ABSTRACT
As biosimilars enter the market, comparisons of product quality are needed. Manufacturing differences may lead to differences in critical quality attributes, which affect efficacy. Therefore, critical quality attributes (structure and biological activity) of Remicade® and of 2 biosimilar products (Flixabi®/Renflexis® and Remsima®/Inflectra®) were determined. We assessed binding to tumor necrosis factor in a fluorescence competitive binding assay; potency in a luciferase reporter gene assay; percentages of galactosylated glycan, afucose plus high mannosylated glycans, and charged glycan; FcγRIIIa (CD16) binding (assessed by 3 methods); and antibody-dependent cell-mediated cytotoxicity (ADCC) in the NK92-CD16a cell line and in peripheral blood mononuclear cells (PBMC). The results of Fab-related activity were similar for all products. Compared with Remicade®, Flixabi® had a lower percentage of charged glycan, and Remsima® had a higher percentage of galactosylated glycan and a lower percentage of afucose plus high mannosylated glycans. Whereas Remsima® and Remicade® are expressed in a Sp2/0 cell line, Flixabi® is expressed in a CHO cell line. Despite this difference, galactosylated glycans from the 3 products were not correlated with the expression system. The results of all 3 methods used in this study indicated that FcγRIIIa binding was lower with Remsima® than with Remicade®. The percentage of ADCC in NK92-CD16a cells was lower with Remsima® and higher with Flixabi® compared with Remicade®, but was similar for all 3 products in PBMC. Surface expression of CD16 was 5.7-fold greater on NK92-CD16a cells than on PBMC. Combined percentages of afucosylated and high mannosylated glycans were positively correlated with FcγRIIIa binding and ADCC in NK92-CD16 cells, while no correlation was observed in PBMC.
Introduction
Remicade® (infliximab) is a monoclonal antibody (mAb) that has been approved by the European Medicines Agency and the United States Food and Drug Administration (FDA) for the treatment of Crohn's disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis.Citation1,2 US federal law defines a biosimilar as a biological product that is highly similar to an FDA-approved biological product, known as the reference product.Citation3 A biosimilar should not have clinically meaningful differences in terms of safety, purity, and potency with respect to the reference product. In addition, there should only be minor differences in clinically inactive components. Biosimilars typically have a lower price than their reference product, and the savings generated by their introduction may reduce the rate of rapidly rising healthcare costs and broaden patient access.
Cell lines and manufacturing processes for biological products may be proprietary to the originator manufacturer. Consequently, biosimilars may be produced using cell lines or manufacturing processes that are different from the originator reference product. Manufacturing process differences may result in differences in critical quality attributes. Critical quality attributes are physical, chemical, or biological properties or characteristics that must be within an appropriate limit, range, or distribution to ensure the desired product quality, safety, and efficacy.Citation4
As a tumor necrosis factor-alpha (TNF-α) inhibitor, the mechanism of action (MOA) of infliximab primarily involves antigen-binding fragment (Fab)-mediated antigen neutralization. However, the level of contribution may differ depending on the indication. While it is generally accepted that in rheumatoid arthritis (RA) the MOA is largely dependent on neutralization of TNF-α, which is Fab-mediated, uncertainties remain regarding the involvement of the Fc region in infliximab in other chronic inflammatory conditions such as inflammatory bowel disease (IBD). For example, the crucial MOA of infliximab for Crohn's disease may be the destruction of TNF-α producing cells through ADCC. The Fc region of infliximab immune complex binds to the Fcγ receptor (FcγR) on immune cells. The FcγR has subclasses (I, II, and III) that are further categorized into subtypes. Interactions between the Fc region of infliximab and FcγRIIIa (CD16) on effector cells lead to the lysis of target cells bound by the antibody Fab region, i.e., antibody-dependent cell-mediated cytotoxicity (ADCC).Citation5,6 The natural killer (NK) cell, one such effector cell, is activated upon FcγRIIIa recognizing and binding to the Fc region of a mAb bound to the surface of a target cell. Activation of NK cells causes the release of cytokines, such as interferon-γ, that signal to other immune cells and cytotoxic mediators such as perforin and granzyme. These mediators enter the target cell and trigger apoptosis. The affinity of FcγR binding to the Fc region of IgG differs with different polymorphisms of FcγRIIIa. For example, the affinity of FcγRIIIa-158F/F for IgG is lower than that of FcγRIIIa-158V/V.
