ABSTRACT
A biosimilar product needs to demonstrate biosimilarity to the originator reference product, and the quality profile of the latter should be monitored throughout the period of the biosimilar's development to match the quality attributes of the 2 products that relate to efficacy and safety. For the development of a biosimilar version of trastuzumab, the reference product, Herceptin®, was extensively characterized for the main physicochemical and biologic properties by standard or state-of-the-art analytical methods, using multiple lots expiring between March 2015 and December 2019. For lots with expiry dates up to July 2018, a high degree of consistency was observed for all the tested properties. However, among the lots expiring in August 2018 or later, a downward drift was observed in %afucose (G0+G1+G2). Furthermore, the upward drift of %high mannose (M5+M6) was observed in the lots with expiry dates from June 2019 to December 2019. As a result, the combination of %afucose and %high mannose showed 2 marked drifts in the lots with expiry dates from August 2018 to December 2019, which was supported by the similar trend of biologic data, such as FcγRIIIa binding and antibody-dependent cell-mediated cytotoxicity (ADCC) activity. Considering that ADCC is one of the clinically relevant mechanisms of action for trastuzumab, the levels of %afucose and %high mannose should be tightly monitored as critical quality attributes for biosimilar development of trastuzumab.
Abbreviations
| 2-AB | = | 2-aminobenzamide |
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| BSA | = | bovine serum albumin |
| CDC | = | complement-dependent cytotoxicity |
| CE-SDS | = | capillary electrophoresis-sodium dodecyl sulfate |
| DMEM/F12 | = | Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 |
| EMA | = | European Medicines Agency |
| EU | = | European Union |
| FBS | = | fetal bovine serum |
| GSH | = | glutathione |
| GST | = | glutathione-S-transferase |
| HMW | = | high molecular weight |
| HPLC | = | high-performance liquid chromatography |
| icIEF | = | imaged capillary isoelectric focusing |
| HRP | = | horseradish peroxidase |
| LC-MS | = | liquid chromatography-mass spectrometry |
| Max | = | maximum |
| Min | = | minimum |
| PLA | = | parallel line analysis |
| SEC | = | size exclusion chromatography |
| US | = | United States |
Introduction
A biosimilar is a medicinal product that is highly similar to an authorized, original biologic drug (called the reference product in this context) in terms of structural and functional properties and the clinical outcome. Similarity in structural and functional properties is expected to translate into no clinical meaningful difference. To demonstrate the similarity to reference product, the cornerstone of biosimilar development is the extensive analytical characterization of both biosimilar product and reference product. The guidelines regarding biosimilar development recommend a stepwise approach, starting with extensive physicochemical and biologic characterization against reference products before initiating clinical studies.Citation1–4 Characterization studies against reference products lay the groundwork for identifying and focusing efficacy, safety, pharmacokinetic, and immunogenicity comparisons in clinical studies. Therefore, extensive physicochemical and biologic characterization of reference products and establishment of a quality target profile are critical steps in the biosimilar development.
Considering inherent properties of biologic therapeutics, biologics are highly sensitive to changes in manufacturing conditions and therefore robust quality management systems ensure process and product consistency.Citation1 Because of the high structural complexity compared with small molecules, even minor alterations (e.g., pH, temperature and manufacturing site) may reduce product consistency and cause drifts in target attributes.Citation5 For example, Schiestl et al. analyzed multiple batches of Aranesp®, Rituxan/Mabthera® and Enbrel® to study the variability in the glycan profiles.Citation6 Different lots of Aranesp® showed different charges resulting from varying numbers of sialic acids per molecule. Different lots of Rituxan/Mabthera® and Enbrel® showed changes of the N- and C-terminal heterogeneity, and the former also showed variation in antibody dependent cell-mediated cytotoxicity (ADCC) activity among batches. In terms of quality target profile establishment for the development of a biosimilar, the physicochemical and functional properties of its reference product should be characterized comprehensively and monitored periodically to establish the target quality profile used to demonstrate similarity in analytical attributes.
