ABSTRACT
Antibody-drug conjugates (ADCs) have exhibited potent clinical benefits in cancer therapy. However, development of ADCs against epidermal growth factor receptor (EGFR) has limitations because of wide expression of EGFR in both normal and tumor tissues. Previously, we developed an anti-EGFR protease-activated antibody (pro-antibody), termed as PanP, which remains inert against EGFR until activated by tumor-specific protease. Herein, we for the first time report a new class of pro-antibody-drug conjugate (PDC) against EGFR, denoted as PanP-DM1. It has been designed to selectively target the EGFR-overexpressing tumor cells and exert greater anti-tumor activity compared with PanP. Our data showed that PanP-DM1 also could be selectively activated by tumor-specific protease 'uPA'. Furthermore, activated PanP-DM1 was potently cytotoxic against EGFR-overexpressing tumor cell lines in vitro. Crucially, our data indicated that PanP-DM1 was significantly more effective in eradicating EGFR-overexpressing tumors in vivo. Additionally, toxicity was preliminarily evaluated in mice as measured by body weight loss. In summary, our study suggests that PanP-DM1, a novel pro-antibody-drug conjugate, has cancer-selectivity, efficacy and safety profile that supports its potential use for EGFR-overexpressing tumors.
Abbreviations
| ADC | = | antibody–drug conjugate |
| CRC | = | colorectal cancer |
| CCK-8 | = | Cell Counting Kit 8 |
| DAR | = | drug to antibody ratio |
| EGFR | = | epidermal growth factor receptor |
| Endo F2 | = | Endo-β-N-acetylglucosaminidase F2 |
| ELISA | = | enzyme-linked immunosorbent assay |
| FACS | = | fluorescence-activated cell sorting |
| mAb | = | monoclonal antibody |
| Pro-antibody | = | protease-activated antibody |
| PDC | = | Pro-antibody-drug conjugate |
| RP-UPLC | = | Reverse-phase Ultra Performance Liquid Chromatography |
| SMCC | = | N-succinimidyl-4-[maleimidomethyl]-cyclohexane carboxylate |
| SEC | = | size-exclusion chromatography. |
Introduction
Epidermal growth factor receptor (EGFR), a transmembrane receptor kinase, plays a pivotal role in tumor progression. Aberrant EGFR activation is associated with tumorigenesis and metastasis.Citation1 Porebska et al revealed its increased expression in 60–80% of colorectal cancer (CRC) cases.Citation2 Furthermore, EGFR expression could be a prognostic marker in many cancers like CRC and breast cancer.Citation3 More importantly, its localization on the cancer cell surface makes it an ideal molecular target for developing EGFR-directed antibodies.Citation4 They specially bind to the extracellular domain III of EGFR, thereby blocking the ligand-binding domain and hindering the extended conformation of the dimerization arm on domain II.Citation5,6 The US Food and Drug Administration (FDA)-approved antibodies against EGFR, cetuximab (Erbitux®) and panitumumab (Vectibix®), are routinely used and produce substantial therapeutic benefits in the treatment of KRAS wild-type advanced CRC.
Although EGFR-blocking antibodies have shown potent clinical efficacy, on-target skin toxicities associated with EGFR inhibition lead to interruption or dose modification, and affect patients' quality of life.Citation7,8 As reviewed by relevant studies, EGFR inhibitors were thought to affect keratinocytes by inducing skin inflammation and innate host defense.Citation9,10 A protease-activated antibody (Pro-antibody) that is inactive in normal tissues and selectively activated by the proteases upregulated in tumor tissues is an attractive approach to reduce side effects caused by target binding in healthy tissues.Citation11,12 Recently, Desnoyers et al designed a pro-antibody based on cetuximab that improved the safety profile without compromising the pre-clinical efficacy.Citation13
Antibody–drug conjugates (ADCs) are emerging as powerful anti-tumor therapeutics that combine tumor-targeted antibodies with active cytotoxic agents.Citation14 Generally speaking, the ADC components consist of an antibody that targets internalized cell surface molecule and a highly cytotoxic compound.Citation15,16 Numerous studies indicated maytansinoid DM1 (derivative of maytansine), a highly potent microtubule polymerization inhibitor, was an ideal payload for developing ADC.Citation17–20 Furthermore, antibody-DM1 conjugates have shown promising results in preclinical and clinical evaluations.Citation21 As a member of the EGFR family, HER2 is a clinically validated ADC target. The FDA-approved HER2-directed ADC, ado-trastuzumab emtansine (T-DM1), is composed of trastuzumab and DM1 for treating patients with HER2-positive breast cancer.Citation14,22 Previous studies demonstrated that EGFR appears to be rapidly internalized when incubated with anti-EGFR antibodies such as panitumumab.Citation23,24 An EGFR-targeted ADC, IMGN289 is currently being evaluated in a Phase 1 clinical trial. Therefore, an EGFR-targeted ADC may be a promising therapeutic, although it potentially increases the severity of the side effects that will be systematically evaluated in clinical trials. Notably, pro-antibody-derived drug conjugates against EGFR that combine the advantage of the pro-antibody's target specificity with a drug's cytotoxicity have not been reported yet. The properties of pro-antibody-drug conjugates (PDCs) should limit the toxicity on normal tissues.
Here, we developed a novel PDC against EGFR, designated PanP-DM1. Previously, we constructed and characterized the cancer-selective pro-antibody termed as PanP. It was engineered by fusing the peptide comprising uPA substrate sequence, blocking peptide and Gly-Ser–rich peptide linkers to the light chain N terminus of Pan that derived from panitumumab.Citation25 In the present study, the maytansine (DM1) was conjugated to PanP through the stable non-reducible thioether linkage. The enhanced anti-tumor effects were assessed using in vitro and in vivo models. Further, we confirmed that PanP-DM1 could be internalized and induce cell cycle arrest. In addition, a preliminary toxicity study was performed in BALB/c mice and tumor-bearing nude mice by comparing the changes on body weight with injection of PanP-DM1.Citation26–28 To conclude, these data suggest that PanP-DM1, the first cancer-selective PDC for EGFR-targeted therapy, holds promise for clinical development because of its high potency and improved cancer selectivity.