Both serum-derived endogenous human mAbs and mAbs produced by engineered mammalian cell lines such as Chinese hamster ovary (CHO) or SP2/0 contain glycans in the CH2 domain of the Fc region (N-glycosylation), and the glycans are highly heterogeneous because of different terminal sugar presence. For mAbs such as infliximab, the terminal sugars of Fc glycans have been shown to be critical for efficacy because Fc glycans influence FcγRIIIa binding and subsequent ADCC activity.Citation7 N-linked glycans on the Fc region carry various degrees of terminal galactosylation and may contain 0 (G0), 1 (G1), or 2 (G2) terminal galactose. These glycans are predominantly fucosylated, in that they contain a fucose attached to the innermost N-acetyl glucosamine (GlcNAc) residue in the core structure. However, small amounts of naturally occurring glycoforms lacking the core fucose have been observed in both human serum-derived and CHO cell-produced mAbs.Citation5,8 IgG1 mAbs lacking the core fucose of the Fc oligosaccharides have been found to elicit higher ADCC in humans than fucosylated mAbs.Citation9 Enhanced ADCC has also been observed previously as a result of high mannosylated (HM) glycans.Citation10 In contrast, reduced ADCC activity has been noted with increased sialylated (charged) Fc glycans, though a recent example has also shown that a 2-3-fold difference in sialylation may not result in reduced activity when the overall level of sialylation is low (e.g., under 1.3%).Citation11,21 Thus, characterization of the N-linked glycosylation profile of biosimilar mAbs is critical because N-linked glycan structures vary with different expression systems, and thus the degree of fucosylation, afucosylation, mannosylation, galactosylation, and sialylation may vary for infliximab products.
As differences in infliximab products may exist in terms of their structure and biological activity, critical quality attributes of Remicade® (infliximab) and of 2 infliximab biosimilars (Flixabi®/Renflexis® and Remsima®/Inflectra®) were determined. The specific aims that we addressed were characterization of the N-glycan profile and determination of FcγRIIIa binding activity, biological activity (ADCC), TNF-α binding activity, and TNF-α neutralization activity. Further, correlation between the percentage of afucose and HM glycans and FcγRIIIa binding activity, and correlation between the percentage of afucose and HM glycans and ADCC activity was calculated.
Results
Comparison of N-glycan profiles
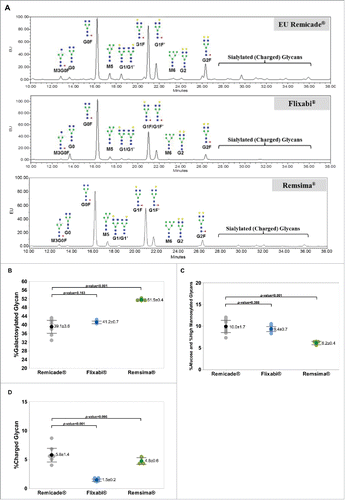
Representative N-glycan profiles of Remicade®, Flixabi®, and Remsima® are shown in . In regard to comparisons with Remicade®, the differences were a higher percentage galactosylated glycan (P < 0.001, ) with Remsima®, a lower percentage afucose plus HM glycans with Remsima® (P < 0.001, ), and a lower percentage charged glycan with Flixabi® (P < 0.001, ).
Figure 1. N-glycan profiles, %Galactosylated, %Afucosylated and %High Mannosylated, and %Charged levels of Remicade®, Flixabi®, and Remsima®. (A) N-glycan profiles of Remicade®, Flixabi®, and Remsima®. EU, emission unit. (B) Percentage of galactosylated glycans. (C) Percentage of afucose and high mannosylated glycans. (D) Percentage of charged glycans. Light color dots: Individual data; Dark color dots: Mean data with mean value and standard deviation; Bar: 95% confidence interval of mean.