N-glycan profile is known to influence important biologic activities and is a critical quality attribute for biosimilar development. N-linked glycosylation in the Fc region can significantly affect Fc effector functions such as Fc receptor binding and complement activation.Citation7,8 Among the various features of N-glycans, the levels of the afucosyl and the high-mannose forms (labeled %afucose and %high mannose, respectively) correlate with ADCC and FcγRIIIa binding activitiesCitation9–11 and the level of galactosylation correlates with the complement-dependent cytotoxicity (CDC) and C1q activities.Citation12
Trastuzumab (Herceptin®) is a monoclonal antibody (of the IgG1 subclass, with a kappa light chain) that contains human framework regions combined with complementarity-determining regions of a murine antibody (with the codename 4D5) that binds to human epidermal growth factor receptor (HER)2. Herceptin® was approved by the US Food and Drug Administration (September 1998) and European Medicines Agency (August 2000) for use in treating certain breast cancers that overexpress HER2. Herceptin®-containing regimens are the standard of care for the treatment of HER2-positive breast cancers, providing significant clinical benefit in adjuvant settings against early as well as advanced or metastatic breast cancers.Citation13
For the development of a biosimilar of Herceptin®, physicochemical and biologic properties of Herceptin® were elucidated by using standard or state-of-the-art methods. Depending on the method, up to 103 unexpired lots in total of EU and US Herceptin® were analyzed, their expiry dates ranging between (and including) March 2015 and December 2019. Interestingly, during characterization of Herceptin®, 2 sequential drifts in the sum of %afucose and %high mannose in N-glycan profiles, FcγRIIIa binding activity and ADCC activity in EU and US Herceptin® lots were observed. Additionally, %galactosylation (G1F + G2F) drifted distinctly downwards. We report here the characterization data for EU and US Herceptin®, and discuss the potential consequences of these drifts from the standpoint of a biosimilar developer.
Results
Physicochemical properties of Herceptin®
For characterization of reference products, physicochemical properties of Herceptin® with expiry dates from March 2015 to December 2019 were analyzed. A high degree of consistency was observed for all the physicochemical properties among the lots expiring between March 2015 and July 2018 ().
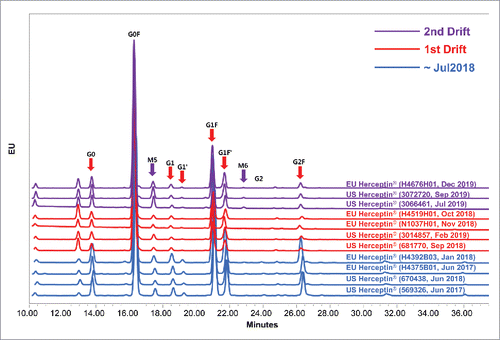
Figure 1. Trends of N-glycan attributes that are related to biologic activities by expiry date. Dotted line shows the min-max range of expiry date before August 2018, 1st drift and 2nd drift periods. Boxplot shows the interquartile range, median and outlier (◊). Statistical significance was assessed with one-way ANOVA (*P ≤ 0.05). (A) %Afucose, (B) %High-mannose, (C) %Afucose + %High-mannose and (D) %Galactosylation.
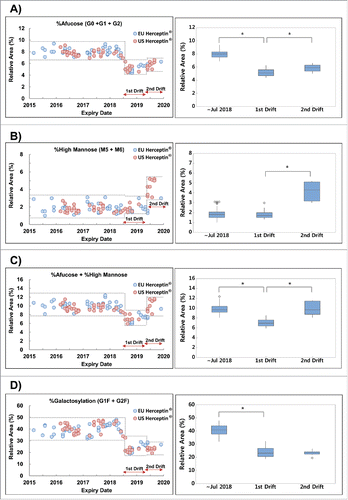
Figure 2. Trends of charge variant by expiry date using icIEF. Dotted line shows the min-max range of expiry date before August 2018, 1st drift and 2nd drift periods. Boxplot shows the interquartile range, median and outlier (◊). (A) %Acidic peak area, (B) %Main peak area, (C) %Basic peak area, (D) Representative icIEF electropherogram of Herceptin® via expiry date.
