectrometry for a comparative assessment. The underlying chains originate from anti-Ang-2 antibody LC06 (heavy chain denoted A and light chain a),Citation27 and bevacizumabCitation28 (Avastin®, heavy chain denoted B and light chain b), which are both isotype IgG1 with kappa CL. LC06 and bevacizumab are both HC allotype G1m1,17, and VH germlines: IGHV1–2–02 (99%) and IGHV3–23–04 (77%), and VL germlines: IGLV3–21–02 (97%) and IGKV1–16–01 or IGKV1–39–01 (88%), respectively.
Results
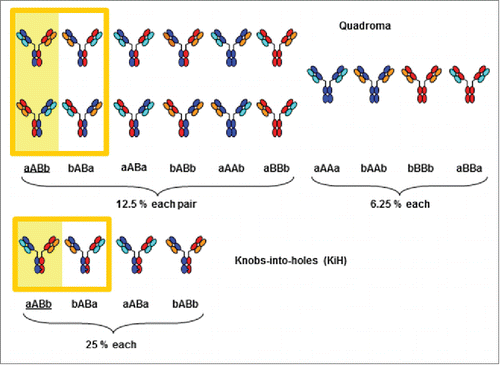
Consider four immunoglobulin chains, one LC (a, cyan) and one HC (A, blue) derived from a first antibody and one LC (b, orange) and one HC (B, red) derived from a second antibody () that are expressed simultaneously. If we assume that, in an ideal case, all components are present at equimolar amounts and all association processes occur with the same probability (which in reality is probably not the case), each of the 4 chains of the resulting antibody may belong to the red or the blue set. Therefore, 2×2×2×2=16 combinations are possible, each occurring at 6.25% in the mixture. Six of the combinations occur twice as a consequence of symmetry and represent the same compounds (aABb,Citation29 bABa, aABa, bABb, aAAb, and aBBb). The remaining 4 combinations (aAAa, bAAb, bBBb and aBBa), among them the parental antibodies aAAa and bBBb, occur only once. We thus expect a mixture of 10 species, 6 of them, including the desired bispecific antibody aABb, occurring at 12.5 %, 4 at 6.25 % (). If heterodimerization of the HCs is enforced by KiH or an equivalent method, the number of possible combinations is reduced to 4 (aABb, bABa, aABa, bABb) and we expect each of them at an amount of 25%, including the desired bispecific antibody aABb (). It is important to note that compounds aABb and bABa have the same mass and thus cannot be distinguished by mass spectrometry of their intact masses. In the case of the quadroma antibody, the number of species theoretically detectable by mass spectrometry is thus reduced from 10 to 9. The same holds true for the knobs-into-holes antibody where the number of observable species is 3 and not 4.
Figure 1. Quadroma and knobs-into-holes antibodies. Theoretical combinations and statistical distribution of quadroma and knobs-into-holes antibodies consisting of heavy chains A (blue) and B (red) and light chains a (cyan) and b (orange). The framed structures aABb (intended bispecific antibody, yellow background) and bABa are isobaric.
For the quadroma experiment, HEK cells were transfected with a 1:1:1:1 plasmid ratio of constructs expressing the chains LC (a) and a HC (A) of an anti-Ang-2 antibody and a LC (b) and a HC (B) of an anti-VEGF-A antibody, all of human IgG1 type without modifications. In a second experiment, HCs with knob and hole mutations were used (knob on the anti-Ang-2 side, blue).