Comparison of FcγRIIIa binding affinity
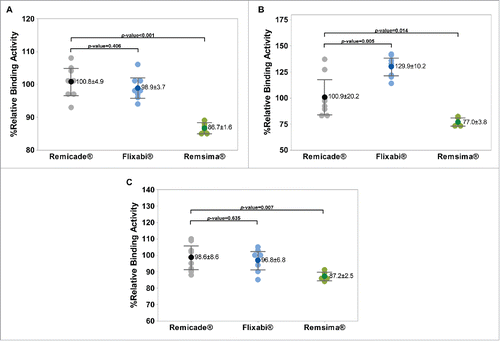
In regard to comparisons with Remicade® as assessed by surface plasmon resonance (SPR), the only difference was a lower percentage relative binding activity to FcγRIIIa with Remsima® (P < 0.001, ). In regard to comparisons with Remicade® as assessed by AlphaScreen, the differences were a higher percentage relative binding activity to FcγRIIIa with Flixabi® (P = 0.005, ) and a lower percentage relative binding activity to FcγRIIIa with Remsima® (P = 0.014, ). In regard to comparisons with Remicade® as assessed by fluorescence-activated cell sorting (FACS), the only difference was a lower percentage relative binding activity to FcγRIIIa with Remsima® (P = 0.007, ).
Figure 2. FcγRIIIa binding activity of Remicade®, Flixabi®, and Remsima®. (A) Percentage of relative binding activity using surface plasmon resonance. (B) Percentage of relative binding activity using AlphaScreen. (C) Percentage of relative binding activity using fluorescence-activated cell sorting. Light color dots: Individual data; Dark color dots: Mean data with mean value and standard deviation; Bar: 95% confidence interval of mean.
Comparison of ADCC activity
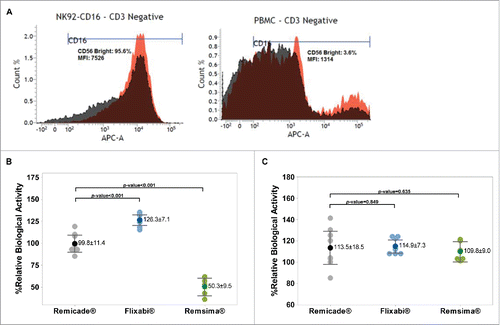
As shown in , higher (5.7-fold) levels of CD16 were observed in the NK92-CD16a cell line (V/V) compared with PBMC (V/F). In the NK92-CD16a cell line, the mean relative ADCC activity was higher with Flixabi® compared with Remicade® (P < 0.001, ). In contrast, Remsima® had lower activity compared with Remicade® (P < 0.001, ). In PBMC, no statistically significant differences were observed between Remicade® and Flixabi® or Remsima® ().
Figure 3. Antibody-dependent cell-mediated cytotoxicity of Remicade®, Flixabi®, and Remsima®. (A) CD16-positive cells (Count %) and mean fluorescence intensities (MFI) in CD3 negative populations from healthy donor peripheral blood mononuclear cells and NK92-CD16a cell line. APC, Allophycocyanin; APC-A, Area of APC detection. (B) Percentage of relative ADCC activity using a NK92-CD16a cell line. (C) Percentage of relative ADCC activity using PBMC from healthy human donors. Light color dots: Individual data; Dark color dots: Mean data with mean value and standard deviation; Bar: 95% confidence interval of mean.
Correlation between percentage of afucosylated and HM glycans and biological activity
The coefficient of determination (R2) for the percentage of afucose plus HM glycans and the percentage of relative FcγRIIIa binding activity were 84.17% as assessed by SPR, 40.03% as assessed by AlphaScreen, and 57.78% as assessed by FACS (). The R2 for the percentage of afucose plus HM glycans and the percentage of relative ADCC activity was 54.17% in NK92-CD16 cells and 0.57% in PBMC (). The data are also presented in . All correlations between the percentage of afucose plus HM glycans and either FcγRIIIa binding or ADCC activity were statistically significant with the exception of the correlation between the percentage of afucose plus HM glycans and the percentage of relative ADCC activity in PBMC (P = 0.739).
Figure 4. Correlation between percentage afucose plus percentage high mannosylated (HM) glycans and Fc functions for Remicade®, Flixabi®, and Remsima®. (A) Correlation between %afucose plus %HM glycan and %relative FcγRIIIa binding activity. (B) Correlation between %afucose plus %HM glycan and %relative ADCC activity.