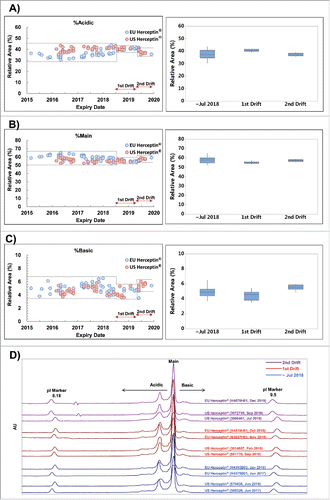
Figure 3. Results of non-reducing CE-SDS and SE-HPLC profile. Dotted line shows the min-max range of expiry date before August 2018, 1st drift and 2nd drift periods. Boxplot shows the interquartile range, median and outlier (◊). (A) Trend of %higher-molecular-weight (%HMW) using non-reducing SE-HPLC, (B) Representative chromatograms for SE-HPLC analysis, (C) Trend of %IgG using non-reducing CE-SDS and (D) Representative CE-SDS electropherogram.
In the lots with expiry dates from August 2018 to December 2019, however, a marked downward trend in the level of %afucose (G0+G1+G2) was observed (). The range between the minimum and maximum (Min-Max range) among the lots expiring after August 2018 did not overlap with those in the earlier lots. Furthermore, a marked upward trend in %high mannose (M5+M6) was also observed among the US Herceptin® lots with expiry dates from June 2019 to December 2019 (). However, this trend in %high mannose (M5±M6) was not observed among the EU Herceptin® counterparts (). When %afucose and %high mannose were summed together as a single attribute (%afucose+%high mannose), this attribute showed 2 marked drifts among the lots expiring between August 2018 and December 2019 (). The %galactosylation (G1F+G2F) decreased among the lots expiring between August 2018 and December 2019 (), and this trend is similar to that seen in %afucose (). All N-glycan species were identified using LC-MS/MS (data not shown) and individual trend of N-glycan attributes (G0, G1, G2, G0F, G1F, G2F, M5 and M6) were shown in the Fig. S1. These results suggest that N-glycan profiles of Herceptin® drifted twice in the lots with expiry dates from March 2015 to December 2019. Hereafter, the first drift was defined when the levels of %afucose and %galactosylation had distinctly fallen and the second drift was defined when the levels of %high mannose were remarkably elevated.
In terms of structural identity, there was no significant change in the N-glycan species between the lots () representing the pre-drift period and the lots representing the drifts, i.e., no additional or missing N-glycan peak was observed (). Although there were subtle differences in the levels of acidic and basic variants by imaged capillary isoelectric focusing (icIEF) between the first and the second drift (), these levels were within the Min-Max range among the pre-drift lots. In all other physicochemical quality attributes, including %higher molecular weight (evaluated by SEC) and %intact IgG (evaluated by non-reducing capillary electrophoresis-sodium dodecyl sulfate (CE-SDS)), a high degree of consistency, i.e., the values being comparable within assay variation, was observed throughout the entire expiry dates monitored ().
Table 1. Information and Characterization Results of the Representative Lots of Herceptin®.
Biological activity of Herceptin®
Given that drifts in %afucose and %high mannose were observed twice during the monitoring period, their effect on the biologic properties of Herceptin® was also assessed. A marked downward shift in the lots of the first drift period and the upward shift in the lots of the second drift period in ADCC activity were also observed (), showing a similar trend with the combination of %afucose and %high mannose content. Moreover, FcγRIIIa binding activity, which is associated with effector binding in the Fc region during ADCC, also exhibited similar trends with ADCC activity and the combination of %afucose and %high mannose content ( and ). However, in all other biologic activities, including anti-proliferative potency, HER2 binding (), and FcRn binding (Fig. S2A), a high degree of consistency was observed throughout the entire expiry dates monitored.
Figure 5. Trend of biologic activities of Herceptin®. Dotted line shows the min-max range of expiry date before August 2018, 1st drift and 2nd drift periods. Boxplot shows the interquartile range, median and outlier (◊). Statistical significance was assessed with one-way ANOVA (*P ≤ 0.05). (A) Relative ADCC activity of Herceptin® (n = 102), (B) Relative FcγRIIIa binding activity of Herceptin® (n = 98), (C) Relative anti-proliferation potency of Herceptin® (n = 102), (D) Relative HER2 binding activity of Herceptin® (n = 102).