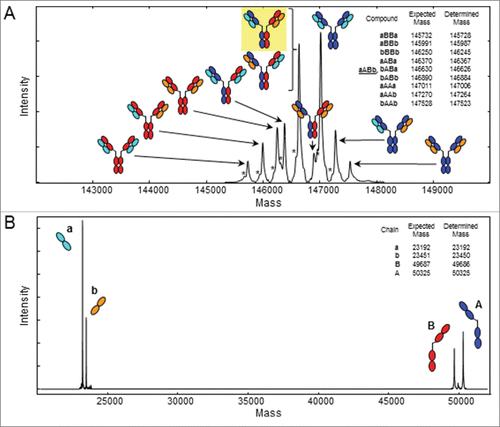
Electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-QTOF MS) is dedicated to qualitative analysis because the time-of-flight detector has a very good resolution and mass accuracy, and can be used for quantitative estimations. When performing ESI-QTOF MS of the deglycosylated and intact quadroma, we were able to identify 9 different molecular masses between 145728 and 147523 Da representing the 10 theoretical antibodies possible by combinations of the 2 different HCs A and B, and 2 different LCs a and b (theoretical masses; 50325, 49687, 23192 and 23451 Da, respectively, ), which in addition were all verified in the mass spectrum of the reduced quadroma (). The second most intense signal in the deconvoluted spectrum is the molecular mass of 146626 Da representing the intended bispecific antibody aABb and the isobaric mispaired antibody bABa ().
Figure 2. ESI-QTOF MS of the quadroma antibody. Deconvoluted spectrum of (A) the deglycosylated, intact quadroma antibody demonstrating the presence of 9 different masses representing the 10 antibodies theoretically possible by combinations of 2 different heavy chains A (blue) and B (red), and 2 different light chains a (cyan) and b (orange). The intended bispecific antibody aABb (yellow background) and bABa are isobaric and thus cannot be distinguished by mass spectrometry of the intact antibody. (B) Deconvoluted spectrum of the deglycosylated, TCEP-reduced quadroma showing the presence of the 2 different heavy chains and 2 different light chains. Expected and determined average masses are listed in the upper right corners. *Without C-terminal Gly.
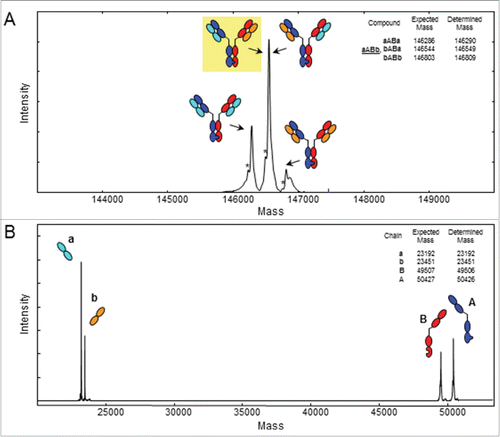
The mass spectrum of the deglycosylated and intact knobs-into-holes antibody revealed the presence of 3 different main masses of 146290, 146549 and 146809 Da, representing the combinations aABa, aABb/bABa and bABb with knob HC denoted A, the hole HC denoted B, and the 2 different LCs denoted a and b (). As with the quadroma, the mass of 146549 Da represent the isobaric compounds aABb and bABa that cannot be discriminated. The heavy and light chains A, B, a and b (theoretical masses: 50427, 49507, 23192 and 23451 Da, respectively) were verified in the mass spectrum of the reduced KiH antibody (). No side products involving knob-knob or hole-hole HCs could be detected in the preparation of the KiH antibody ().
Figure 3. ESI-QTOF MS of the knobs-into-holes antibody. Deconvoluted spectrum of (A) the deglycosylated, intact knobs-into-holes antibody demonstrating the presence of 3 different masses representing the 4 antibodies theoretically possible by combinations of the knob heavy chain A (blue), the hole heavy chain B (red), and 2 different light chains a (cyan) and b (orange). The intended bispecific antibody aABb (yellow background) and bABa are isobaric and thus cannot be distinguished by mass spectrometry of the intact antibody. (B) Deconvoluted spectrum of the deglycosylated, TCEP-reduced knobs-into-holes antibody showing the presence of the knob and hole heavy chains and 2 different light chains. Expected and determined average masses are listed in the upper right corners. *Without C-terminal Gly.