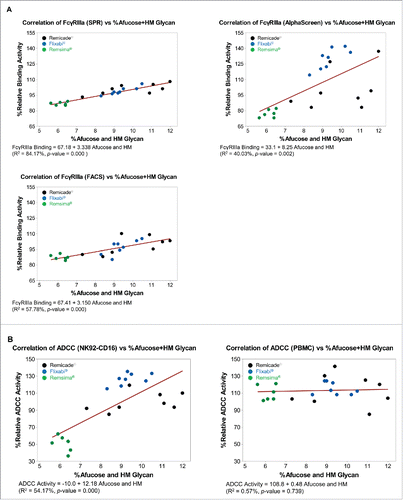
Table 1. %Afucose plus high mannosylated glycan and %relative ADCC activity of infliximab products. %RBA: %relative biological activity.
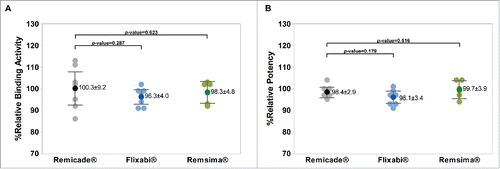
Comparison of TNF-α binding activity and TNF-α neutralization activity
No significant differences between Remicade®, Flixabi®, and Remsima® were noted for the mean percentage relative binding activity and the mean percentage relative potency ( and ).
Figure 5. Fab-related TNF-α binding and TNF-α neutralization activities of Remicade®, Flixabi®, and Remsima®. (A) Percentage of relative binding activity assessed by fluorescence resonance energy transfer. (B) Percentage of relative potency measured by a luciferase reporter gene assay. Light color dots: Individual data; Dark color dots: Mean data with mean value and standard deviation; Bar: 95% confidence interval of mean.
Discussion
Previous studies have separately reported the analytical (physicochemical and biological) evidence that supported the conclusion that Remsima® and Flixabi® are biosimilars to Remicade®.Citation19,20 However, the head-to-head analytical comparability of both biosimilars to the reference product had not been previously assessed, representing a key data gap for physicians faced with deciding between several approved versions of infliximab. To our knowledge, this is the first study to begin addressing this data gap. We characterized 2 biosimilars in comparison to the originator product Remicade® with respect to attributes associated with glycosylation (percentage of galactosylated glycan, percentage of afucose plus HM glycans, and percentage of charged glycan), ADCC activity (in NK92-CD16a cells and in PBMC), and FcγRIIIa activity (3 different assays). We considered that comparing Fc-related quality attributes could provide insights in the similarity of infliximab products, especially when they are used for indications other than rheumatoid arthritis. When compared with Remicade®, no statistically significant differences were found in 5 of 8 assessments of Flixabi® (percentage of galactosylated glycan, percentage of afucose plus HM glycans, percentage relative ADCC activity in PBMC, and FcγRIIIa binding activity by both SPR and FACS) and in 2 of 8 assessments of Remsima® (percentage relative ADCC activity in PBMC and percentage charged glycan).
The availability of both Flixabi® and Remsima® samples was limited. Selection of Flixabi® and Remsima® product lots based on low, mid, and high %afucosylated and HM glycan was not possible due to the limited supply, and all available lots were used. In contrast, selection of US- and EU-licensed Remicade® was based on the glycosylation profile (percentage of afucosylated and HM glycans), which ranged from 7.3–12.8%. The data for the %afucosylated and HM glycan are more tightly clustered for both Flixabi® and Remsima®. The narrower range is also reflected in the correlations between the %afucosylated and HM glycan and Fc functions.
Variations in cell culture conditions and in cell lines have been shown to affect the glycosylation of recombinant IgGs. Thus, the terminal galactosylation of recombinant IgGs may be affected by such variations. Indeed, wide variations in terminal galactosylation exist regardless of the system for recombinant IgG production (e.g., CHO cells or mouse myeloma cells).Citation12,13 Our data also showed such variation, as Flixabi® (produced using CHO cells) and Remicade® (produced using SP2/0 cells) had similar percentages of galactosylated glycans. In contrast, the percentage of galactosylated glycans in Remsima® (produced using SP2/0 cells) was higher than in the other 2 products.
The effect of charged glycans on ADCC activity was not assessed in this study, but a higher level of terminal sialic acid (charged glycan) could have a negative effect on ADCC activity.Citation7,11 Of the 3 products, Flixabi® had the lowest percentage of charged glycan, and it had greater ADCC activity compared with Remicade® and Remsima® in NK92-CD16a cells.