Results
Characterization of PanP-DM1
PanP-DM1, a conjugate where lysine residues were modified with DM1 via a non-reducible thioether linker, succinimidyl-4-[maleimidomethyl]-cyclohexane carboxylate (SMCC), was prepared as described in Materials and Methods. Schematic representation of PDC was shown in . The resulting PanP-DM1 was firstly characterized by SDS-PAGE (). Under reducing conditions, the heavy chain of PanP-DM1 had a slightly higher molecular weight than that of PanP, suggesting that the linker drug preferentially attaches to lysine residues in the heavy chain as previously reported.Citation16 In addition, only little aggregation was observed at non-reducing conditions; the content was then determined to be below 3% using size-exclusion chromatography (Fig S1).
Figure 1. Generation and characterization of PanP-DM1. (A) Architecture of PDC: PanP is a pro-antibody against EGFR. (B) PanP-DM1 conjugate was constructed , as evidenced by 10% nonreducing and reducing SDS-PAGE. Lane 1, PanP; Lane 2, PanP-DM1. (C) The deconvoluted mass spectrum showing the drug distribution of PanP-DM1 (included raw MS data). The labels D0 to D7, Dn indicated PanP species linked to n DM1 molecules. The conjugation of one DM1 molecule through SMCC to PanP increases the theoretical mass of the molecule by 958.5 Da.
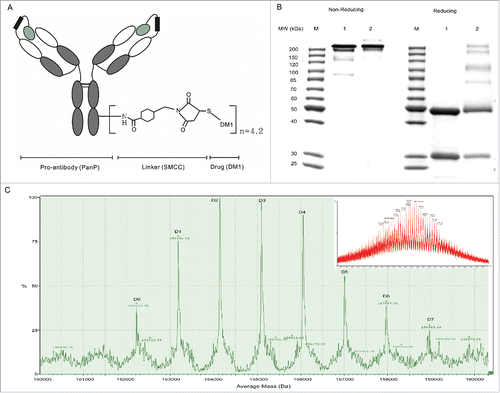
Moreover, drug-to-antibody ratio (DAR) of PanP-DM1 was determined to be 4.2 using UV/VIS spectroscopic analysis, which is similar to that of commercial T-DM1.Citation29 To further evaluate the drug distribution, deglycosylated PanP-DM1 was analyzed by ESI-TOF-MS. As illustrated in , the spectrum shows 8 prominent peaks, which are equally spaced and correspond to antibody linked to zero to 7 DM1 molecules. Taken together, these data showed that PanP-DM1 has been successfully constructed in structure.
PanP-DM1 displays comparable property to PanP
An enzyme-linked immunosorbent assay (ELISA) was performed to investigate whether PanP-DM1 still maintains the binding properties of PanP. PanP-DM1 showed slightly reduced EGFR binding compared to PanP, suggesting that conjugation apparently did not affect the antigen binding (). It is important to note that PanP-DM1 displayed 12-fold weaker binding to immobilized EGFR than Pan (parental antibody of PanP) DM1 conjugate, indicating that PanP-DM1 retained masked binding of PanP.
Figure 2. PanP-DM1 retains the property of PanP. (A) ELISA evaluating the masked binding activity of PanP-DM1 to immobilized EGFR. EC50 for Pan, Pan-DM1, PanP and PanP-DM1 were 0.1265 µg/mL (95% confidence interval [CI], 0.1089–0.1469 µg/mL), 0.2034 µg/mL (95% CI, 0.1805–0.2293 µg/mL), 2.257 µg/mL (95% CI, 1.861–2.739 µg/mL) and 2.569 µg/mL (95% CI, 2.044–3.229 µg/mL), respectively. (B) FACS assay assessing the binding activity of activated PanP-DM1 (Ac PanP-DM1) to EGFR expressed on surface of A431 cells at 1 µg/ml or 10 µg/ml. (C) uPA-activated PanP-DM1 restored binding comparable to Pan-DM1, as tested by ELISA. EC50 for activated PanP-DM1, PanP-DM1 and Pan-DM1 were 0.3198 µg/mL (95% CI, 0.2804 - 0.3648 µg/mL), 2.414 µg/mL (95% CI, 1.950 – 2.987 µg/mL) and 0.2251 (95% CI, 0.1964 – 0.2579 µg/mL), respectively. Error bars, SEM.