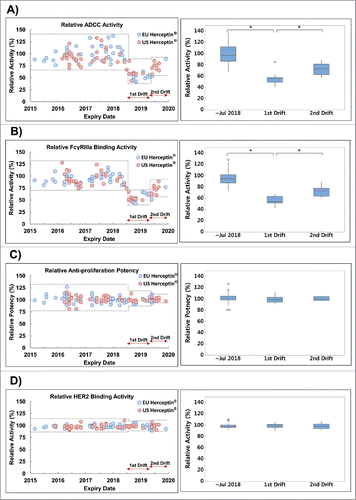
%Galactosylation is known to affect CDC, which is mediated through C1q binding of the Fc region. As shown in , %galactosylation decreased for lots after August 2018. Because of no or only insignificantly low level of in vitro CDC activity in breast cancer cell lines by trastuzumab,Citation14,15 the impact of %galactosylation drift was confirmed by C1q binding activity which showed a uniform behavior in the entire monitoring period (Fig. S2B). In our assay system, it seems that the influence of %galactosylation change is not large enough to observe in C1q binding activity. These results suggest that the 2 sequential drifts in N-glycan profiles of Herceptin® affected only ADCC and FcγRIIIa binding activities.
Discussion
Glycosylated proteins, including monoclonal antibodies, are complex molecules that may exist in more than a hundred isoforms even when the amino acid sequence is fixed and the production well-controlled.Citation6 In particular, the glycosylation pattern is often inherently variable from one batch to another, and changes in manufacturing process such as process improvements, scale changes, and site changes can add to the variability. Although manufacturers try to prevent associated changes in quality attributes, such changes cannot be avoided in every case.Citation6
In developing the quality product profile (QPP) of trastuzumab biosimilar, the reference product, Herceptin®, has been continually monitored on quality attributes over 5 years, and the present paper reports the data on the main physicochemical and biologic properties of up to 103 lots being marketed in the EU and the US. Most physicochemical and biologic attributes, including N-glycan profiles, FcγRIIIa binding and ADCC activities showed consistent trends in the lots with expiry dates from March 2015 to August 2018. However, starting with lots with expiry dates of August 2018, a marked downward drift was observed in the level of afucosylated glycans (%afucose; equal to %G0+%G1+%G2), referred to as the “first drift,” as well as in the level of galactosylated glycans (%galactosylation; equal to %G1F+%G2F). %Afucose correlates with the ADCC and FcγRIIIa binding activities,Citation9-11,16 while %galactosylation correlates with the CDC and C1q binding activities.Citation12 A congruous, downward drift was observed in ADCC and FcγRIIIa binding among the lots in the first drift. However, the C1q binding remained uniform throughout these lots.
While the first drift was still in effect, an upward drift was observed in the level of high mannose (%high mannose; equal to %M5+%M6), referred to as the “second drift,” among the US lots with expiry dates from June 2019 to December 2019. High-mannose glycans on the Fc region of therapeutic IgG antibodies enhance ADCC activity.Citation11,17 A congruous, upward drift was observed in ADCC and FcγRIIIa binding among the lots in the second drift.
In HER2-overexpressing breast cancer cells, trastuzumab may elicit an ADCC response upon the binding of the Fab region to HER2 on the cancer cells and the binding of the Fc region to FcγRs on immune effector cells.Citation18,19 In the HER2 binding and anti-proliferation activities, which are Fab-related biologic activities, a high degree of consistency and uniformity was observed throughout the entire expiry dates monitored. This data suggest that the changes in ADCC and FcγRIIIa binding associated with the 2 drifts would not be causally related to the Fab region. Therefore, the downward drift of ADCC and FcγRIIIa binding activity in the first drift period seems to be due to %afucosylated glycan and the upward drift in the second drift period due to %high mannosylated glycans.
Though charge heterogeneity showed a different trend between the first and second drift, this charge difference was overlapped in results of the lots with expiry dates before August 2018 and did not affect Fab-related biologic activity. Moreover, it was observed that the %acidic variant of US Herceptin® was slightly higher than that of EU Herceptin®. It is likely that the dose and lyophilization process of EU Herceptin® (150 mg/vial) and US Herceptin® (440 mg/vial) were different. Nevertheless, the results of Fab-related biologic activity and Fc-related biologic activity showed that this difference did not affect the efficacy.