The percentage of afucosylated glycans and HM glycans was similar for Remicade® and Flixabi®, and this level was higher than the percentage with Remsima®. In NK92-CD16a cells, Flixabi® and Remicade® had the highest ADCC activity (126.3% and 99.8%, respectively). Our data are consistent with other data that have shown increased ADCC activity with products that have a high percentage of afucose or HM glycans.Citation9,10
Our data are also consistent with other published data that suggest a positive correlation of combined afucosylated (percentage of afucose) and HM (percentage of HM) glycans with ADCC activity and with FcγRIIIa binding activity.Citation14-16
A NK92-CD16a cell line was used because it is a more sensitive platform and thus increased the potential to detect any differences between infliximab products. In this sensitive system, Flixabi® had greater ADCC activity than both Remicade® and Remsima®. In contrast, ADCC activity was similar for all 3 products when PBMC were used. The lower R2 value and lack of statistical significance in PBMC may be due to the decreased sensitivity to detect differences with the products; PBMC had a lower surface expression of CD16. ADCC activity is correlated with the surface expression level of CD16a on effector cells, suggesting that the expression level of cell surface CD16a can have an effect on ADCC activity.Citation6
The observed differences using NK92-CD16a cells may illustrate the discriminatory power of the state-of-the-art analytics being developed to assess the similarity between molecules. Indeed, the ADCC assay using NK cells constitutively expressing CD16a offers improved performance in terms of assay sensitivity and ability to discriminate very small differences in ADCC activity.Citation17,18 However, our data, and those previously shared, demonstrate that these differences need to be placed into context by adding an orthogonal assessment of ADCC in the physiologically relevant PBMC cells.Citation22 We propose that future biosimilar development programs for ADCC-relevant products should include both assessments of ADCC to provide a more balanced picture: if differences are observed in both assay systems (with NK92-CD16 and PBMC cells alike), this could leave room for concern.
Although no statistical comparisons were made directly between Flixabi® and Remsima®, this comparability assessment between Flixabi® or Remsima® and Remicade® builds on the totality of evidence presented to support the approval of Flixabi® as a second biosimilar to infliximab and provides the first insight to how it compares to both previously approved infliximabs for selected relevant critical quality attributes. The comparative data may be of clinical relevance when health care providers are faced with choosing among 3 approved infliximab products, especially when their efficacy may be largely related to the Fc-related function assessed in this study. In this case, our data should provide additional confidence to health care providers, since Flixabi® was generally shown to fit within the frame of reference established by the 2 previously approved infliximabs. While differences were observed with the highly sensitive ADCC assay using NK92-CD16 cells and not with the physiologically relevant PBMC cells, these in vitro differences should not translate into clinically meaningful differences.
Materials and methods
Samples
Four lots each of US- and EU-licensed Remicade® were selected from more than 50 lots of reference product inventory; lots with expiration dates during the study were excluded. Selection was based on the glycosylation profile (percentage of afucosylated and HM glycans), which ranged from 7.3–12.8%. Specifically, the 4 lots selected represented low, mid, and high %afucosylated and HM glycan. All available lots of Flixabi® (n = 8) were manufactured in the EU; the source of 5 lots of Remsima® was the EU, and the source of 1 additional lot was the Republic of Korea. Remicade® and Remsima® is produced by a SP2/0 host cell line (mouse myeloma-derived),Citation19 and Flixabi® is produced by a CHO cell line.Citation20
Peripheral blood mononuclear cells
PBMC were isolated from leukopacks that were ethically collected from healthy donors and purchased from CTL (CTL-UP1). The PBMC were frozen in CTL-CryoABC™ serum-free freezing medium. The PBMC were thawed and stabilized with RPMI-1640 medium (Gibco, 11875) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, 10082) before use.
Enriched NK cells were derived from healthy donor PBMC. PBMC were thawed in Dulbecco's modified Eagle's medium (DMEM) (Gibco, 11995) with an anti-aggregate wash supplement (CTL, CTL-AA-005). Thawed PBMCs were labeled with MACS® MicroBeads and isolated magnetically by using a MACS® separation column (Miltenyi Biotec, 130–042–201) and a human NK isolation kit (Miltenyi Biotec, 130–092–657).