Quantitative evaluations of the m/z spectra of the intact quadroma and KiH revealed compound distributions close to the theoretical expectations (). For the quadroma the isobaric compounds aABb and bAba were estimated to be 24%, which should be compared to a theoretical 25% (2x 12.5%, ). The compounds aABa, bABb, aAAb and aBBb were all estimated to between 6–11%; theoretically expected at 12.5% each. aBBa, bAAb, and bBBb are represented by relatively 3–9%; theoretically all expected at 6.25% (). Only compound aAAa exceeded the expected relative level; estimated 28% as compared to the expected 6.25% (). The equivalent relative numbers for the KiH antibody resulted in 61% for aABb/bABa (theoretically 2x 25% = 50%), 27% aABa and 12% bABb (both theoretically 25%) ().
Table 1. Comparison of the statistical distribution of quadroma, knobs-into-holes and CrossMabFab antibodies with an experimentally determined estimated distribution analyzed by ESI-QTOF mass spectrometry. *HC dimers not included
Discussion
The relative distribution of the quadroma and KiH compounds could be strongly affected by small differences in the association energies/kinetics between the individual heavy and light chains, and by whether the chains are expressed close to equimolar ratios or not. Although the HEK cells in this study were transfected with 1:1:1:1 plasmid ratios of constructs expressing the chains A, B, a, and b, the chains might not be present at exactly equimolar ratios because the protein level is dependent on parameters like plasmid number, transcription and translation efficiency or chain stability. In the case of the quadroma and KiH antibodies, the intensity of the LC a in the reduced MS spectra is about double the intensity of the LC b (). This could indicate that there is more LC a present than LC b in the samples and could also explain the higher relative level of the aAAa compound in the case of the quadroma antibody and the lower level of bABb in the KiH compared to the statistically expected levels (). With estimated 9%, the VEGF-A antibody (compound bBBb) is slightly more abundant than statistically expected (6.25%) in the quadroma approach; this although LC b is less present compared to LC a. Whether this is contributed by a stronger pairing of the LC b with HC B than with HC A is unknown. Preferential parent heavy and light chain pairing would have been a prerequisite for a high yield of bispecific antibodies from quadromas. Early in vitro studies involving re-association of 2 different competing LCs with a limited amount of HC proposed preferential association of parent heavy and light chains.Citation30,31,32 However, later quantitative analysis of fractionations of IgGs produced by 8 quadromas by De Lau et al.Citation33 concluded that heavy and light chains associate in a random fashion. Studies by Hamel et al.,Citation34,35,36 who also did in vitro competitive heavy and light chain recombination studies, concluded that the LCs show no preference for their parent HCs. Also previous studies did not find any evidence for preferences in pairing of VH and VL families.Citation37 When Tiller et al.Citation38 analyzed the frequency of VH/VL pairs in the human antibody repertoire, the most prevalent pairs were mainly composed of the most frequently occurring VH and VL segments. The suggestion by Jayaram et al.Citation39 that the human VH1 family shows a strong pairing preference to the kappa-3 family, indicates that preferential HC-LC assembly might take place.
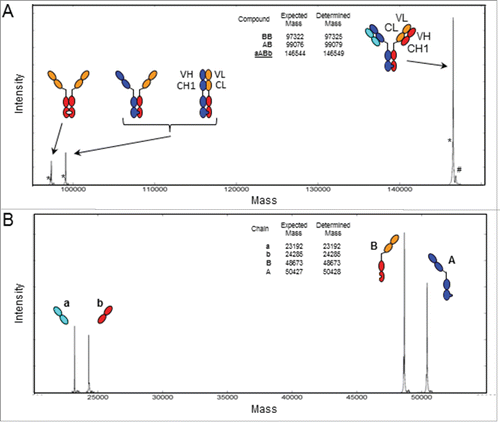
The discrepancies we observed could also be contributed by the fact that ESI-QTOF MS is semi-quantitative due to differences in ionization properties between chains with different amino acid sequences. However, the assumption that LC a and b might not to be present at equimolar level in the quadroma and KiH approaches in this study is supported by observations with our CrossMab bsAbs. The CrossMabs involve the KiH technology, and correct light chain pairing is enforced by a “crossed” light chain that does not associate with the heavy chain Fab region of the other arm of the bsAb.Citation20 When analyzed by MS, our deglycosylated CrossMabFab, based on the same sequences as used in the quadroma and KiH antibodies, exhibits only the correct bsAb (compound aABb), besides some hole-hole and knob-hole HC dimer (). Consequently, the LCs are present at equimolar levels in the example with the CrossMabFab, and the intensities of the pairs of LCs in the ESI-MS spectrum of the reduced CrossMabFab are similar (). The presence of the isobaric compound bABa in the CrossMabFab could be excluded because only the “correct” Fabs (“aA” and “Ba”) were detected in the MS spectra following Fab fragment generation (data not shown).
