ABSTRACT
Various studies have demonstrated that Fc engineering to enhance neonatal Fc receptor (FcRn) binding is effective for elongating half-life or increasing cellular uptake of IgG. A previous study has shown that a N434H mutation to enhance FcRn binding resulted in increased binding to rheumatoid factor (RF) autoantibody, which is not desirable for therapeutic use in autoimmune disease. In this study, we first showed that all the existing Fc variants with enhanced FcRn binding also show increased RF binding, and then identified specific mutations that could be introduced to those Fc variants to reduce the RF binding. Furthermore, we generated novel Fc variants that do not increase RF binding and show half-lives of 45 d in cynomolgus monkey, which is longer than those of previously reported Fc variants. In addition, we generated novel Fc variants with antigen sweeping activity that do not increase RF binding. We expect that these novel Fc variants will be useful as antibody therapeutics against autoimmune diseases.
Introduction
Monoclonal antibodies are well established as therapeutic agents for various diseases, including autoimmune and chronic inflammatory diseases.Citation1-3 In recent years, their potent efficacy and safety profiles made it possible to offer novel treatment options for rheumatoid arthritis (RA),Citation1-4 Crohn's disease,Citation1,3 ulcerative colitis,Citation3 multiple sclerosis,Citation1,3,5 and other autoimmune diseases.Citation1-3 Monoclonal antibodies against tumor necrosis factor, interleukin-6 receptor (IL-6R), CD20, and other targets are available as treatment of these autoimmune diseases,Citation1-3 and some patients receiving these therapeutics can achieve remission.
Generally, a lengthy half-life is an important property for therapeutic antibodies, especially for those used to treat chronic diseases. Whereas an antibody with a short half-life requires frequent or high-dose injections to achieve therapeutic efficacy, the frequency of administration or the dosage amount can be reduced if an antibody has a long half-life. Moreover, since a low dosage (e.g., less than 2 mg/kg) is generally required for single-site subcutaneous injection, a long-lasting antibody can offer treatments that are more convenient for both patients and medical staff.
Antibody engineering technologies have been developed to improve the half-life and the duration of target neutralization.Citation6-9 Both the Fab and Fc regions can be engineered to improve their pharmacokinetic properties. Fc engineering to increase the binding affinity to FcRn in endosomal condition (acidic pH) is an effective approach to prolonging the pharmacokinetics of monoclonal antibodies.Citation10-14 FcRn is a heterodimer of an α-chain and β-2-microglobulin (β2m),Citation15 and is known as a salvage receptor of IgG because it can bind to IgG at endosomal acidic pH and form a complex of FcRn/IgG that is recycled back from early endosome to the cell surface.Citation16 The binding affinity of IgG to FcRn is weak in the neutral pH of the extracellular condition, so IgG dissociates from FcRn and is released into plasma.Citation17 Therefore, the length of the half-life of IgG depends on its binding affinity to FcRn in the endosome. Previous studies have demonstrated that Fc engineering to increase the binding affinity to FcRn at acidic pH improved the endosomal recycling efficiency and prolonged the pharmacokinetics of the antibody. For example, M252Y/S254T/T256E (YTE),Citation10 M428L/N434S (LS),Citation11 and N434HCitation12,13 variants improved the half-life relative to wild-type human IgG1 in human-FcRn transgenic mouse, cynomolgus monkey, or human. While conventional IgG1 antibodies have a half-life of ∼2–3 weeks in human, the YTE variant of the anti-RSV IgG1 antibody has demonstrated a strikingly long half-life of 70–100 d in human.Citation14 Such mutations to increase FcRn binding are located in the CH2-CH3 interface region, which is the binding site for FcRn. The advantage of such Fc engineering is that it can be easily applied to various antibodies by simply introducing these mutation(s) into its Fc.
On the other hand, the pharmacokinetics are also known to be influenced by the amino acid sequence of the variable region, and an antibody with a lower isoelectric point (pI) has been shown to have a longer half-life; therefore, lowering the pI by engineering the variable region could reduce the non-specific clearance of an IgG antibody and improve its pharmacokinetics.Citation6,7 Moreover, a novel modality, called recycling antibody, has been developed to improve the duration of target neutralization. An antibody with pH- or calcium-dependent binding to an antigen binds strongly in plasma (neutral pH, high calcium ion condition), but weakly in the endosome (acidic pH, low calcium ion condition), and thus can dissociate from the antigen in the endosome. Such a recycling antibody can bind and neutralize the antigen repeatedly, thereby prolonging the duration of target neutralization.Citation8 In addition, a sweeping antibody, which was generated by combining a recycling antibody with a mutated Fc to increase the binding affinity to FcRn or Fc gamma receptor IIB (FcγRIIB) in the extracellular condition, both prolongs the duration of target neutralization and also actively eliminates the soluble antigen from circulation.Citation9,18-20 Our previous reports of sweeping antibodies with improved binding to FcRnCitation9 and FcγRIIBCitation20 have demonstrated that they can reduce accumulation of soluble antigen by more than 100-fold compared with a conventional antibody that has a non–pH-dependent antigen-binding Fab and intact Fc. Sweeping antibody technology allows the antibody to target soluble antigens with high plasma concentration or high turnover that a conventional antibody cannot target.
Rheumatoid factor (RF) is an autoantibody that binds to the Fc portion of human IgG. RFs are frequently detected in patients with RA or other autoimmune diseases, but are also observed in patients with non-rheumatic conditions and even in healthy subjects.Citation21 Although their detailed function still remains uncertain, it was suggested that RFs are likely to play an important role in the host defense against infection in normal people, where RFs can contribute to the formation and clearance of an immune complex (IC) derived from an anti-pathogen antibody, and RF-positive B-cells can promote an anti-pathogen response through the antigen presentation mechanism. However, when such RF responses occur excessively, they may cause autoimmune diseases.Citation22,23
Recently, it was reported that a humanized anti-CD4 IgG1 antibody with the N434H mutation, which was introduced to prolong the half-life by enhancing FcRn binding, elicited significant RF binding.Citation24 Detailed study has confirmed that the N434H mutation unexpectedly increased the binding of RF to the Fc region of the antibody compared with the parent human IgG1. Such RF binding to the therapeutic antibody may have an effect on pharmacokinetics, as well as antibody immunogenicity, because the interaction with RF can cause cellular uptake by antigen-presenting cells (APCs) and may induce an immune response against the antibody. Therefore, increased RF binding to engineered Fc, such as human IgG1 with the N434H mutation, could cause various issues, especially when the antibody is used to treat autoimmune diseases. Although the position of the epitope in RF clones may vary, it generally seems to be located at the CH2-CH3 interface region, which could overlap with the FcRn binding site.Citation25-27 Since the N434H mutation is located in the CH2-CH3 interface, it seems reasonable that the mutation altered the binding affinity to some of the RF clones. To date, increased RF binding to Fc with enhanced binding to FcRn has only been reported for the N434H mutation, and it is not known whether other previously reported Fc variants with enhanced FcRn binding also have increased RF binding.