Cell lines
NK92-CD16a cells (Biogen), a human NK cell line expressing CD16a (FcγRIIIa V/V), were grown in Myelocult H5100 medium (Stemcell, 5150) supplemented with 25 ng/mL IL-2 (Cell Signaling, 8907-LC) and 5 μg/mL puromycin (Sigma, T8532).
293-NFκB-luc cells (Panomics, RC0014), a human embryonic kidney cell line stably transfected with a luciferase gene downstream of NFκB binding sequences, were grown in DMEM supplemented with 10% FBS (Gibco, 10082) and 0.1 mg/mL hygromycin B (Roche, 10843555001).
3T3-mTNF cells (Biogen), a mouse embryonic fibroblast cell line that overexpresses human membrane TNF-α on the cell surface, were grown in DMEM supplemented with 10% FBS (Gibco, 10082) and 0.5 mg/mL Geneticin® (Gibco, 10131).
Quality attributes related to Fc function
Glycosylation profiling by 2-aminobenzamide (2-AB) labeling and hydrophilic interaction chromatography-ultra-performance liquid chromatography analysis
Infliximab samples (100 µg) were denatured in sodium dodecyl sulfate and dithiothreitol and treated with PNGase F for about 18 hours to release N-glycan moieties. Released N-glycan was precipitated in cold ethanol and dried. The precipitated N-glycan fraction was then labeled with 2-AB for 3 hours. Samples were injected onto an ultra-performance liquid chromatography ethylene bridged hybrid (UPLC BEH) column (2.1 mm × 150 mm, 1.7 μm). Labeled N-glycan was separated at a flow rate of 0.5 mL/min with mobile phase A (50 mM ammonium formate) and mobile phase B (100% acetonitrile). The signal was detected by a fluorescence detector at an excitation wavelength of 330 nm and an emission wavelength of 420 nm. Relative quantity of N-glycan species (galactosylated, afucosylated, HM, and sialylated [charged]) was determined. Statistical analysis of glycan species was performed using Student's t test with 95% confidence level.
FcγRIIIa binding assay
Infliximab sample binding activity of the Fc region to FcγRIIIa was assessed with FACS, SPR, and AlphaScreen-based binding assay.
Flow cytometry analysis
FACS analysis was used to measure the binding of infliximab samples to FcγRIIIa (158V/F) on NK cells from healthy donor PBMCs. The only available PBMC lots were 158V/F; genotyping and screening were conducted using multiple lots of PMBC before test method establishment. The available lots showed enough activity to set up the assays. Infliximab samples (4 µg/mL) were incubated with enriched NK cells. After incubation, the mixtures were centrifuged, remaining samples were washed using FACS buffer (phosphate-buffered saline supplemented with 1% bovine serum albumin), and then phycoerythrin (PE)-conjugated anti-human IgG Fc secondary antibody (Jackson ImmunoResearch, 109–116–098) were added. After the staining, the binding of the infliximab samples to FcγRIIIa on the surface of NK cells was measured using Flow cytometry (BD Biosciences, 65115). Binding activity relative to reference standard (EU-licensed Remicade®) was calculated using the PE-A value of each sample. Statistical analysis was performed using the Student's t test with 95% confidence interval (Minitab Software, ver. 17).
SPR analysis
Binding affinity of infliximab samples were measured using Biacore T100 (GE Healthcare, T100). Purified FcγRIIIa (158V/V) (R&D Systems, 4325-FC) was immobilized on a CM5 sensor chip (GE Healthcare, BR-1005–30) using N-hydroxysuccinimide/N-ethyl-N’-(-3-dimethylamino-propyl) carbodiimide (NHS/EDC) (GE Healthcare, BR-1000–50). Infliximab samples were prepared with various concentrations (2-fold 7-point dilution, 4000 nM to 62.5 nM) in HBS-EP buffer (GE Healthcare, BR-1001–88) and injected into the flow cell for association and dissociation. The surface was regenerated by injecting the HBS-EP buffer for 30 seconds. The affinity constant (KD) was calculated from the sensorgrams using the BIA evaluation software affinity model (GE Healthcare). Results were reported as relative to the reference standard. Statistical analysis was performed using the Student's t test with 95% confidence level (Minitab Software, ver. 17).