Several findings have supported that ADCC is one of the major mechanisms of trastuzumab.Citation10,20 A xenografts study proposed that natural killer cells contribute to killing of HER2-overexpressing target cells opsonized by trastuzumab via CD16-dependent ADCC.Citation21 Since then, this concept has been strongly supported by several in vitro and in vivo studies.Citation22–25 For example, an afucosylated version of trastuzumab with similar Fab-related activity was shown to have enhanced efficacy against cancer cells in animal models using HER2-overexpressing breast cancer.Citation10 Furthermore, Boero and colleagues suggest that ADCC-favored conditions are correlated with therapeutic efficacy or response of trastuzumab in a clinical setting.Citation26 Considering that ADCC is one of clinically relevant mechanisms of action for trastuzumab, if trastuzumab with drift of both the N-glycan profile and ADCC activity are used in a clinical study, the quality change should be taken into account to interpret the clinical outcomes.
As the characterization of the reference product is a fundamental first step in the development of biosimilars, the quality profile of the reference product should be monitored throughout the entire period of biosimilar development according to the EMA Quality 2015 guideline to take into account the “product shift” common to manufacturing changes and its possible significance during the life cycle of a monoclonal antibody. In the case of trastuzumab biosimilar, the levels of %afucose and %high mannose of Herceptin® should be closely monitored to determine in case there is any clinical bearing, and to match them in the biosimilars.
Materials and methods
Reference products
EU and US Herceptin® lots (up to 103 lots) were purchased from local distributors and then stored according to the manufacturer's instructions. The reference standard for the bioassays was prepared from one lot of EU Herceptin® and used for the characterization of the reference products.
Materials
The CellTiter-Blue® and CytoTox-Glo® kits were obtained from Promega (Madison, WI, USA). The BrdU cell proliferation ELISA kits were obtained from Roche (Penzberg, Germany). The PNGase F kit was obtained from New England Biolabs (Ipswich, MA, USA). The 2-AB labeling kit for N-glycan analysis was obtained from Ludger (Oxfordshire, UK). The glutathione (GSH)-coated donor beads, human IgG-conjugated acceptor beads, and streptavidin-coated donor beads were obtained from Perkin Elmer (Waltham, MA, USA). The glutathione-S-transferase (GST)-tagged FcγRIIIa and HER2-Fc were obtained from Biogen (Cambridge, MA, USA).
Software and equipment
Parallel line analysis 2.0 (PLA) was obtained from Stegmann Systems GmbH (Rodgau, Germany). The high performance liquid chromatography system (Waters 2695 separations module and 2489 dual λ absorbance detector) and the Empower™3 software were obtained from Waters (Milford, MA, USA). The high-performance capillary electrophoresis system (PA 800 plus Pharmaceutical Analysis System) was obtained from Beckman Coulter (Brea, CA, USA). Envision Multi-label Plate Reader was obtained from Perkin Elmer (Wallac Oy, Finland), and SpectraMax M3 Microplate Reader was obtained from Molecular Devices (Sunnyvale, CA, USA)
Cell lines and cell culture
SKBR-3 cells were obtained from American Type Culture Collection and incubated at 37°C, 5% CO2 in McCoy's 5A (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA). BT-474 cells were obtained from American Type Culture Collection and incubated at 37°C, 5% CO2 in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, Gibco, Grand Island, NY, USA) containing 10% FBS (Hyclone, Logan, UT, USA). NK92-CD16 cells were obtained from Biogen (Cambridge, MA, USA) and incubated at 37°C, 5% CO2 in Myelocult H5100 (Stemcell Technology, Vancouver, Canada) containing human IL-2 (Cell Signaling Technology, Beverly, MA, USA) and puromycin (Gibco, Grand Island, NY, USA).