Figure 4. ESI-QTOF MS of the CrossMabFabantibody. Deconvoluted spectrum of (A) the deglycosylated, intact CrossMabFab demonstrating the presence of the intended product aABb consisting of the knob heavy chain A (blue), the hole CL-VL “heavy chain” B (red-orange), the wt light chain a (cyan) and the CH1-VH “light chain” b (red), and hole-hole and knob-hole heavy chain dimer side-products. (B) Deconvoluted spectrum of the deglycosylated, TCEP-reduced CrossMabFab showing the presence of the 4 different chains. Expected and determined average masses are listed. *Without C-terminal Gly. #Phosphate adduct.
In conclusion, the quadroma experiment, co-expression of 4 antibody chains, has been performed using a LC and a HC of an anti-Ang-2 antibody and a LC and a HC of an anti-VEGF-A antibody, all of human IgG1 type. In a second experiment, HCs with knobs and holes were used. The expression mixture was purified by Protein A and size-exclusion chromatography and investigated by ESI-QTOF mass spectrometry. All nine molecular masses expected in the mixture could be detected for the quadroma (3 in the case of the KiH antibody); the reduced spectra seem to suggest that the LC of the VEGF-A part is underrepresented in both experiments. Overall, we have experimentally demonstrated the existence of all expected heavy and light chain combinations of quadroma and KiH antibodies produced in this study. We also conclude that, within the limits of ESI-QTOF mass spectrometry, the measured relative amounts of the individual species correspond approximately to the prediction. This example shows that antibodies heavy and light chain association occurs largely as expected from statistical consideration.
Materials and Methods
Recombinant expression of quadroma, knobs-into-holes and CrossMab antibodies
All antibody HC and LC genes were ordered as gene syntheses and cloned via unique restriction sites using standard cloning procedures into separate expression vectors for each chain enabling secretory expression in HEK cells growing in suspension. The KiH mutations described by Ridgway et al.Citation8 were used (Knob: T366W; Hole: T366S, L368A, and Y407V). In addition 2 Cys residues were introduced in the CH3 domains (S354C in the knob chain and Y349C in the hole chain) that form a stabilizing disulfide bridge.Citation20 Transfection (1:1:1:1 plasmid ratios) into HEK293-F cells (Invitrogen, 510029) was performed according to the cell supplier's instructions using Maxiprep (Qiagen, 12163) preparations of the antibody vectors, Opti-MEM I medium (Invitrogen, 31985) 293fectin (Invitrogen, 31985070), and an initial cell density of 1–2 × 106 viable cells/mL in serum-free FreeStyle 293 expression medium (Invitrogen, 12338018). Antibody containing cell culture supernatants were harvested after 7 d of cultivation in shake flasks by centrifugation at 14,000 × g for 30 min and filtered through a 0.22 μm sterile filter (Thermo Scientific, 566–0020). The antibodies were purified directly from the supernatant, or the supernatant was stored at −80 °C until purification.