![Figure 2. PanP-DM1 retains the property of PanP. (A) ELISA evaluating the masked binding activity of PanP-DM1 to immobilized EGFR. EC50 for Pan, Pan-DM1, PanP and PanP-DM1 were 0.1265 µg/mL (95% confidence interval [CI], 0.1089–0.1469 µg/mL), 0.2034 µg/mL (95% CI, 0.1805–0.2293 µg/mL), 2.257 µg/mL (95% CI, 1.861–2.739 µg/mL) and 2.569 µg/mL (95% CI, 2.044–3.229 µg/mL), respectively. (B) FACS assay assessing the binding activity of activated PanP-DM1 (Ac PanP-DM1) to EGFR expressed on surface of A431 cells at 1 µg/ml or 10 µg/ml. (C) uPA-activated PanP-DM1 restored binding comparable to Pan-DM1, as tested by ELISA. EC50 for activated PanP-DM1, PanP-DM1 and Pan-DM1 were 0.3198 µg/mL (95% CI, 0.2804 - 0.3648 µg/mL), 2.414 µg/mL (95% CI, 1.950 – 2.987 µg/mL) and 0.2251 (95% CI, 0.1964 – 0.2579 µg/mL), respectively. Error bars, SEM.](/cms/asset/19b484dc-d801-4ff7-94bb-482dbc9dad63/kmab_a_1127491_f0002_c.gif)
To further evaluate the binding of uPA-activated PanP-DM1 on cell-surface EGFR, we used a fluorescence-activated cell sorting (FACS) assay. Activated PanP-DM1 was obtained by incubating PanP-DM1 with recombinant uPA in vitro as described previously and characterized using SDS-PAGE (Fig S2).Citation13,25 Activated PanP-DM1 and activated PanP exhibited mostly equivalent ability to bind to EGFR-overexpressing A431 cells at saturating (10 µg/ml) and subsaturating (1 µg/ml) concentrations, suggesting that attachment of DM1 did not affect the uPA-mediated activation (). Moreover, PanP-DM1 showed about an order of magnitude increase in binding activity after exposure to uPA as determined by ELISA and FACS, which is comparable to Pan-DM1 (; Fig S3; Table S1). Thus, these results revealed that PanP-DM1 remains masked against EGFR binding until activated by uPA, which is similar to PanP.
PanP-DM1 has superior in vitro anti-tumor activity compared with PanP
Next, we compared the inhibitory effects of PanP-DM1, activated PanP-DM1 and activated PanP on 3 EGFR-overexpressing cancer cell lines (A431, H292 and DiFi cells).Citation30–32 After 48 or 72h incubation, activated PanP-DM1 inhibited the growth of DiFi, H292 and A431 cancer cells in a dose-dependent manner (). Importantly, the growth inhibition was significantly stronger compared with activated PanP at the concentration of 1 µg/ml (P<0.001). Furthermore, as expected, activated PanP-DM1 exhibited good inhibitory activity compared to non-activated PanP-DM1 against these cells at a low concentration (0.1 µg/mL), indicating the functionally masked effect in the intact PDC (). These results demonstrated that PanP-DM1 had superior in vitro anti-tumor activity compared with PanP.
Figure 3. Activated PanP-DM1 is highly active against EGFR-overexpressing cancer cells. (A) EGFR-overexpressing cells were treated with increasing concentrations of Pan-DM1, activated PanP-DM1, PanP-DM1 and activated PanP. Anti-TNF DM1 was used as control. Cell growth relative to an untreated control was measured after 48 or 72 hours using CCK-8 assay. (B) Statistical analysis showing the percentage of surviving cells when treated with activated PanP-DM1 compared with PanP-DM1 in the same low concentration (0.1 µg/mL). (C) Non-targeted inhibitory effects were also examined on breast cancer cells with low expression of EGFR. Error bars, SEM. ** P < 0.01, *** P < 0.001.
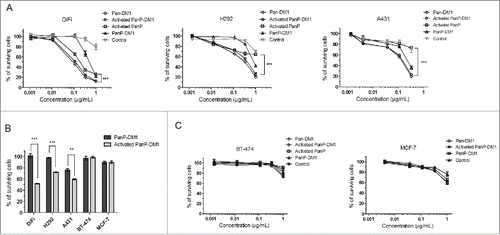
In addition, non-targeted inhibitory effects of PanP-DM1 conjugate were also evaluated on breast cancer cell lines expressing low levels of EGFR (BT-474 and MCF7).Citation33,34 As a result, activated PanP-DM1 only showed minimal dose-dependent inhibitory activity in these cell lines (), which is equal to that of a control ADC, anti-tumor necrosis factor (TNF) mAb-DM1. Only activated PanP-DM1 at a high concentration (1 µg/ml) inhibited the growth of BT-474 or MCF-7 cells, indicating a non-specifically inhibitory effect.
Effect of PanP-DM1 on A431 cell cycle arrest
To further evaluate PanP-DM1s mechanism of action, we analyzed the conjugate-induced cell cycle arrest on A431 cells. Internalization of activated PanP-DM1, which is a necessary process for delivering DM1 into cells and triggering cell cycle arrest, was first evaluated in A431 cells. The surface level of activated PanP-DM1 markedly decreased on cells when shifted to 37°C over the course of 90 minutes study (), suggesting rapid internalization of the conjugate into A431 cells.
Figure 4. The enhanced potency of PanP-DM1 correlates with its rapid internalization and induced cell cycle arrest in A431 cell line. (A) A431 cells were incubated with saturating level of activated PanP-DM1 (10 µg/mL) for 30 minutes at 4 °C. Unbound antibody conjugates were removed by washing cells. Cells were then incubated at either 4°C or 37°C. At the indicated time points, samples were detected by flow cytometry. (B) Representative cell cycle distribution in A431 cells treated with activated PanP, activated PanP-DM1 or PanP-DM1 compared with non-treated cells (Control). Cells were exposed to indicated drugs at the same concentration (1 µg/mL) for 3 h at 37°C, washed, and incubated in medium for an additional 18 h at 37°C. The DNA content was then measured by flow-cytometric analysis as described in Materials and Methods. (C) Statistical analysis showing G2/M population in A431 cells induced by indicated drugs. Error bars, SEM.