In this study, we first show that not only N434H but also YTE and LS variants have increased binding to RF derived from patients with RA. Second, we identified specific mutations that can be incorporated into N434H, YTE, or LS variants so that the increase in RF binding caused by these mutations can be significantly reduced. Third, we have identified a novel Fc variant with optimized binding affinity to FcRn that does not have increased binding affinity to RF and demonstrated its significantly improved half-life in cynomolgus monkey. Fourth, we have also identified novel Fc variants without RF binding for use in a sweeping antibody that can actively eliminate plasma antigen.
Results
RF binding to Fc variants with enhanced FcRn binding
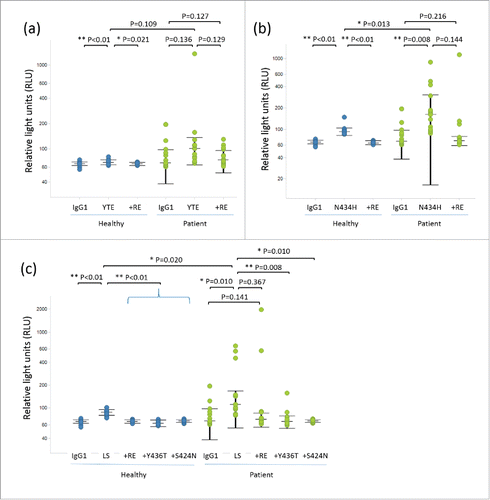
First, we selected YTE, LS, and N434H variants to evaluate the RF binding because they had already been tested in clinical studies. We prepared test antibodies with the reported Fc variants and evaluated their binding affinity to human FcRn (hFcRn). Consistent with previous reports, they showed increased hFcRn binding at acidic pH compared with intact human IgG1 (Table S1). To evaluate the binding property of these variants to RFs, we set up an assay by referring to the method established by Araujo et al.Citation24 We applied an electrochemiluminescence (ECL) immunoassay system, which is commonly used to detect anti-drug antibody (ADA), and optimized the assay condition to detect the RF binding to the test antibody with high sensitivity. As a source of RF, we used serum samples from 15 RA patients. We were able to reproduce the previous study that showed the N434H variant has RF binding in some donors (4 out of 15 patients). Interestingly, we found that both the YTE and LS variants also showed an increased response in some donors ( and Fig. S1), although the response was less frequent compared with N434H (out of 15 patients, one for YTE and 3 for LS). In contrast, serum samples from healthy donors did not show any significant response for any of the variants tested (data not shown). Therefore, only an average value for 14 healthy donors is shown in each figure.
Table 1. Dissociation constants (KD) for binding of the novel Fc variants to human and cynomolgus monkey FcRn at pH 6.0.
Identification of the Q438R/S440E mutation to reduce RF binding
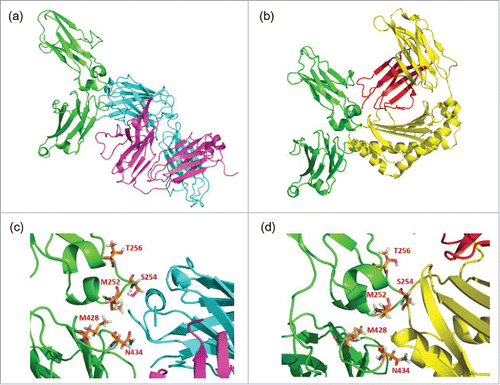
The structures of complexes of human Fc/RF (PDB ID. 1ADQ)Citation27 and rat Fc/FcRn (PDB ID: 1FRT)Citation28 are available from the Protein Data Bank and are shown in and , respectively. In addition, residues of M252, S254, T256 (included in YTE), M428 (LS), and N434 (LS and N434H) are shown as sticks in the Fc/RF () and Fc/FcRn () structures. These figures were generated using PyMOL (Schrödinger, LLC).Citation29 The structures indicate that the mutations to enhance the FcRn binding affinity are located in the FcRn binding site in the Fc structure, and importantly, they are also located in the epitope of the RF, which is recognized by the heavy chain complementarity-determining regions (CDRs) of the RF Fab. Thus, it can be assumed that the mutations to enhance FcRn may affect the binding of RF and inadvertently increase the binding affinity of specific RF clones.
Figure 2. Complex structures of (a) CH2 and CH3 domains of Fc (green) with RF heavy chain (light blue) and light chain (purple), and of (b) CH2 and CH3 domains of Fc (green) with FcRn α chain (yellow) and β-2-microgloblin (red). Enlarged figures of (a) and (b) are shown as (c) and (d), respectively, and the positions mutated to enhance FcRn binding (252, 254, 256, 428 and 434) are shown as orange sticks.