N-Glycan analysis
The relative content of N-glycan species (G0, G1, G2, G0F, G1F, G2F, M5 and M6) was determined by hydrophilic-interaction ultra-performance liquid chromatography. Herceptin® samples were first treated with PNGase F to release the N-glycans. The released N-glycans were separated using ethanol precipitation, dried, and labeled with 2-aminobenzamide (2-AB). Next, they were separated through an Acquity™ BEH glycan column (2.1 mm x 150 mm, 1.7 µm; manufactured by Waters) on an Acquity™ ultra-performance liquid chromatography system (Waters). A mobile phase gradient was performed by 50 mM ammonium formate (solution A) and 100% acetonitrile (solution B) with a column flow rate of 0.5 mL/min. Eluting N-glycans were detected by fluorescence at a λex of 330 nm and λem of 420 nm. Each glycan species was then identified by LC-MS. The relative content of each N-glycan species was represented by the area under the curve of each peak relative to the total area under the curve covering all peaks.
Capillary electrophoresis-sodium dodecyl sulfate analysis
CE-SDS analysis was done with non-reduced samples and used a high-performance capillary electrophoresis system (PA 800 plus Pharmaceutical Analysis System; Beckman). In non-reduced-sample CE-SDS, sample (200 µg) was mixed with 2 µL of a 10 kDa internal standard, 95 µL of SDS-MW sample buffer (Beckman coulter, USA), and 5 µL of iodoacetamide and then boiled at 70°C for 5 minutes. The sample was electro-kinetically introduced onto a capillary (Beckman Coulter, bare fused-silica capillary, 50 µm/30.2 cm) by applying voltage at −5 kV for 20 seconds and was separated in the capillary cartridge. Electrophoresis was performed at a constant voltage with an applied field strength of −497 V and monitored by UV detection (λ = 220 nm) through the capillary window and aperture (Beckman Coulter, 100 × 200 µm). Data were acquired and processed by 32 Karat software with integration capabilities (Beckman Coulter).
Imaged capillary isoelectric focus analysis
The analysis of charge heterogeneity was performed by the iCE3 analyzer (ProteinSimple). 60 μg of sample was added to 200 μL of icIEF mixing buffer containing 70 μL of 1% MC, 4 μL of pharmalyte 3–10, 8 μL of pharmalyte 8–10.5, 0.5 μL of 8.18 pI marker, 2 μL of 9.50 pI marker, and 115.5 μL of water. Samples were focused at 1500 V for 1 min and 3000 V for 9 min. The absorbance of the focused proteins was monitored at 280 nm. The resulting electropherograms were integrated using the Chrom Perfect program.
Size exclusion chromatography (SEC-HPLC) analysis
Sample (100 µg) was directly injected onto a TSK-GEL G3000 SWXL analytical column (Tosoh, 008541, 5 µm/7.8 mm × 300 mm) at 25°C, which was connected to a Waters HPLC system; monitoring was done by UV (UV) detection (λ = 280 nm). A mobile phase consisting of 100 mM sodium phosphate with 200 mM sodium chloride, pH 6.8, was used. The flow rate was 0.5 mL/min, and monomers and impurities were detected at a UV wavelength of 280 nm. Data were acquired and processed by the Empower™3 (Waters) software.
Anti-proliferation assay
The anti-proliferation potency was determined by CellTiter-Blue® or BrdU proliferation assay. BT-474 cells were incubated with 10 concentrations (0.008–1.0 μg/mL) of Herceptin® for 4 d at 37°C, 5% CO2 in DMEM/F12 containing 10% FBS. In the case of CellTiter-Blue® assay, anti-proliferation potency was measured by CellTiter-Blue® cell viability assay kit (Promega) according to manufacturer's instruction. The relative number of viable cells was quantified by measuring the fluorescence (excitation at 560 nm and emission at 590 nm) using a SpectraMax M3 (Molecular Devices). In the case of BrdU assay, the difference of DNA synthesis in trastuzumab-treated cells was measured by BrdU cell proliferation ELISA kit (Roche) according to the manufacturer's instruction. The amounts of BrdU incorporated were quantified by measuring the photo generation from a chemiluminescent reaction with an Envision Multi-reader (Perkin Elmer). Data were analyzed using PLA software to calculate relative anti-proliferation potency.