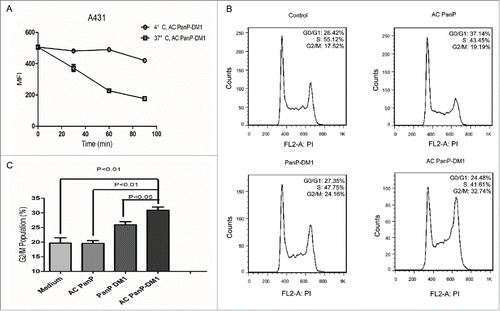
The effect of activated PanP-DM1 on cell cycle was subsequently determined by flow cytometry. Treatment with activated PanP-DM1 resulted in the remarkable G2/M phase arrest, which may directly lead to cell apoptosis ().Citation27,35 The percentage of cells in the G2/M phase increased from 19.69 ± 1.809% to 30.92 ± 1.097% in activated PanP-DM1-treated culture and from 19.69 ± 1.809% to 25.92 ± 1.092 % in PanP-DM1-treated culture (). These results revealed that non-activated PanP-DM1 was less effective than activated PanP-DM1 in inducing G2/M arrest on A431 cells (P<0.05), which is likely attributable to reduced antigen-binding ability. In addition, in line with previous studies, activated PanP induced G0/G1 arrest, which is a known mechanism of action of panitumumab and cetuximab ().Citation36,37 Together, these data implied that PanP-DM1 exerts enhanced anti-tumor effect through targeted delivery of DM1 and inducing G2/M cell cycle arrest.
Therapeutic effects of PanP-DM1 in A431 and H292 xenograft tumor models
Next, the therapeutic effects of PanP-DM1 and PanP were examined in nude mice bearing established A431 and H292 xenograft tumors. The two cell lines can express and secrete uPA as previously described.Citation38,39 As shown in , both PanP and PanP-DM1 were effective in delaying the A431 xenograft tumor progression. It is particularly noteworthy that treatment with PanP-DM1 resulted in rapid and nearly complete tumor regression from day 7 post injection, whereas only partial tumor growth inhibition was observed in tumor-bearing mice treated with PanP. At the end of observation period (day 21), tumors were absent in all mice (n = 10) treated with PanP-DM1, whereas mice treated with PanP already reached tumor volume of 555.8 ± 70.58 mm3. Evidently, PanP-DM1 inhibited tumor growth much more effectively than PanP in A431 tumor models.
Figure 5. In vivo efficacy of PanP-DM1 in the A431 and H292 xenograft tumor models. (A) Nude mice were injected s.c. with 1 × 106 A431 cells. Treatment (arrows) with PanP-DM1 (35 mg/kg, i.p., n=10), PanP (35 mg/kg, i.p., n=10) or control (human IgG, 35 mg/kg, i.p., n=10) was started when tumor volumes reach of about 100 mm3. (B) Nude mice were injected s.c. with 1 × 106 H292 cells. Treatment (arrows) with PanP-DM1 (20 mg/kg, i.p., n=10), PanP (20 mg/kg, i.p., n=10) or control (human IgG, 20 mg/kg, i.p., n=10) was started when tumor volumes reach of about 130 mm3. Arrows indicate the time point of injection. Tumor volumes were recorded at regular intervals. Data are shown as means ± SEM. * P < 0.05, *** P < 0.001.
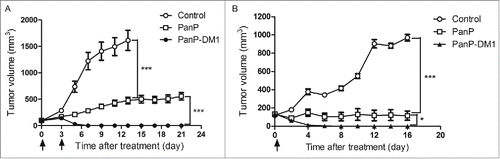
We then confirmed the anti-tumor effect of PanP-DM1 in the H292 xenograft tumor model (). A single intraperitoneal (i.p.) injection of PanP-DM1 at 20 mg/kg achieved complete tumor regression in all mice (n = 10). Notably, PanP-DM1 inhibited tumor development more effectively than PanP, although the difference was less pronounced than on faster growing A431 tumors. In conclusion, consistent with in vitro results, PanP-DM1 also exhibited significant benefit over PanP in the A431 and H292 xenograft tumor models.
Evaluation of PanP-DM1' non-specific toxicity in mice
To assess the therapy-related unspecific toxicity on PanP-DM1 treatment, body weight was monitored in nude mice bearing established subcutaneous A431 and H292 tumor xenografts. For A431 tumor-bearing mice, treatment with PanP-DM1 was well tolerated and the average body weight markedly recovered after marginal weight loss post the second injection (). Similar results were also observed on H292 tumor-bearing mice treated with PanP-DM1. Over the study period (about 18 days), these mice did not significantly lose body weight and behaved normally.
Figure 6. Toxicity and tolerability evaluation of PanP-DM1 in mice. (A) Effect of PanP-DM1 on nude mice body weight was determined using A431 or H292 tumor-bearing nude mice. Mice were weighed at regular intervals during the whole period to monitor therapy-related toxicity. (B) Body weight change of BALB/c mice on treatment with different dose schedules of PanP-DM1. Mice were randomly divided into groups of 5 mice each and treated on day 0 with a single i.p. dose of PanP-DM1 (0, 25, 50, 75 or 100 mg/kg). Changes in body weight over time relative to day 0 were plotted. Arrows indicate the time point of injection. Data are shown as means ± SEM. ** P < 0.01, *** P < 0.001.
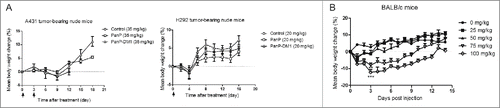
As PanP does not recognize mouse EGFR, the antigen-independent effect of PanP-DM1 was measured on BALB/c mice by body weight loss at multiple doses (). Of the tested dose, PanP-DM1 treatment at 0, 25 or 50 mg/kg did not exhibit body weight loss. However, administration of PanP-DM1 at a dose of 75 or 100 mg/kg resulted in considerable transient body weight loss (-8% or -12%) in the first 3 days, indicating that a single-dose of PanP-DM1 above 75 mg/kg exerted a toxic effect on BALB/c mice. Overall, PanP-DM1 at multiple doses appears to be well tolerated.