AlphaScreen analysis
AlphaScreen is a technology that uses hydrogel-coated donor and acceptor beads to provide functional groups for conjugation to biomolecules. In this assay, the acceptor beads were coated with human IgG1 mAb (infliximab), and donor beads were coated with reduced glutathione (GSH) (PerkinElmer, 6765301). FcγRIIIa (158V/V), manufactured and purified by Biogen, was tagged with glutathione-S-transferase (GST). Unlabeled samples competed with human IgG1-acceptor bead for the opportunity to bind to GST-FcγRIIIa. Infliximab samples were prepared as an 8-point, 1:3 dilution series (40 µg/mL to 0.0183 µg/mL). All 3 components were mixed with infliximab samples in an assay plate, and the plate was incubated at 22°C for 2 hours with mild agitation. Luminescence signals were measured by a microplate reader (EnVision multilabel reader, PerkinElmer, 2104).
Laser excitation at 680 nm of a photosensitizer present on the donor bead results in the conversion of ambient oxygen to a more excited singlet state. The singlet-state oxygen molecules diffuse and react with a thioxene derivative on the acceptor bead, leading to chemiluminescence at a wavelength of 370 nm. This further activates fluorophores contained on the same bead. The fluorophores subsequently emit light at wavelengths of 520-620 nm. The luminescent signal is generated only when the 3 components (human IgG1-conjugated acceptor bead, GST-tagged FcγRIIIa, and GSH-coated donor bead) bind to each other. Therefore, the measured luminescence is inversely proportional to the FcγRIIIa binding activity of the mAbs. Results are reported relative to the reference standard. Statistical analysis was performed using the Student's t test with 95% confidence level (Minitab Software, ver. 17).
ADCC assay
An ADCC assay was performed with 3T3mTNF cells as a target cell. Two kinds of cells were used as effector cells; healthy donor PBMCs (FcγRIIIa 158V/F) and a NK92-CD16a cell line (FcγRIIIa 158V/V). CD16 surface expression level of these 2 effector cells was assessed using flow cytometry because ADCC activity is correlated with the surface expression level of CD16 on effector cells.Citation6 Ten million (1 × 106) cells (NK92-CD16a and PBMCs) were separately prepared and incubated for 30 minutes at 2-8°C with human normal IgG to block non-specific binding sites. After incubation, 3 detection antibodies (anti-human CD3-PerCP [mouse BALB/c IgG1, κ] (BD PharMingen, 347344), anti-human CD56-PE [mouse IgG1, κ] (BD PharMingen, 561903), and anti-human CD16-APC [mouse BALB/c IgG1, κ] (BD PharMingen, 561248) were diluted to assay working concentration with FACS buffer (phosphate-buffered saline supplemented with 1% bovine serum albumin) and incubated with each cell for an additional 30 minutes. After staining, tubes were centrifuged, the supernatant was discarded, and cells were washed twice with 500 μL FACS buffer. Fluorescence-minus-one controls were set up for standardization. To assess ADCC activity, the 3T3mTNF cells were treated first with an infliximab sample and second with an effector cell sample before incubation at 37°C. The cell plate was incubated with a luminogenic peptide substrate (alanyl-alanyl-phenylalanyl-aminoluciferin; AAF-Glo Substrate, Promega, G9292), and the resulting signal was quantified with a luminometer. Results were reported as relative value to the reference standard. For NK92-CD16a cells, results were analyzed based on parallel line analysis. For PBMCs, results were analyzed by percent cell lysis. Statistical analysis was performed using the Student's t test with 95% confidence level (Minitab Software, ver. 17).
Quality attributes related to fab function
TNF-α neutralization assay (NFκB-luc reporter gene assay)
TNF-α neutralization activity of infliximab samples was measured by an assay using a luciferase reporter gene system. The assay used a 293-NFκB-luc stable reporter cell line (Panomics, RC0014) that was engineered to contain regulatory elements upstream of a luciferase reporter gene. Mediated by TNF-α binding to its receptor, activation of the regulated gene resulted in expression of the luciferase reporter gene. TNF-α (NIBSC, 12/154) was mixed with the infliximab sample and then incubated at room temperature for 20 to 40 minutes in a 96-well tissue culture plate. After incubation, the 293-NFκB-luc cells were transferred to each well of the 96-well tissue culture plate. The mixture was incubated at 37°C for 24 hours. Luciferase activity was measured by the Steady-Glo Luciferase Assay System (Promega, E2520) after 24 hours of incubation. The resulting signal was quantified with a luminometer. Measured luminescence was inversely proportional to the level of TNF-α neutralization. Results were analyzed based on parallel line analysis and reported as relative value to the reference standard. Statistical analysis was performed using the Student's t test with 95% confidence level (Minitab Software, ver. 17).