HER2 binding assay
The competitive inhibitory HER2 binding assay was performed using time-resolved fluorescence resonance energy transfer technology. In this system, 12 concentrations (0.0488–100 μg/mL) of Herceptin® were incubated with fixed volume of Europium chelate labeled trastuzumab and Cy5-labeled HER2 in assay diluent [1x phosphate-buffered saline, 0.1% bovine serum albumin (BSA), and pH 7.4]. The mixture was then incubated at 25°C for 1 hour with moderate agitation. After incubation, the fluorescent signal was obtained on the Envision™ Multilabel Plate Reader (PerkinElmer) at a wavelength of 665 nm. Data were analyzed by using PLA software to calculate the relative binding activity.
ADCC assay
The ADCC assay was performed to assess Fc-related biologic activity of Herceptin® against a HER2-overexpressing human breast cancer cell line (SKBR3). NK92-CD16 cells, a human natural killer cell line expressing CD16, were used as effector cells. Experimentally, Herceptin® was incubated with SKBR3 and NK92-CD16 cells in flat-bottom 96-well plates for 4 hours at 37°C, 5% CO2 in McCoy's 5A media. After incubation, the SKBR3/NK92-CD16/sample mixture was incubated with a luminogenic peptide substrate (Alanyl-alanylphenylanyl-aminoluciferin; AAF-Glo Substrate) included as part of the CytoTox-Glo® kit. The luminescence signals (dead cell signals) were quantified with an Envision Multi-reader (Perkin Elmer). Data were analyzed by using PLA software to calculate relative ADCC activity.
FcγRIIIa binding assay
The FcγRIIIa binding assay was performed to assess Fc-related biologic activity of Herceptin® using αscreen™ technology. In this system, 12 concentrations (0.0488–100 μg/mL) of Herceptin® were incubated with fixed volume of GSH coated donor/human IgG conjugated acceptor beads and GST-tagged FcγRIIIa. The mixture was incubated at 22°C for 3.5 hours with moderate agitation. After incubation, the fluorescent signal was obtained on the Envision™ Multilabel Plate Reader (PerkinElmer) with excitation at 680 nm and reading emission at 520–620nm. Data were analyzed by using PLA software to calculate the relative binding activity.
Statistical analysis
Statistical analysis was performed with Minitab statistic software package (Leadtools Technologies Inc., Version 17.1.0). Comparisons between groups were performed with one-way analysis of variance (1-way ANOVA). A value of P ± 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_Material.zip
Download Zip (1.1 MB)Acknowledgments
We would like to thank Laurel Riemann, PharmD (Med Communications, Inc.) for editing the manuscript. We would also like to thank Jaewoong Hwang, Jae-Il Lee, Young-Phil Lee, Kyung Ah Kim (Samsung Bioepis) for internal review and proofreading of the manuscript.
Funding
This work was funded by Samsung Bioepis Co., Ltd.
References
- Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products containing monoclonal antibodies-non clinical and clinical issues. EMEA/CHMP/BMWP/42832/2005 Rev1, European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal product. CHMP/437/04 Rev. 1. European Medicines Agency 2005. Available from:http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf
- Food and Drug Administration Center for Drug Evaluation and Research (FDA). Guidance for Industry: scientific considerations in demonstrating biosimilarity to a reference product. FDA-2011-D-0605, U.S. Department of Health and Human Services 2015. Available from: http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biologic medicinal products containing biotechnology-derived protein as active substance: quality issue (revision 1). EMA/CHMP/BWP/247713/2012, European Medicines Agency 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/06/WC500167838.pdf
- Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs 2014; 28(4):363-72; PMID:24567263; http://dx.doi.org/10.1007/s40259-014-0088-z
- Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol 2011; 29(4):310-2; PMID:21478841; http://dx.doi.org/10.1038/nbt.1839
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277(30):26733-40; PMID:11986321; http://dx.doi.org/10.1074/jbc.M202069200