Discussion
To our knowledge, this is the first study to describe a PDC against EGFR. Our previous study revealed that PanP has enhanced anti-tumor potency and target selectivity. In the present study, our data showed that PanP-DM1 retained the properties of PanP and was much more active in eradicating established tumors. As noted in our previous study, 50 mg/kg dose of PanP (2 times per week) for 3 weeks ultimately eradicated established A431 tumors in tumor bearing mice. Strikingly, 2 doses of 35 mg/kg PanP-DM1 conjugate in one week resulted in complete regressions in A431 tumor-bearing mice, suggesting its potential advantage for clinical therapy.
Generally speaking, conjugates with lesser or higher drug load are likely to result in compromised anti-tumor effects or increased systemic toxicities. PanP-DM1, with a DAR of 4.2:1, has exhibited improved therapeutic effect and was well tolerated in mice, demonstrating the ratio seemed to be favorable for developing PDC against EGFR. A previous study reported that the paclitaxel-cetuximab conjugate has enhanced cytotoxicity compared to intact antibody in vitro. However, the lack of a significant difference between cetuximab and the conjugate was found in the in vivo model, which may be explained by insufficient dose of mAb-delivered paclitaxel or untimely release of it.Citation40 Therefore, we chose the stable thioether linker SMCC and highly potent agent DM1 to construct PanP-DM1. As a result, it has shown strong anti-tumor potency in vitro and in vivo, indicating that the linker and cytotoxic drug play important roles in governing the efficacy and viability of conjugates.
Skin toxicities were observed in patients after treatment with anti-EGFR antibodies.Citation41,42 ADCs targeting EGFR could potentially increase the severity of the side effects. AMG 595, an ADC directed to the tumor-specific antigen EGFRvIII was designed to minimize on-target skin toxicities that might be observed in ADCs against wild-type EGFR.Citation43 In addition, PDCs that could be selectively activated in tumors have the potential to limit on-target skin toxicities without compromising the therapeutic effect. Data shown here revealed that PanP-DM1 exhibited attenuated functional activities on antigen binding, cell growth inhibition and cell cycle arrest, but, when activated by uPA, it showed significantly enhanced activities in these assays. A previous study concluded that stronger binding clearly leads to higher toxicities among EGFR-targeted antibodies.Citation44 Thus, PanP-DM1 has the potential to achieve desired tolerability profile with less binding on normal cells than other conventional anti-EGFR ADCs.
Furthermore, the toxicity evaluation provides a valuable reference for further safety assessment of PDC in cynomolgus monkeys. Notably, PanP-DM1 eradicated A431 and H292 xenografts in mice with no obvious side effects at the effective doses. Since PanP-DM1 has no cross-reactivity with mouse tissues, the potential safety benefits conferred by cancer-selectivity of PanP-DM1 could not be sufficiently evaluated in our present study. And further studies will be focused on evaluating the on-target cutaneous toxicity in nonhuman primates.
Drug resistance with the current established anti-EGFR antibodies such as cetuximab creates the need for better therapeutic avenue.Citation45 ADC has become a powerful treatment strategy to overcome drug resistance like trastuzumab resistance.Citation35 The efficacy of PanP-DM1 on the cancer cells that are refractory to EGFR-directed antibodies will be explored in a future research publication.
Collectively, the data presented here suggest that PanP-DM1 has the potential not only to dramatically improve the therapeutic effect of PanP, but also to exhibit cancer-selective activity. Therefore, development of PDC provides a new strategy for targeting EGFR in a cancer-specific manner, which contributes to enhancing therapeutic effect and improving the safety index over conventional ADC.
Materials and methods
Cell lines, animals and other reagents
The human epithelial carcinoma cell line A431, human breast cancer cell lines MCF-7 and BT-474 and human lung cancer cell line NCI-H292 were obtained from the American Type Culture Collection (ATCC). The human colorectal cancer cell line DiFi was obtained from MD Anderson Cancer Center. The anti-EGFR mAb Pan and PanP were produced by State Key Laboratory of Antibody Medicine and Targeted Therapy (Shanghai, China). Recombinant human uPA was purchased from Sino Biological Inc. DM1-SMCC was purchased from Levena Biopharma Co, Ltd (China). Six-week-old female BALB/c nude mice and BALB/c mice were obtained from the Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China). All animals were treated in accordance with guidelines of the Committee on Animals of the Second Military Medical University.
Conjugation and characterization of pro-antibody-drug conjugate (PDC)
DM1 conjugated PanP, termed PanP-DM1, was generated by conjugation of DM1-SMCC on antibody lysine groups as described in US 2011/0003969 A1. In brief, antibody at 5 mg/mL was mixed with ~6.4 mg/mL DM1-SMCC in a phosphate buffer (50mM, PH = 7.5) containing 2mM EDTA and 50 mM NaCl for 2 hours. The anti-EGFR mAb Pan was also conjugated to DM1-SMCC to form Pan-DM1. In addition, anti-TNF DM1 conjugate was prepared as control. DAR was determined by UV-VIS absorbance at 280 and 252 nm and calculated according to US 2010/0092495 A1. Drug distribution was then analyzed by reverse-phase ultra-performance liquid chromatography (RP-UPLC)/ESI-MS. The resulting conjugates were resolved on 10% SDS–PAGE under reducing conditions and nonreducing conditions, followed by Coomassie brilliant blue staining.
RP-UPLC /ESI-MS analysis of deglycosylated PanP-DM1
Samples were analyzed using reverse-phase chromatography coupled online with ESI-TOF-MS. Firstly, deglycosylation was performed by incubating 100 µg of PanP-DM1 with 7.14 µg endo-β-N-acetylglucosaminidase F2 (Endo F2) (Zhangjiang Biotech, Inc.) at 37°C for 4 h. Approximately 2 µg of deglycosylated PanP-DM1 was loaded onto a Waters C4 column (2.1 × 50 mm, 1.7 µm) and eluted with a mobile phase of 25–35% aqueous acetonitrile gradient containing 0.1% FA in 6min at 60°C with a flow rate of 0.4mL/min. Finally, Biopharmalynx was used to analyze the obtained mass spectrum.