To counteract the increased RF binding of these Fc variants, we took a mutagenesis approach to the Fc region. As shown in , the RF heavy chain recognizes the CH2-CH3 interface in the Fc, while the light chain recognizes the CH3 domain. Positions 438 and 440 (numbered according to the EU index), which are far from the FcRn-binding site, seem to be especially important for the interaction with the RF light chain. Therefore, we assumed that mutagenesis at position 438 or 440 could alter the binding profile against RF without affecting FcRn binding. We introduced Q438R/S440E (RE) mutations into the YTE, LS, and N434H variants. As expected, these new variants showed similar binding affinity to human FcRn compared with the original variants (Table S1). Next, we performed a RF binding assay on the Fc variants with RE mutations. While the YTE variant showed a strong response in one donor, #12 (Fig. S1b), this response was completely reduced by RE mutations (Fig. S1e). For the LS variant, RE mutations reduced the response in donor #11, increased it in #3, and left it the same in #12 (Fig. S1c vs. 1f). N434H variants showed a clear effect in several patients (#7, #11 and #12), while RE mutations increased the binding in patient #3 (Fig. S1d vs 1g). These results suggest that RE mutations can alter the RF binding property without compromising FcRn binding affinity.
Identification of other mutations to reduce RF binding
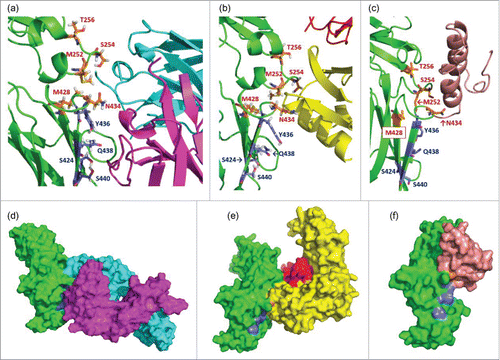
RE mutations clearly reduced RF binding in the YTE and N434H variants, but the effect was less clear in the LS variant. Therefore, we next focused on the Y436 residue, which is also located at the interaction site with the RF molecule, as shown in . We found that a single Y436T mutation in the LS variant could effectively reduce the RF binding in multiple donors (#3, #11 and #12) (Fig. S1c vs 1h) while the binding affinity against hFcRn was maintained (Table S1). Furthermore, we tested another approach to reducing the RF binding of the LS variant by introducing an S424N mutation onto the LS variant so as to create an additional N-linked glycosylation site (NxS sequence) at position 424 to 426, which is also a RF binding site. The S424N variant was produced by HEK293 cells, and SDS-PAGE analysis suggested that Asn introduced at position 424 was successfully glycosylated (data not shown). Results of the RF binding of the S424N variant are shown in Fig. S1i. As expected, there was no response of RF binding, which shows that the additional N-linked glycosylation at position 424 could significantly reduce the increased RF binding of the LS variant.
Figure 3. Enlarged complex structures of (a) Fc and RF, (b) Fc and FcRn, and (c) Fc and Protein A. In each figure, the positions mutated to enhance FcRn binding (252, 254, 256, 428, and 434) are shown as orange sticks, and those to reduce RF binding (424, 436, 438, and 440) are shown as blue sticks. The colors of ribbons are the same as in . The blue sticks interact with the light chain of RF but not with FcRn or protein A. Surface figures of each complex structure are shown in (d), (e), and (f). The colors of the molecular surface are the same in as the ribbon structures. The blue regions, which indicate the positions mutated to reduce RF binding, are exposed to the molecular surface in (e) and (f), but not in (d).
displays RF binding response for each individual as dot plots with median and interquartile range (+/− IQR). The medians in healthy donor and RA patient samples showed similar tendency that YTE, N434H, and LS variants showed the increased RF binding compared with intact human IgG1 while RE mutation, Y436T, and S424N reduced their increased RF binding. For healthy donor samples, although the overall binding responses were smaller than that of RA patients, YTE, N434H, and LS variants increased the response compared with intact human IgG1 while RE mutation, Y436T, and S424N reduced their increased RF binding with a statistical significance (). For RA patient samples, LS with Y436T and S424N showed statistically significant reduction of RF binding compared with LS (). However, although tendency toward reduction of RF binding by RE mutation was observed for YTE, N434H and LS, the effect was not statistically significant due to large variation among individuals. As a result, 4 mutations—S424N, Y436T, Q438R, and S440E—were obtained to reduce the RF binding that was increased by the YTE, N434H, and LS mutations. The position of these mutations in the structure are described in .
Generation of novel Fc variants with prolonged half-life and without increased RF binding
Novel Fc variants were then designed for prolonged half-life without increased RF binding compared with wild-type human IgG1. Since protein A or its variants are generally used for affinity purification in the manufacturing processes of IgG antibodies, the binding affinity to protein A should be maintained when we design novel Fc variants to allow for protein A purification. As shown in c, the interaction site of the Fc with protein A is also located in the CH2-CH3 interface region. On the other hand, the mutated positions for reducing RF binding are located far from the interaction site for protein A. We carefully designed novel Fc variants that had increased FcRn binding in acidic pH, but did not have increased RF binding or lose the ability to be purified by protein A. We identified which combinations of mutations were effective, and finally designed 5 novel Fc variants, ACT1 to ACT5 (). These variants could be purified with the conventional protein A affinity column without any issue.
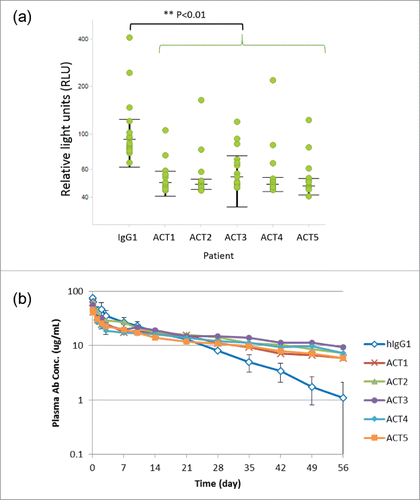
We evaluated the binding affinity to human and cynomolgus monkey FcRn. As shown in , these variants showed increased binding affinity against both human and cynomolgus monkey FcRn. Next, we evaluated RF binding in these novel variants. In this study, we used plasma from 15 RA patients, and individual results are shown in and Fig. S2. Surprisingly, all ACT1 to ACT5 variants showed even significantly lower RF binding than intact IgG1 (p<0.01). Finally, we examined the pharmacokinetic profiles of these variants in cynomolgus monkey. Interestingly, they all showed an improved pharmacokinetic profile compared with intact IgG1 ( and Table S2). The half-life of these variants was improved by 2- to 4-fold over the intact IgG1 (11.9 days), and ACT3 showed a particularly remarkable long half-life (45.1 days).