Protein purification
Proteins were purified from filtered cell culture supernatants referring to standard protein A protocols. The antibodies were captured by affinity chromatography using HiTrap MabSelect SuRe (GE Healthcare, 11–0034–93) equilibrated with PBS. Elution of antibodies was achieved at pH 3.0 followed by immediate neutralization of the sample. Aggregated protein, or in the case of the CrossMabFab a light chain heterodimer, was separated from monomeric antibodies by size exclusion chromatography (Superdex 200; GE Healthcare, 17–5175–01) in 20 mM histidine, 140 mM NaCl, pH 6.0. Monomeric antibody fractions were pooled, concentrated if required using a 30 molcular weight cut-off Millipore Amicon Ultra (Millipore, UFC803096) centrifugal concentrator, and stored at −80°C. The protein concentration of antibody preparations was determined by measuring the optical density (OD) at 280 nm, using the molar extinction coefficient calculated on the basis of the amino acid sequence.
ESI-QTOF mass spectrometry
The assembly of the quadroma and KiH antibodies was analyzed by electrospray ionization mass spectrometry of the deglycosylated and non-reduced molecules. 100 µg of the antibodies was deglycosylated with N-Glycosidase F (Roche, 11 836 552 001) at 37°C for 16 hours in a 100 mM phosphate buffer and subsequently denatured with or without 25 mM TCEP and 0.8 M GuA-HCl at 37°C for 30 min. Subsequently, the samples were desalted via HPLC on a Sephadex G25 column (GE Healthcare, 17–0032–02) using 40% acetonitrile with 2% formic acid (v/v). The total mass was determined via ESI-QTOF MS on a maXis 4G UHR-QTOF MS system (Bruker Daltonik) equipped with a TriVersa NanoMate source (Advion). Calibration was performed with the low concentration tuning mix (Agilent Technologies, G1969–85000). Data acquisition was done at 600–2000 m/z (ISCID: 0.0 eV), 1000–4000 m/z (ISCID: 130.0 eV), for the reduced and non-reduced samples, respectively. The raw mass spectra were evaluated and transformed into individual relative molar masses using an in-house developed Roche software tool. The quantitative evaluation of the mass spectra was performed by summing up contributions of m/z ion intensities of all charge states forming the dominant part (larger than 20%) of the charge state envelope as observed for the most abundant individual product mass. Then all peak contributions (fitted as Gaussians) of all signals in these charge states were used to calculate the relative contents of the individual species. For example, the quadroma main and side products were evaluated using the non-reduced quadroma m/z dataset in the range 2690–3640 m/z equivalent to charge states +40 to +54 of the most abundant form aAAa.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Oksana Tyshchuk and Eileen Petzold for contributing to the sample characterization and Dr. Hans Koll for helpful discussions.
References
- Riethmüller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immun 2012; 12:12; PMID:22896757
- Kontermann RE. Dual targeting strategies with bispecific antibodies. Mabs 2012; 4:182-97; PMID:22453100; http://dx.doi.org/10.4161/mabs.4.2.19000
- Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol 2015; 67:95-106; PMID:25637431; http://dx.doi.org/10.1016/j.molimm.2015.01.003
- Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature 1983; 305:537-40; PMID:6137772; http://dx.doi.org/10.1038/305537a0
- Suresh MR, Cuello AC, Milstein C. Advantages of bispecific hybridomas in one-step immunocytochemistry and immunoassays. Proc Natl Acad Sci U S A 1986; 83:7989-93; PMID:2429324; http://dx.doi.org/10.1073/pnas.83.20.7989