EGFR binding measurements by ELISA and Flow Cytometry
The assay was performed as described previously.Citation25 Briefly, 96–well plates (Nunc) were coated with EGFR-Fc and blocked with phosphate-buffered saline (PBS) containing 10% nonfat dried milk. Indicated concentrations of antibodies were added to the plates and incubated for 1 hour at room temperature. After a wash step, bound antibody was detected with horseradish peroxidase (HRP)–conjugated anti-human F(ab')2 (Sigma) for 30 min at 37°C followed by the addition of 3,3',5,5 –tetramethylbenzidine (TMB) substrate reagent. Absorbance was read at 450 nm. Data were fitted using a sigmoidal 4-parameter curve.
For flow cytometric analysis, A431 cells overexpressed EGFR were incubated on ice with indicated agents in fluorescence activated cell sorting (FACS) buffer (PBS buffer containing 1% fetal bovine serum) for 1 hour. Cells were washed with FACS buffer and incubated with FITC-labeled goat anti-human IgG (H+L) secondary antibody (Life Technologies) on ice for 30 min. Then all samples were washed using FACS buffer, followed by FACS analysis using a BD FACSCalibur system (BD Biosciences).Citation46
In vitro cytotoxicity assays
The effects of PanP and PanP-DM1 conjugate on tumor cell viability were assessed using CCK-8 kit (Dojindo, Japan). Cells in medium containing 1% fetal bovine serum were plated in 96-well culture plates (5,000 per well for BT-474, MCF-7 and DiFi; 3,000 cells per well for H292; 2,000 cells per well for A431) and allowed to adhere overnight at 37°C. The next day, the indicated concentrations of activated-PanP, activated PanP-DM1, PanP-DM1 or anti-TNF-DM1 as control were added to each well and the cells were incubated for varying periods of time (48 h for A431 cells; 72 h for other cells). Then cell viability was determined by CCK-8 kit according to the manufacturer's instructions.
The percentage of surviving cells was calculated using the following formula: [(A450 of experiment - A450 of background) / (A450 of untreated control - A450 of background)] *100.
Cell cycle analysis
This assay was performed according to previous report.Citation28 Cells (1 × 105/mL) were incubated with activated PanP (1 µg/mL), activated PanP-DM1 (1 µg/mL) or PanP-DM1 (1 µg/mL) for 3 h at 37°C, washed, and replaced with drug-free medium for an additional 18 h at 37°C. Cells were then fixed with 1 mL of 70% ethanol, and DNA content was determined after staining with propidium iodide by flow cytometry. Flow cytometric data were analyzed using FlowJo 7.6 software.
Internalization determination of PanP-DM1
This assay was conducted as previously described.Citation47 A431 cells were treated with the saturating concentration of activated PanP-DM1 conjugate at 4°C for 30 minutes. Unbound conjugate was removed by washing cells. Cells were then incubated at either 4°C or 37°C. At the indicated time points, the cell surface-bound PDC was detected by flow cytometry with goat anti-human IgG FITC.
In vivo efficacy study
Mice were injected subcutaneously with 0.2 mL of 1 × 106 A431 cells or 1 × 106 NCI-H292 cells suspended in PBS into the right flank. All mice were randomly divided into groups of 10 mice each when tumor volumes reached an average of about 100 mm3 (for A431 cells) or 130 mm3 (for H292 cells). In the 2 models mentioned above, PanP (35 or 20 mg/kg), PanP-DM1 conjugate (35 or 20 mg/kg), or human IgG as control (35 or 20 mg/kg) was given i.p. (one or 2 injections as indicated). Tumor size, calculated as length × width2 × 0.5. Mice were euthanized with CO2 asphyxiation. All experiments were conducted in accordance with institutional guidelines and under an Institutional Animal Care and Use Committee protocol.
In vivo toxicity study
Antigen-independent toxicity of the PDC was determined in BALB/c mice (5 mice per dose) by a single i.p. injection of PanP-DM1 at 0, 25, 50, 75 or 100 mg/kg body weight.Citation26 In addition, toxicity associated with therapeutic dose was evaluated in nude mice bearing A431 and NCI-H292 cells (10 mice per group). Mice were observed for ~15–20 d Toxicity was evaluated by observing mouse body weight loss.
Statistical analysis
GraphPad Prism 5 Software was used for statistic calculations. Data are showed as mean ± SEM. Statistical analysis was conducted by Student's 2 sided unpaired t-test to identify significant differences unless otherwise indicated. Differences were considered significant at P < 0.05 (*). Nonlinear regression analyses were used to fit curves.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KMAB_A_1127491_supplementary_material.docx
Download MS Word (2.6 MB)Acknowledgments
We thank Shuangshuang Lu, Zhangsheng Lu and Tianlin Wang for their expert technical assistance. This work was supported by grants from the Natural Science Foundation of China (81330061), Ministry of Science and Technology of China (973 projects 2010CB833605 and 863 projects 2011AA020114, 2014AA021004), State Key Project for New Drug Development (2013ZX09101021; 2013ZX09401303), and Shanghai Rising-Star Program and for Infectious Diseases (2012ZX10002012–009), Shanghai Key Laboratory of Cell Engineering (14DZ2272300), and Shanghai Excellent technical leader (13XD1424000) and Shanghai Leading Academic Discipline Project (B905).