Figure 4. Novel Fc variants ACT1 to ACT5 (the ACT series). (a) Dot plots and statistical significance in RF binding assay for ACT variants. Each individual response of RA patients is shown with median and interquartile range (+/− IQR). Every ACT variant showed significant lower RF binding than intact IgG1 (p<0.01). (b) Time course of plasma antibody concentration in a cynomolgus monkey pharmacokinetic study. Test antibodies have an Fc region of intact human IgG1 (hIgG1) or novel variants (ACT series). The data of several monkeys who showed rapid clearance of a test antibody and were suspected to develop ADA are excluded from the result.
Generation of novel Fc variants capable of antigen sweeping without increased RF binding
As we reported previously, a sweeping antibody (i.e., an engineered monoclonal antibody with pH-dependent antigen binding and increased binding to FcRn at neutral pH) provides potential advantages over conventional antibodies by effectively eliminating soluble antigen from plasma.Citation9 However, we found that the Fc variants used as a sweeping antibody also increase RF binding. Fc variant v3, which is described in the previous reportCitation9 and has M252Y/N434Y mutations, showed significant binding to RF (Fig. S3a). We examined the effect of RE mutations, and generated 2 variants, type A and B, with sweeping activity and reduced RF binding. As shown in Table S3, they have mutations for increasing the FcRn binding, reducing the FcγR binding (L235R/S239K), and also RE mutations. No RF binding was detected in these variants (Fig. S3b and c). Furthermore, we examined the in vivo sweeping efficacy of the variants in a hFcRn transgenic mouse steady-state model,Citation9 and found that they reduced the antigen concentration by 10-fold compared with intact human IgG1, while antibody pharmacokinetics were comparable to IgG1 (Fig. S3d and e).
Discussion
In this study, we examined the RF binding of several Fc variants in which FcRn binding has been enhanced. Enhancing FcRn binding has 2 objectives: 1) elongating the half-life by improving the binding in acidic pH, and 2) acquiring a sweeping function by improving the binding at neutral pH. We found that RF binding is generally observed for such Fc variants, and is a potential risk for their clinical application in autoimmune diseases. We successfully developed a way to avoid the RF binding and, for the first time, generated novel Fc variants that have increased FcRn binding without increased RF binding. Furthermore, we revealed some findings about the interaction between RF and the mutated Fc.
We found that all the Fc variants with enhanced binding affinity to FcRn, including N434H, YTE, LS, and v3, showed increased binding to RF. On the other hand, mutations to modify the FcγR binding did not show increased RF binding (data not shown). These results indicate that increased RF binding is a general issue when inserting Fc mutations to enhance the FcRn binding. This may be because the epitope of RF is mainly located in the CH2-CH3 junction region,Citation25-27 where FcRn binds, and only a small number of RF can recognize the FcγR binding region.Citation30-33
RF binding to a therapeutic antibody may be problematic for the immunogenicity and pharmacokinetics of the antibody. It is known that IC formation of a drug and an ADA can elicit a variety of downstream effects and further immunogenic responses.Citation34 A similar effect can be expected when the ADA is an RF. The complex of the therapeutic antibody and RF will be easily taken up into APCs, and a further immunogenic response against the therapeutic antibody may be elicited. In addition, such RF binding would interfere with the assessment of ADA in clinical development, because RF may be detected as a pre-existing ADA and complicate the ADA assessment.Citation24,35 Other reports suggest that RF could influence the efficacy or safety of therapeutics because RFs are reported to amplify the inflammatory response of macrophagesCitation36 and to inhibit the effector function of rituximab.Citation37 Moreover, since RF binds the FcRn-binding site of the Fc, RF could inhibit FcRn-mediated recycling of the antibody.Citation38 Therefore, Fc variants in which FcRn binding has been enhanced to elongate the half-life or the sweeping activity would have risks in terms of efficacy and safety, and such risks should be minimized.
RFs are polyclonal autoantibodies against the Fc region of human IgG. Some RF clones can recognize the native structure of a therapeutic antibody that has wild-type human IgG, but some clones may incidentally have higher affinity to human IgG that has specific mutation(s). Since the former type of RF clones can also bind to endogenous human IgG (which has the same amino acid sequence as therapeutic IgG), the binding of these RF clones to a therapeutic antibody that has wild-type human IgG would be mostly inhibited by having to compete with the excess amount of endogenous human IgG present at much higher concentration in plasma. In such a case, RF would not cause any serious issues, such as an immune reaction caused by RF binding to the therapeutic antibody. On the other hand, the latter type of RFs recognize specific mutation(s) introduced in the therapeutic antibody, and could preferably bind to the therapeutic antibody with higher binding affinity than to wild-type human IgG. These RFs could cause an undesired immune reaction, increased ADA incidence, and enhanced clearance of the therapeutic antibody. Also, we found that not all serum samples from RA patients showed increased binding to the Fc variants. This indicates that some, but not all, RA patients have the latter type of RFs, which can bind to a therapeutic antibody with the mutations and may be problematic.
Our study demonstrated that there are RFs that can specifically recognize the mutated CH2-CH3 junction. Considering the nature of antibody diversity, these RFs should be polyclonal and would include a variety of CDR sequences and epitopes that recognize the mutated region. So it was quite surprising that just one or 2 additional mutations could substantially reduce the binding of polyclonal RF, and even RF from different RA patients. Although the exact nature of the interactions is not clear, there may be some specific recognition pattern of RF that binds to the mutated CH2-CH3 junction, and the mutations for reducing RF binding inhibited this recognition. Such RFs may be derived from specific germline sequences that have a similar structure to recognize the mutated Fc.
Duquerroy et al. reported the structure of a RF/Fc complex where the RF recognizes the C-terminal region of the CH3 domain, not the CH2-CH3 junction.Citation39 This RF can recognize an intact IgG Fc, but would not recognize the mutated residues used to enhance FcRn binding at the CH2-CH3 junction. Interestingly, however, RE mutations seem to inhibit the binding of this type of RF. Some RF binding data indicate that Fc variants with RE mutations often show less RF binding than intact IgG1. These results may suggest RE mutations could inhibit the binding of both types of RF: those that recognize the intact region and those that recognize the mutated region of the Fc.