- Moldenhauer G. Bispecific antibodies from hybrid hybridoma. In: Kontermann RE (ed.), Bispecific Antibodies, Springer-Verlag Berlin Heidelberg 2011:29-46
- Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol. 1995; 155:219-25; PMID:7602098
- Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 1996; 9:617-21; PMID:8844834; http://dx.doi.org/10.1093/protein/9.7.617
- Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, Ng SB, Born T, Retter M, Manchulenko K, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem 2010; 285:19637-46; PMID:20400508; http://dx.doi.org/10.1074/jbc.M110.117382
- Moore GL, Bautista C, Pong E, Nguyen DH, Jacinto J, Eivazi A, Muchhal US, Karki S, Chu SY, Lazar GA. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. Mabs 2011; 3:546-57; PMID:22123055; http://dx.doi.org/10.4161/mabs.3.6.18123
- Kitazawa T, Igawa T, Sampei Z, Muto A, Kojima T, Soeda T, Yoshihashi K, Okuyama-Nishida Y, Saito H, Tsunoda H, et al. A bispecific antibody to factors IXa and × restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012; 18:1570-4; PMID:23023498; http://dx.doi.org/10.1038/nm.2942
- Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol 2012; 420:204-19; PMID:22543237; http://dx.doi.org/10.1016/j.jmb.2012.04.020
- Von Kreudenstein TS, Escobar-Carbrera E, Lario PI, D'Angelo I, Brault K, Kelly J, Durocher Y, Baardsnes J, Woods RJ, Xie MH, et al. Improving biophysical properties of a bispecific antibody scaffold to aid developability: quality by molecular design. Mabs 2013; 5:646-54; PMID:23924797; http://dx.doi.org/10.4161/mabs.25632
- Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, Brinkmann U, Kettenberger H, Regula JT, Schaefer W. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. Mabs 2012; 4:653-63; PMID:22925968; http://dx.doi.org/10.4161/mabs.21379
- Carter P. Bispecific human IgG by design. J Immunol Methods 2001; 248:7-15; PMID:11223065; http://dx.doi.org/10.1016/S0022-1759(00)00339-2
- Jackman J, Chen Y, Huang A, Moffat B, Scheer JM, Leong SR, Lee WP, Zhang J, Sharma N, Lu Y, et al. Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling. J Biol Chem 2010; 285:20850-9; PMID:20444694; http://dx.doi.org/10.1074/jbc.M1-10.113910
- Igawa T, Tsunoda H, Kikuchi Y, Yoshida M, Tanaka M, Koga A, Sekimori Y, Orita T, Aso Y, Hattori K, Tsuchiya M. VH/VL interface engineering to promote selective expression and inhibit conformational isomerization of thrombopoietin receptor agonist single-chain diabody. Protein Eng Des Sel 2010; 23:667-77; PMID:20576629; http://dx.doi.org/10.1093/protein/gzq034
- Liu Z, Leng EC, Gunasekaran K, Pentony M, Shen M, Howard M, Stoops J, Manchulenko K, Razinkov V, Liu H. et al. A novel antibody engineering strategy for making monovalent bispecific heterodimeric IgG antibodies by electrostatic steering mechanism. J Biol Chem 2015; 290:7535-62; PMID:25583986; http://dx.doi.org/10.1074/jbc.M114.620260
- Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol 2014; 32:191-8; PMID:24463572; http://dx.doi.org/10.1038/nbt.2797
- Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A 2011; 108:11187-92; PMID:21690412; http://dx.doi.org/10.1073/pnas.1019002108
- Kienast Y, Klein C, Scheuer W, Raemsch R, Lorenzon E, Bernicke D, Herting F, Yu S, The HH, Martarello L, et al. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res 2013; 19:6730-40; PMID:24097868; http://dx.doi.org/10.1158/1078-0432.CCR-13-0081
- Cheung GCM, Barathi VA, Tun BB, Yeo SW, Gan PP, Nyein CL, Regula J, Hartman G. Dual inhibition of angiopoietin-2 and vascular endothelial growth factor-A with Crossmab RG7716 suppressed laser-induced choroidal neovascularization in a non-human primate model. Invest Ophthalmol Vis Sci 2014; 55:1174; http://dx.doi.org/10.1167/iovs.13-11636
- Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol 1995; 155:219-25; PMID:7602098
- Suresh MR, Cuello AC, Milstein C. Bispecific monoclonal antibodies from hybrid hybridomas. Meth Enzymol 1986; 121:210-28; PMID:3724461; http://dx.doi.org/10.1016/0076-6879(86)21019-8
- Smith W, Jarrett AL, Beattie RE, Corvalan JR. Immunoglobulins secreted by a hybrid-hybridoma: analysis of chain assemblies. Hybridoma 1992; 11:87-98; PMID:1737643; http://dx.doi.org/10.1089/hyb.1992.-11.87
- Tada H, Toyoda Y, Iwasa S. Bispecific antibody-producing hybrid hybridoma and its use in one-step immunoassays for human lymphotoxin. Hybridoma. 1989; 8:73-83; PMID:2647617; http://dx.doi.org/10.1089/hyb.1989.8.73
- Thomas M, Kienast Y, Scheuer W, Bähner M, Kaluza K, Gassner C, Herting F, Brinkmann U, Seeber S, Kavlie A, et al. A Novel Angiopoietin-2 Selective Fully Human Antibody with Potent Anti-Tumoral and Anti-Angiogenic Efficacy and Superior Side Effect Profile Compared to Pan-Angiopoietin-1/-2 Inhibitors. PLoS One 2013; 8:e54923; PMID:23405099; http://dx.doi.org/10.1371/journal.pone.0054923
- Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997; 57:4593-9; PMID:9377574
- In all cases, including Table and Figures, the intended bispecific antibody is underlined
- De Préval C, Fougereau M. Specific interaction between VH and VL regions of human monoclonal immunoglobulins. J Mol Biol. 1976; 102:657-78; http://dx.doi.org/10.1016/0022-2836(76)90340-5
- Kranz DM, Voss EW Jr. Restricted reassociation of heavy and light chains from hapten-specific monoclonal antibodies. Proc Natl Acad Sci U S A 1981; 78:5807-11; PMID:6795637; http://dx.doi.org/10.1073/pnas.78.9.5807
- Stevenson GT, Mole LE. The specificity of chain interactions among immunoglobulins. Combinations of gamma chains with kappa chains of the same subgroup as in the parent immunoglobulin G. Biochem J 1974; 139:369-74; PMID:4217181; http://dx.doi.org/10.1042/bj1390369
- De Lau WB, Heije K, Neefjes JJ, Oosterwegel M, Rozemuller E, Bast BJ. Absence of preferential homologous H/L chain association in hybrid hybridomas. J Immunol 1991; 146:906-14; PMID:1899099
- Hamel PA, Isenman DE, Klein MH, Luedtke R, Dorrington KJ. Structural basis for the preferential association of autologous immunoglobulin subunits: role of the J region of the light chain. Mol Immunol. 1984; 21:277-83; PMID:6427603; http://dx.doi.org/10.1016/0161-5890(84)90098-1
- Hamel PA, Klein MH, Dorrington KJ. The role of the VL- and VH-segments in the preferential reassociation of immunoglobulin subunits. Mol Immunol 1986; 23:503-10; PMID:3092029; http://dx.doi.org/10.1016/0161-5890(86)90113-6
- Hamel PA, Klein MH, Smith-Gill SJ, Dorrington KJ. Relative noncovalent association constant between immunoglobulin H and L chains is unrelated to their expression or antigen-binding activity. J Immunol 1987; 139:3012-20; PMID:3117886
- Brezinschek HP, Foster SJ, Dörner T, Brezinschek RI, Lipsky PE. Pairing of variable heavy and variable kappa chains in individual naive and memory B cells. J Immunol 1998; 160:4762-7; PMID:9590222
- Tiller T, Schuster I, Deppe D, Siegers K, Strohner R, Herrmann T, Berenguer M, Poujol D, Stehle J, Stark Y, et al. A fully synthetic human Fab antibody library based on fixed VH/VL framework pairings with favorable biophysical properties. MAbs. 2013; 5:445-70; PMID:23571156; http://dx.doi.org/10.4161/mabs.24218
- Jayaram N, Bhowmick P, Martin AC. Germline VH/VL pairing in antibodies. Protein Eng Des Sel 2012; 25:523-9; PMID:22802295; http://dx.doi.org/10.1093/protein/gzs043