References
- Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation 2007; 75:770-87; PMID:17999740; http://dx.doi.org/10.1111/j.1432-0436.2007.00238.x
- Porebska I, Harlozinska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol 2000; 21:105-15; PMID:10686540; http://dx.doi.org/10.1159/000030116
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001; 37 Suppl 4:S9-15; PMID:11597399; http://dx.doi.org/10.1016/S0959-8049(01)00231-3
- Wang X, Zhu J, Zhao P, Jiao Y, Xu N, Grabinski T, Liu C, Miranti CK, Fu T, Cao BB. In vitro efficacy of immuno-chemotherapy with anti-EGFR human Fab-Taxol conjugate on A431 epidermoid carcinoma cells. Cancer Biol Ther 2007; 6:980-7; PMID:17534145; http://dx.doi.org/10.4161/cbt.6.6.4197
- Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005; 7:301-11; PMID:15837620; http://dx.doi.org/10.1016/j.ccr.2005.03.003
- Iida M, Brand TM, Starr MM, Li C, Huppert EJ, Luthar N, Pedersen MW, Horak ID, Kragh M, Wheeler DL. Sym004, a novel EGFR antibody mixture, can overcome acquired resistance to cetuximab. Neoplasia 2013; 15:1196-206; PMID:24204198; http://dx.doi.org/10.1593/neo.131584
- Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 2006; 6:803-12; PMID:16990857; http://dx.doi.org/10.1038/nrc1970
- Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol 2009; 4:107-19; PMID:19452131; http://dx.doi.org/10.1007/s11523-009-0114-0
- Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol 2003; 163:303-12; PMID:12819035; http://dx.doi.org/10.1016/S0002-9440(10)63654-1
- Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, Schrumpf H, Boelke E, Ansari P, Mackenzie C, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med 2013; 5:199ra11; PMID:23966300; http://dx.doi.org/10.1126/scitranslmed.3005886
- Sandersjoo L, Jonsson A, Lofblom J. A new prodrug form of Affibody molecules (pro-Affibody) is selectively activated by cancer-associated proteases. Cell Mol Life Sci 2015; 72:1405-15; PMID:25287047; http://dx.doi.org/10.1007/s00018-014-1751-8
- Baumann A, Flagella K, Forster R, de Haan L, Kronenberg S, Locher M, Richter WF, Theil FP, Todd M. New challenges and opportunities in nonclinical safety testing of biologics. Regul Toxicol Pharmacol 2014; 69:226-33; PMID:24755365; http://dx.doi.org/10.1016/j.yrtph.2014.04.005
- Desnoyers LR, Vasiljeva O, Richardson JH, Yang A, Menendez EE, Liang TW, Wong C, Bessette PH, Kamath K, Moore SJ, et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci Transl Med 2013; 5:207ra144; PMID:24132639; http://dx.doi.org/10.1126/scitranslmed.3006682
- de Goeij BE, Satijn D, Freitag CM, Wubbolts R, Bleeker WK, Khasanov A, Zhu T, Chen G, Miao D, van Berkel PH, et al. High turnover of Tissue Factor enables efficient intracellular delivery of antibody-drug conjugates. Mol Cancer Ther 2015; 14(5):1130-40; PMID:25724665; http://dx.doi.org/10.1158/1535-7163
- Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol 2010; 14:529-37; PMID:20643572; http://dx.doi.org/10.1016/j.cbpa.2010.06.170
- Flygare JA, Pillow TH, Aristoff P. Antibody-drug conjugates for the treatment of cancer. Chem Biol Drug Des 2013; 81:113-21; PMID:23253133; http://dx.doi.org/10.1111/cbdd.12085
- Liu C, Tadayoni BM, Bourret LA, Mattocks KM, Derr SM, Widdison WC, Kedersha NL, Ariniello PD, Goldmacher VS, Lambert JM, et al. Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc Natl Acad Sci U S A 1996; 93:8618-23; PMID:8710920; http://dx.doi.org/10.1073/pnas.93.16.8618
- Polson AG, Williams M, Gray AM, Fuji RN, Poon KA, McBride J, Raab H, Januario T, Go M, Lau J, et al. Anti-CD22-MCC-DM1: an antibody-drug conjugate with a stable linker for the treatment of non-Hodgkin's lymphoma. Leukemia 2010; 24:1566-73; PMID:20596033; http://dx.doi.org/10.1038/leu.2010.141
- Isakoff SJ, Baselga J. Trastuzumab-DM1: building a chemotherapy-free road in the treatment of human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2011; 29:351-4; PMID:21172881; http://dx.doi.org/10.1200/JCO.2010.31.6679
- Oroudjev E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, Kovtun Y, Chari R, Jordan MA. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol Cancer Ther 2010; 9:2700-13; PMID:20937595; http://dx.doi.org/10.1158/1535-7163.MCT-10-0645
- Lopus M. Antibody-DM1 conjugates as cancer therapeutics. Cancer Lett 2011; 307:113-8; PMID:21481526; http://dx.doi.org/10.1016/j.canlet.2011.03.017
- LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res 2011; 17:6437-47; PMID:22003071; http://dx.doi.org/10.1158/1078-0432.CCR-11-0762
- Niu G, Li Z, Xie J, Le QT, Chen X. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J Nucl Med 2009; 50:1116-23; PMID:19525473; http://dx.doi.org/10.2967/jnumed.109.061820
- Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, Kragh M. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010; 70:588-97; PMID:20068188; http://dx.doi.org/10.1158/0008-5472.CAN-09-1417
- Yang Y, Guo Q, Xia M, Li Y, Peng X, Liu T, Tong X, Xu J, Guo H, Qian W, et al. Generation and characterization of a target-selectively activated antibody against epidermal growth factor receptor with enhanced anti-tumor potency. MAbs 2015; 7:440-50; PMID:25679409; http://dx.doi.org/10.1080/19420862.2015.1008352