RE mutations to reduce RF binding were obtained by screening Fc variants with mutations at positions 386, 387, 422, 424, 438, and 440, which are located in the RF-binding region and are distant from the FcRn-binding region. Variants were generated in which the positions were changed one at a time to Arg as a basic residue, Glu as an acidic residue, and Ser as a hydrophilic residue or to combinations thereof, and their binding to RF and FcRn evaluated. As a result, several variants showed reduced binding to RF and, interestingly, combinations of these mutations showed additive effects or synergistic effects. The combination of RE mutations selected as a result of these experiments was 438Arg and 440Glu. Furthermore, we revealed that 438Arg (basic residue) can be replaced by Lys (basic residue), but not by Glu (acidic residue). On the other hand, 440Glu can be replaced by Asp (acidic residue), but not by Arg. These results suggest that Fc recognition by an RF is mediated by specific charge interactions, and the reduced RF binding achieved by RE mutations is produced by charge-based repulsion between the Fc and RF.
N-linked glycosylation is known to bestow high solubility and a bulky structure compared with an amino acid side chain. We successfully introduced an artificial N-linked glycosylation site to eliminate RF binding. An engineered N-linked glycosylation site can be used not only to reduce RF binding, but also to avoid other unfavorable protein–protein interactions, and several cases of artificial N-linked glycosylation have been reported. For example, an additional N-linked glycosylation into the antibody CDR was able to improve the solubility without impairing the binding affinity to the antigen,Citation40 and another study reports that an additional N-glycosylation rescued the human LHb-subunit from aggregation.Citation41 Furthermore, it is known that N-glycosylation is closely related to immunogenicity and antigenicity of the glycoproteins, and it could positively or negatively affect its immunogenicity and antigenicity.Citation42-46 Our study also demonstrated that N-glycosylation can alter the antigenicity feature of Fc variants.
The novel engineered Fc ACT series, which has improved FcRn binding without increased RF binding, showed an extended pharmacokinetic profile in cynomolgus monkey, especially that of ACT3, which has a half-life of 45.1 d. The half-life in cynomolgus monkey of previously existing Fc variants with enhanced FcRn binding is 14.5 days,Citation17 21.2 days,Citation10 31.1 days,Citation11 11.6 days,Citation47 26.5 days,Citation48 and 24 d.Citation49 To the best of our knowledge, the ACT3 variant has the longest half-life in cynomolgus monkey, and importantly has significantly lower RF binding than intact IgG1 using RA patient samples (p<0.01). This means that ACT variants could provide benefits to patients who have RFs by not only extending the half-life but also reducing RF binding to the level even lower than intact IgG1. The immunogenicity risk of the ACT series variants derived from non-natural mutation in the Fc domain was assessed by in silico analysis using EpiMatrix (EpiVax, Inc.)Citation50 and an in vitro Th cell assay,Citation51,52 and these studies confirmed that the ACT series variants have no increased risk of immunogenicity (data not shown). Also, we confirmed that RE mutations did not impair the stability of the antibody, and the ACT series variants maintained good physical properties and stability. These results support the view that the Fc variant ACT3 could be readily applicable as a therapeutic antibody for chronic autoimmune diseases that require long-term treatment.
We have previously reported that sweeping antibody technology, which can effectively eliminate soluble antigen from plasma, is an attractive approach for inhibiting a large amount of antigen using a small amount of antibody. The v3 variant with enhanced FcRn binding at neutral pH showed increased binding to RF, similarly to the N434H, LS, and YTE variants, but the RE mutations effectively eliminated the increased RF binding while showing the sweeping activity in vivo. In addition, the sweeping activity and the antibody pharmacokinetics of the novel Fc variants were consistent with our previous reports,Citation9 in which results for PH-v3 or v4 were similar to those of the novel variants. In this way we confirmed that the RE mutations did not impair the sweeping activity and antibody pharmacokinetics.
Our findings can be summarized as follows: First, all tested Fc variants with enhanced FcRn binding had increased RF binding. Second, several mutations were obtained that reduced the RF binding, despite the polyclonal feature of RF. Third, novel Fc variants without increased RF binding and considerably extended half-life were obtained. Fourth, novel Fc variants for sweeping antibody without increased RF binding were obtained. Based on our results, we conclude that Fc-engineered therapeutic antibodies without increased RF binding can play an important role in developing therapeutic antibodies for autoimmune diseases.
Materials and methods
Ethics statement
Animal studies were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Chugai Pharmaceutical Co., Ltd. under the approval of the company's Institutional Animal Care and Use Committee. The company is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (http://www.aaalac.org).
The blood samples for RF binding assays were donated by 14 healthy Japanese volunteers after written informed consent was obtained, and the samples were anonymized. The use of human-derived test materials was approved by the Research Ethics Committee of Chugai Pharmaceutical Co., Ltd.
Generation of Fc variants and FcRn binding analysis
Fc variants were generated by site-directed mutagenesis of a humanized monoclonal antibody with human IgG1 and kappa chain. Binding analyses of each of the antibodies for human or cynomolgus monkey FcRn were performed using Biacore T200 (GE Healthcare). To mimic the condition in the endosome, we used pH 6.0 for the analysis in acidic pH, and to reflect the condition in plasma, we used pH 7.0 for the neutral pH analysis instead of pH 7.3–7.4 because the binding of wild type IgG was too weak at pH 7.3–7.4.
An appropriate amount of Protein L (ACTIGEN) to give 2000–2500 RU was fixed onto Sensor chip CM4 (GE Healthcare) by the amine coupling method to capture the test antibodies. Next, a diluted FcRn solution (prepared in house) or a running buffer (used as a reference solution) was injected and the binding response to the test antibody was analyzed. For the running buffer, 50 mM sodium phosphate, 150 mM NaCl, and 0.05% (w/v) Tween 20 at pH 6.0 or pH 7.0 was used, and it was also used to dilute FcRn. The antibodies described in and IgG1 in Table S1 were evaluated with 0, 50, 100, 200, 400, 800, or 1600 nM FcRn, and the antibodies other than IgG1 in Tables S1 and 3 were evaluated with 0, 25, 50, 100, 200, 400, or 800 nM hFcRn. To regenerate the sensor chip, 10 mM glycine-HCl at pH 1.5 was used. All measurements were performed at 25°C. The equilibrium dissociation constant (KD (M)) for FcRn was calculated for each antibody based on the steady-state analysis. The fitting curves for the calculation are shown in Fig. S4. The Biacore T200 Evaluation Software (GE Healthcare) was used to calculate each parameter.