- Upton R, Bell L, Guy C, Caldwell P, Estdale S, Barran PE, Firth D. Orthogonal assessment of biotherapeutic glycosylation; A case study correlating N-Glycan Core Afucosylation of Herceptin with mechanism of action. Anal Chem 2016; 88(20):10259-10265; PMID:27620140; http://dx.doi.org/10.1021/acs.analchem.6b02994
- Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG, et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 2008; 112(6):2390-9; PMID:18566325; http://dx.doi.org/10.1182/blood-2008-03-144600
- Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, Crocker L, Pabonan O, Baginski T, Meng G, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res 2010; 70(11):4481-9; PMID:20484044; http://dx.doi.org/10.1158/0008-5472.CAN-09-3704
- Yu M, Brown D, Reed C, Chung S, Lutman J, Stefanich E, Wong A, Stephan JP, Bayer R. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. MAbs 2012; 4(4):475-87; PMID:22699308; http://dx.doi.org/10.4161/mabs.20737
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol 2008; 20:471-8; PMID:18606225; http://dx.doi.org/10.1016/j.coi.2008.06.007
- Pivot X, Curtit E, Lee YJ, Golor G, Gauliard A, Shin D, Kim Y, Kim H, Fuhr R. A randomized Phase I pharmacokinetic study comparing biosimilar candidate SB3 and Trastuzumab in healthy male subjects. Clin Ther 2016; 38(7):1665-1673; PMID:27368117; http://dx.doi.org/10.1016/j.clinthera.2016.06.002
- Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, Bartsch R, Jensen-Jarolim E, Bergmann M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med 2013; 11:307; PMID:24330813; http://dx.doi.org/10.1186/1479-5876-11-307
- Prang N, Preithner S, Brischwein K, Göster P, Wöppel A, Müller J, Steiger C, Peters M, Baeuerle PA, Da Silva AJ. Cellular and complement-dependent cytotoxicity of Ep-CAM-specific monoclonal antibody MT201 against breast cancer cell lines. Br J Cancer 2005; 92(2):342-9; PMID:15655555; http://dx.doi.org/10.1038/sj.bjc.6602310
- Iida S, Kuni-Kamochi R, Mori K, Misaka H, Inoue M, Okazaki A, Shitara K, Satoh M. Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer 2009; 9:58; PMID:19226457; http://dx.doi.org/10.1186/1471-2407-9-58
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, Culm-Merdek K, Park A, Pan C, Edmunds T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng 2008; 99:652-665; PMID:17680659; http://dx.doi.org/10.1002/bit.21598
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26:1789-1796; PMID:18347005; http://dx.doi.org/10.1200/JCO.2007.14.8957
- Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Ménard S, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res 2007; 67:11991-11999; PMID:18089830; http://dx.doi.org/10.1158/0008-5472.CAN-07-2068
- Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2012; 2:62; PMID:22720269; http://dx.doi.org/10.3389/fonc.2012.00062
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cyto-toxicity against tumor targets. Nat Med 2000; 6:443-446; PMID:10742152; http://dx.doi.org/10.1038/74704
- Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cyto-toxicity mechanism? Br J Cancer 2006; 94:259-267; PMID:16404427; http://dx.doi.org/10.1038/sj.bjc.6602930
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol 2001; 31:3016-3025; PMID:11592078; http://dx.doi.org/10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016>3.0.CO;2-J
- Kubo M, Morisaki T, Kuroki H, Tasaki A, Yamanaka N, Matsumoto K, Nakamura K, Onishi H, Baba E, Katano M. Combination of adoptive immunotherapy with Herceptin for patients with HER2-expressing breast cancer. Anticancer Res 2003; 23:4443-4449; PMID:14666732
- Yamaguchi Y, Hironaka K, Okawaki M, Okita R, Matsuura K, Ohshita A, Toge T. HER2-specific cytotoxic activity of lymphokine-activated killer cells in the presence of trastuzumab. Anticancer Res 2005; 25(2A):827-32; PMID:15868915
- Boero S, Morabito A, Banelli B, Cardinali B, Dozin B, Lunardi G, Piccioli P, Lastraioli S, Carosio R, Salvi S, et al. Analysis of in vitro ADCC and clinical response to trastuzumab: possible relevance of FcγRIIIA/FcγRIIA gene polymorphisms and HER-2 expression levels on breast cancer cell lines. J Transl Med 2015; 13:324; PMID:26450443; http://dx.doi.org/10.1186/s12967-015-0680-0