- Feng L, Yao HP, Wang W, Zhou YQ, Zhou J, Zhang R, Wang MH. Efficacy of anti-RON antibody Zt/g4-drug maytansinoid conjugation (Anti-RON ADC) as a novel therapeutics for targeted colorectal cancer therapy. Clin Cancer Res 2014; 20:6045-58; PMID:25294907; http://dx.doi.org/10.1158/1078-0432.CCR-14-0898
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008; 68:9280-90; PMID:19010901; http://dx.doi.org/10.1158/0008-5472.CAN-08-1776
- Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK, Erickson HK, Sun X, Wilhelm S, Ab O, et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res 2010; 70:2528-37; PMID:20197459; http://dx.doi.org/10.1158/0008-5472.CAN-09-3546
- Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, Girish S, Tibbitts J, Yi JH, Sliwkowski MX, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 2010; 28:2698-704; PMID:20421541; http://dx.doi.org/10.1200/JCO.2009.26.2071
- Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012; 18:221-3; PMID:22270724; http://dx.doi.org/10.1038/nm.2609
- Reusch U, Sundaram M, Davol PA, Olson SD, Davis JB, Demel K, Nissim J, Rathore R, Liu PY, Lum LG. Anti-CD3 x anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res 2006; 12:183-90; PMID:16397041; http://dx.doi.org/10.1158/1078-0432.CCR-05-1855
- Ono N, Yamazaki T, Tsukaguchi T, Fujii T, Sakata K, Suda A, Tsukuda T, Mio T, Ishii N, Kondoh O, et al. Enhanced antitumor activity of erlotinib in combination with the Hsp90 inhibitor CH5164840 against non-small-cell lung cancer. Cancer Sci 2013; 104:1346-52; PMID:23863134; http://dx.doi.org/10.1111/cas.12237
- Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, Hung MC. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 2008; 13:385-93; PMID:18455122; http://dx.doi.org/10.1016/j.ccr.2008.03.015
- Golubovskaya V, Beviglia L, Xu LH, Earp HS, 3rd, Craven R, Cance W. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem 2002; 277:38978-87; PMID:12167618; http://dx.doi.org/10.1074/jbc.M205002200
- Barok M, Tanner M, Koninki K, Isola J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res 2011; 13:R46; PMID:21510863; http://dx.doi.org/10.1186/bcr2868
- Jakobovits A, Amado RG, Yang X, Roskos L, Schwab G. From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat Biotechnol 2007; 25:1134-43; PMID:17921999; http://dx.doi.org/10.1038/nbt1337
- Khan MH, Alam M, Yoo S. Epidermal growth factor receptor inhibitors in the treatment of nonmelanoma skin cancers. Dermatol Surg 2011; 37:1199-209; PMID:21615602; http://dx.doi.org/10.1111/j.1524-4725.2011.02038.x
- Liu G, Shuman MA, Cohen RL. Co-expression of urokinase, urokinase receptor and PAI-1 is necessary for optimum invasiveness of cultured lung cancer cells. Int J Cancer 1995; 60:501-6; PMID:7829264; http://dx.doi.org/10.1002/ijc.2910600413
- Corti A, Nolli ML, Soffientini A, Cassani G. Purification and characterization of single-chain urokinase-type plasminogen activator (pro-urokinase) from human A431 cells. Thromb Haemost 1986; 56:219-24; PMID:3101222
- Safavy A, Bonner JA, Waksal HW, Buchsbaum DJ, Gillespie GY, Khazaeli MB, Arani R, Chen DT, Carpenter M, Raisch KP. Synthesis and biological evaluation of paclitaxel-C225 conjugate as a model for targeted drug delivery. Bioconjug Chem 2003; 14:302-10; PMID:12643740; http://dx.doi.org/10.1021/bc020033z
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 2005; 16:1425-33; PMID:16012181; http://dx.doi.org/10.1093/annonc/mdi279
- Weidle UH, Tiefenthaler G, Georges G. Proteases as activators for cytotoxic prodrugs in antitumor therapy. Cancer Genomics Proteomics 2014; 11:67-79; PMID:24709544
- Hamblett KJ, Kozlosky CJ, Siu S, Chang WS, Liu H, Foltz IN, Trueblood ES, Meininger D, Arora T, Twomey B, et al. AMG 595, an anti-EGFRvIII Antibody Drug Conjugate, Induces Potent Anti-Tumor Activity Against EGFRvIII Expressing Glioblastoma. Mol Cancer Ther 2015; 14(7):1614-24; PMID:25931519 doi: http://dx.doi.org/10.1158/1535-7163
- Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, Arvind AS. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs 2009; 1:41-8; PMID:20046573; http://dx.doi.org/10.4161/mabs.1.1.7509
- Quesnelle KM, Grandis JR. Dual kinase inhibition of EGFR and HER2 overcomes resistance to cetuximab in a novel in vivo model of acquired cetuximab resistance. Clin Cancer Res 2011; 17:5935-44; PMID:21791633; http://dx.doi.org/10.1158/1078-0432.CCR-11-0370
- Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, Bhakta S, Nguyen T, Dugger DL, Li G, et al. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res 2010; 16:4769-78; PMID:20805300; http://dx.doi.org/10.1158/1078-0432.CCR-10-0987
- Law CL, Cerveny CG, Gordon KA, Klussman K, Mixan BJ, Chace DF, Meyer DL, Doronina SO, Siegall CB, Francisco JA, et al. Efficient elimination of B-lineage lymphomas by anti-CD20-auristatin conjugates. Clin Cancer Res 2004; 10:7842-51; PMID:15585616; http://dx.doi.org/10.1158/1078-0432.CCR-04-1028