RF binding assay
RF binding of the test antibodies was detected by ECL immunoassay at pH 7.4. The assays were performed with the serum of 15 individual RA patients (Proteogenex) and 14 healthy volunteers (in-house). Due to the limited amount of serum samples from RA patients, most of the experiments were conducted as N = 1. The test antibodies were labeled with biotin and SULFO-TAG NHS Ester (Meso Scale Discovery), respectively. Next, 50-fold diluted serum samples, 1 μg/mL test antibody labeled with biotin, and labeled with SULFO-TAG NHS Ester were mixed and incubated for 3 hr at room temperature. Then, the mixtures were added to streptavidin-coated MULTI-ARRAY® 96-well plates (Meso Scale Discovery), and the plates were incubated for 2 hr at room temperature and washed. After Read Buffer T (x4) (Meso Scale Discovery) was added to each well, plates were immediately set on the SECTOR imager 2400 Reader (Meso Scale Discovery) and relative light units (RLU) were measured.
Cynomolgus monkey PK study
A single dose of each test antibody was intravenously administered at 2 mg/kg to cynomolgus monkeys (N = 3). Blood samples were collected at 5 min, 4 hr, 1, 2, 3, 7, 10, 14, 21, 28, 35, 42, 49, and 56 d after administration. The collected blood was immediately centrifuged at 4°C and 15,000 rpm for 10 minutes to obtain plasma. The separated plasma was stored in a freezer set to −60°C or below until measurements were taken. Heparin was used as an anticoagulant. The antibody concentration in cynomolgus monkey plasma was measured by an ECL immunoassay. Since several monkeys showed rapid clearance of the test antibody and were suspected of developing ADA, we excluded them from the results; therefore, averages of N = 2 are shown for ACT1, 2, 3, and 5 data. However, variations in the antibody concentration between the 2 animals in each group were small (data not shown). T1/2 was calculated with WinNonlin6.3 (Certara, Princeton, USA).
In vivo study in hFcRn transgenic mice steady-state model
An infusion pump (alzet) filled with 92.8 mg/mL hsIL-6R was implanted under the skin on the back of hFcRn-Tgm (B6.mFcRn2/2.hFcRn Tg line 32+/+ mouse, Jackson Laboratories)Citation53 to prepare model mice with a constant plasma concentration of hsIL-6R. Monoclonal anti-mouse CD4 antibody GK1.554 was administered by intravenous (i.v.) injection to inhibit the production of mouse antibody against hsIL-6R by depleting CD4+ T-cells. Antibodies against hsIL-6R were administered at 1 mg/kg to hFcRn-Tgm with a single i.v. injection of 1 g/kg of hIgG (Sanglopor®, CSL Behring) to mimic endogenous human IgG. Blood samples were collected at 15 min, 7 hr, 1, 2, 3, 7, 14, 21, and 28 d after administration. The plasma hsIL-6R and antibody concentration were determined as described previously.Citation9
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Materials.docx
Download MS Word (846.2 KB)Funding
This work was fully supported by Chugai Pharmaceutical Co., Ltd. Funding to pay the Open Access publication charges for this article was provided by Chugai Pharmaceutical Co., Ltd.
References
- Kalden JR, Burkhardt H. Autoimmune disease: Treatment. Encyclopedia of Life Sciences 2009; https://doi.org/10.1002/9780470015902.a0001437.pub2
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: An update. BMC Med 2013; 11:88; PMID: 23557513; https://doi.org/10.1186/1741-7015-11-88
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 2010; 10:301-16; PMID: 20414204; https://doi.org/10.1038/nri2761
- Wang D, Li Y, Liu Y, Shi G. The use of biologic therapies in the treatment of rheumatoid arthritis. Curr Pharm Biotechnol 2014; 15:542-8; PMID: 25213363; https://doi.org/10.2174/138920101506140910150612
- Lycke J. Monoclonal antibody therapies for the treatment of relapsing-remitting multiple sclerosis: Differentiating mechanisms and clinical outcomes. Ther Adv Neurol Disord 2015; 8:274-93; PMID: 26600872; https://doi.org/10.1177/1756285615605429
- Igawa T, Tsunoda H, Tachibana T, Maeda A, Mimoto F, Moriyama C, Nanami M, Sekimori Y, Nabuchi Y, Aso Y, et al. Reduced elimination of IgG antibodies by engineering the variable region. Protein Eng Des Sel 2010; 23:385-92; PMID: 20159773; https://doi.org/10.1093/protein/gzq009
- Igawa T, Tsunoda H, Kuramochi T, Sampei Z, Ishii S, Hattori K. Engineering the variable region of therapeutic IgG antibodies. mAbs 2011; 3:243-52; PMID: 21406966; https://doi.org/10.4161/mabs.3.3.15234
- Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, Moriyama C, Watanabe T, Takubo R, Doi Y, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol 2010; 28:1203-7; PMID: 20953198; https://doi.org/10.1038/nbt.1691
- Igawa T, Maeda A, Haraya K, Tachibana T, Iwayanagi Y, Mimoto F, Higuchi Y, Ishii S, Tamba S, Hironiwa N, et al. Engineered monoclonal antibody with novel antigen-sweeping activity in vivo. PloS one 2013; 8:(5):e63236; PMID:23667591; https://doi.org/10.1371/journal.pone.0063236
- Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 2006; 281:23514-24; PMID: 16793771; https://doi.org/10.1074/jbc.M604292200
- Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010; 28:157-9; PMID: 20081867; https://doi.org/10.1038/nbt.1601
- Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, Lowman HB, Fielder PJ, Prabhu S. Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab Dispos 2010; 38:600-5; PMID: 20071453; https://doi.org/10.1124/dmd.109.031310
- Zheng Y, Scheerens H, Davis JC, Jr., Deng R, Fischer SK, Woods C, Fielder PJ, Stefanich EG. Translational pharmacokinetics and pharmacodynamics of an FcRn-variant anti-CD4 monoclonal antibody from preclinical model to phase I study. Clin Pharmacol Ther 2011; 89:283-90; PMID: 21191378; https://doi.org/10.1038/clpt.2010.311
- Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother 2013; 57:6147-53; PMID: 24080653; https://doi.org/10.1128/AAC.01285-13
- West AP, Jr., Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor. Biochemistry 2000; 15; 39(32):9698-708; PMID: 10933786; https://doi.org/10.1021/bi000749m
- Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol 2000; 18:739-66; PMID: 10837074; https://doi.org/10.1146/annurev.immunol.18.1.739
- Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, Starovasnik MA, Lowman HB. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol 2009; 182:7663-71; PMID: 19494290; https://doi.org/10.4049/jimmunol.0804182
- Haraya K, Tachibana T, Iwayanagi Y, Maeda A, Ozeki K, Nezu J, Ishigai M, Igawa T. PK/PD analysis of a novel pH-dependent antigen-binding antibody using a dynamic antibody-antigen binding model. Drug Metab Pharmacokinet 2016; 31:123-32; PMID: 26944099; https://doi.org/10.1016/j.dmpk.2015.12.007
- Igawa T, Haraya K, Hattori K. Sweeping antibody as a novel therapeutic antibody modality capable of eliminating soluble antigens from circulation. Immunol Rev 2016; 270:132-51; PMID: 26864109; https://doi.org/10.1111/imr.12392
- Iwayanagi Y, Igawa T, Maeda A, Haraya K, Wada NA, Shibahara N, Ohmine K, Nambu T, Nakamura G, Mimoto F, et al. Inhibitory FcgammaRIIb-Mediated soluble antigen clearance from plasma by a pH-Dependent Antigen-Binding antibody and its enhancement by Fc engineering. J Immunol 2015; 195:3198-205; PMID: 26320252; https://doi.org/10.4049/jimmunol.1401470
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013; 35:727-34; PMID: 24324289; https://doi.org/10.1155/2013/726598
- Newkirk MM. Rheumatoid factors: Host Resistance or Autoimmunity?. Clin Immunol 2002; 104:1-13; PMID: 12139942; https://doi.org/10.1006/clim.2002.5210
- Carson DA, Chen PP, Kipps TJ. New roles for rheumatoid factor. J Clin Invest 1991; 87:379-83; PMID: 1991824; https://doi.org/10.1172/JCI115007
- Araujo J, Zocher M, Wallace K, Peng K, Fischer SK. Increased rheumatoid factor interference observed during immunogenicity assessment of an Fc-engineered therapeutic antibody. J Pharm Biomed Anal 2011; 55:1041-9; PMID: 21466939; https://doi.org/10.1016/j.jpba.2011.03.008
- Sasso EH, Barber CV, Nardella FA, Yount WJ, Mannik M. Antigenic specificities of human monoclonal and polyclonal IgM rheumatoid factors. The C gamma 2-C gamma 3 interface region contains the major determinants. J Immunol 1988; 140:3098-107; PMID: 2452199
- Artandi SE, Calame KL, Morrison SL, Bonagura VR. Monoclonal IgM rheumatoid factors bind IgG at a discontinuous epitope comprised of amino acid loops from heavy-chain constant-region domains 2 and 3. Proc Natl Acad Sci U S A 1992; 89:94-8; PMID: 1370358; https://doi.org/10.1073/pnas.89.1.94
- Corper AL, Sohi MK, Bonagura VR, Steinitz M, Jefferis R, Feinstein A, Beale D, Taussig MJ, Sutton BJ. Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody-antigen interaction. Nat Struct Biol 1997; 4:374-81; PMID: 9145108; https://doi.org/10.1038/nsb0597-374
- Martin WL, West AP, Jr., Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell 2001; 7:867-77; PMID: 11336709; https://doi.org/10.1016/S1097-2765(01)00230-1
- Rigsby RE, Parker AB. Using the PyMOL application to reinforce visual understanding of protein structure. Biochem Mol Biol Educ 2016; 44:433-7; PMID: 27241834; https://doi.org/10.1002/bmb.20966
- Kiyoshi M, Caaveiro JM, Kawai T, Tashiro S, Ide T, Asaoka Y, Hatayama K, Tsumoto K. Structural basis for binding of human IgG1 to its high-affinity human receptor FcgammaRI. Nat Commun 2015; 6:6866; PMID: 25925696; https://doi.org/10.1038/ncomms7866
- Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, Powell MS, Tan PS, Cendron AC, Wines BD, et al. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol 2011; 187:3208-17; PMID: 21856937; https://doi.org/10.4049/jimmunol.1101467
- Mimoto F, Katada H, Kadono S, Igawa T, Kuramochi T, Muraoka M, Wada Y, Haraya K, Miyazaki T, Hattori K. Engineered antibody Fc variant with selectively enhanced FcgammaRIIb binding over both FcgammaRIIa(R131) and FcgammaRIIa(H131). Protein Eng Des Sel 2013; 26:589-98; PMID: 23744091; https://doi.org/10.1093/protein/gzt022
- Mimoto F, Kadono S, Katada H, Igawa T, Kamikawa T, Hattori K. Crystal structure of a novel asymmetrically engineered Fc variant with improved affinity for FcgammaRs. Mol Immunol 2014; 58:132-8; PMID: 24334029; https://doi.org/10.1016/j.molimm.2013.11.017
- Krishna M, Nadler SG. Immunogenicity to Biotherapeutics - The role of Anti-drug immune complexes. Front Immunol 2016; 7:21; PMID: 26870037; https://doi.org/10.3389/fimmu.2016.00021
- Tatarewicz S, Miller JM, Swanson SJ, Moxness MS. Rheumatoid factor interference in immunogenicity assays for human monoclonal antibody therapeutics. J Immunol Methods 2010; 357:10-6; PMID: 20347831; https://doi.org/10.1016/j.jim.2010.03.012
- Laurent L, Anquetil F, Clavel C, Ndongo-Thiam N, Offer G, Miossec P, Pasquali JL, Sebbag M, Serre G. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann Rheum Dis 2015; 74:1425-31; PMID: 24618262; https://doi.org/10.1136/annrheumdis-2013-204543
- Jones JD, Shyu I, Newkirk MM, Rigby WF. A rheumatoid factor paradox: inhibition of rituximab effector function. Arthritis Res Ther 2013; 15(1):R20; ; PMID: 23351360; https://doi.org/10.1186/ar4152
- Zhou J, Pop L, Ghetie V. Hypercatabolism of IgG in mice with lupus-like syndrome. Lupus 2005; 14:458-66; PMID: 16038110; https://doi.org/10.1191/0961203305lu2129oa
- Duquerroy S, Stura EA, Bressanelli S, Fabiane SM, Vaney MC, Beale D, Hamon M, Casali P, Rey FA, Sutton BJ, et al. Crystal structure of a human autoimmune complex between IgM rheumatoid factor RF61 and IgG1 Fc reveals a novel epitope and evidence for affinity maturation. J Mol Biol 2007; 368:1321-31; PMID: 17395205; https://doi.org/10.1016/j.jmb.2007.02.085
- Wu SJ, Luo J, O'Neil KT, Kang J, Lacy ER, Canziani G, Baker A, Huang M, Tang QM, Raju TS, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel 2010; 23:643-51; PMID: 20543007; https://doi.org/10.1093/protein/gzq037
- Suzuki S, Furuhashi M, Suganuma N. Additional N-glycosylation at Asn13 rescues the human LHb-subunit from disulfide-linked aggregation. Mol Cell Endocrinol 2000; 160:157-63; PMID: 10715549; https://doi.org/10.1016/S0303-7207(99)00213-0
- Dowling W, Thompson E, Badger C, Mellquist JL, Garrison AR, Smith JM, Paragas J, Hogan RJ, Schmaljohn C. Influences of glycosylation on antigenicity, immunogenicity, and protective efficacy of ebola virus GP DNA vaccines. J Virol 2007; 81:1821-37; PMID: 17151111; https://doi.org/10.1128/JVI.02098-06
- Wang W, Lu B, Zhou H, Suguitan AL, Jr., Cheng X, Subbarao K, Kemble G, Jin H. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J Virol 2010; 84:6570-7; PMID: 20427525; https://doi.org/10.1128/JVI.00221-10
- Fournillier A, Wychowski C, Boucreux D, Baumert TF, Meunier JC, Jacobs D, Muguet S, Depla E, Inchauspe G. Induction of hepatitis C virus E1 envelope protein-specific immune response can be enhanced by mutation of N-glycosylation sites. J Virol 2001; 75:12088-97; PMID: 11711599; https://doi.org/10.1128/JVI.75.24.12088-12097.2001
- Hutter J, Rodig JV, Hoper D, Seeberger PH, Reichl U, Rapp E, Lepenies B. Toward animal cell culture-based influenza vaccine design: viral hemagglutinin N-glycosylation markedly impacts immunogenicity. J Immunol 2013; 190:220-30; PMID: 23225881; https://doi.org/10.4049/jimmunol.1201060
- Hyakumura M, Walsh R, Thaysen-Andersen M, Kingston NJ, La M, Lu L, Lovrecz G, Packer NH, Locarnini S, Netter HJ. Modification of Asparagine-Linked glycan density for the design of Hepatitis B Virus Virus-Like particles with enhanced immunogenicity. J Virol 2015; 89:11312-22; PMID: 26339047; https://doi.org/10.1128/JVI.01123-15
- Borrok MJ, Wu Y, Beyaz N, Yu XQ, Oganesyan V, Dall'Acqua WF, Tsui P. pH-dependent binding engineering reveals an FcRn affinity threshold that governs IgG recycling. J Biol Chem 2015; 290:4282-90; PMID: 25538249; https://doi.org/10.1074/jbc.M114.603712
- Datta-Mannan A, Chow CK, Dickinson C, Driver D, Lu J, Witcher DR, Wroblewski VJ. FcRn affinity-pharmacokinetic relationship of five human IgG4 antibodies engineered for improved in vitro FcRn binding properties in cynomolgus monkeys. Drug Metab Dispos 2012; 40:1545-55; PMID: 22584253; https://doi.org/10.1124/dmd.112.045864
- Ng CM, Loyet KM, Iyer S, Fielder PJ, Deng R. Modeling approach to investigate the effect of neonatal Fc receptor binding affinity and anti-therapeutic antibody on the pharmacokinetic of humanized monoclonal anti-tumor necrosis factor-alpha IgG antibody in cynomolgus monkey. Eur J Pharm Sci 2014; 51:51-8; PMID: 23999033; https://doi.org/10.1016/j.ejps.2013.08.033
- De Groot AS, Martin W. Reducing risk, improving outcomes: bioengineering less immunogenic protein therapeutics. Clin Immunol 2009; 131:189-201; PMID: 19269256; https://doi.org/10.1016/j.clim.2009.01.009
- Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin Immunol 2013; 149:534-55; PMID: 24263283; https://doi.org/10.1016/j.clim.2013.09.006
- Wullner D, Zhou L, Bramhall E, Kuck A, Goletz TJ, Swanson S, Chirmule N, Jawa V. Considerations for optimization and validation of an in vitro PBMC derived T cell assay for immunogenicity prediction of biotherapeutics. Clin Immunol 2010; 137:5-14; PMID: 20708973; https://doi.org/10.1016/j.clim.2010.06.018
- Roopenian DC, Christianson GJ, Sproule TJ. Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol Biol 2010; 602:93-104; PMID: 20012394
- Rashid A, Auchincloss H Jr., Sharon J. Comparison of GK1.5 and chimeric rat/mouse GK1.5 anti-CD4 antibodies for prolongation of skin allograft survival and suppression of alloantibody production in mice. J Immunol 1992; 148:1382-8