TNF-α binding assay using fluorescence resonance energy transfer
Fab-related binding activity of infliximab samples to TNF-α was determined using a fluorescence resonance energy transfer (FRET)-based competitive binding assay. Infliximab samples were labeled with fluorescent Europium chelate and TNF-α was labeled with Cy5 fluorophore. As a result of Europium-labeled infliximab binding to Cy5-labeled TNF-α, a fluorescence signal was generated. Unlabeled infliximab sample competed against Europium-labeled infliximab for binding to Cy5-labeled TNF-α. Measured fluorescence was inversely proportional to the binding of unlabeled sample. Samples were prepared as a dilution series in an assay plate. Fixed concentrations and volumes of Europium-labeled infliximab and Cy5-labeled TNF-α were added to the assay plate and incubated at ambient temperature with moderate agitation. The plate was read by a microplate reader using time-resolved fluorimetry (EnVision® multilabel reader, PerkinElmer, 2104), and relative binding activity was calculated by parallel line analysis. The results were reported as relative value to the reference standard. Statistical analysis was performed using the Student's t test with 95% confidence level (Minitab Software, ver. 17).
Disclosure of potential conflicts of interest
Changsoo Lee, Min Jeong, JongAh Joanne Lee, Saebom Seo, and Sung Chun Cho are employees of Samsung Bioepis Co., Ltd., which funded the study. Wei Zhang and Orlando Jaquez are employees of Biogen.
Acknowledgments
We would like to thank Zoran Sosic, from Biogen, Inc., and Michael Flores from Samsung Bioepis Co., Ltd. for providing valuable insights and reviewing this manuscript. We also thank Laurel Riemann, PharmD, BCPS and Julia C. Jones, PharmD, PhD, MWC™, of Med Communications, Inc., for their writing and editing assistance.
Funding
This work was funded by Samsung Bioepis Co., Ltd.
References
- European Medicines Agency. REMICADE® (infliximab). European Union: London, UK; 2016 Nov 21 [accessed 2017 March 8]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000240/human_med_001023.jsp&mid=WC0b01ac058001d124
- Janssen Biotech. REMICADE® (infliximab). Titusville, NJ: Janssen Immunology; 2016 December [accessed 2017 March 8]. http://www.remicade.com
- Public Health Services Act. 42 U.S.C. § 262(k)(2)(A)(i)(I)
- Reusch D, Tejada ML. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015; 25:1325-34; PMID:26263923; https://doi.org/10.1093/glycob/cwv065
- Chung S, Quarmby V, Gao X, Ying Y, Lin L, Reed C, Fong C, Lau W, Qiu ZJ, Shen A, et al. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcγ receptor binding and antibody-dependent cell-mediated cytotoxicity activities. MAbs 2012; 4:326-40; PMID:22531441; https://doi.org/10.4161/mabs.19941
- Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, Modica M, Cao Y, Manning RJ, Leleu X, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the FcγRIIIa-158 V/V and V/F polymorphism. Blood 2007; 110(7):2561-4; PMID:17475906; https://doi.org/10.1182/blood-2007-01-070656
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol 2008; 20:471-8; PMID:18606225; https://doi.org/10.1016/j.coi.2008.06.007
- Routier FH, Davies MJ, Bergemann K, Hounsell EF. The glycosylation pattern of humanized IgGI antibody (D1.3) expressed in CHO cells. Glycoconj J 1997; 14:201-7; PMID:9111137; https://doi.org/10.1023/A:1018589704981
- Iida S, Kuni-Kamochi R, Mori K, Misaka H, Inoue M, Okazaki A, Shitara K, Satoh M. Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer 2009; 9:58; PMID:19226457; https://doi.org/10.1186/1471-2407-9-58
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, Culm-Merdek K, Park A, Pan C, Edmunds T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng 2008; 99:652-65; PMID:17680659; https://doi.org/10.1002/bit.21598
- Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol 2007; 44:1524-34; PMID:17045339; https://doi.org/10.1016/j.molimm.2006.09.005
- Raju TS, Jordan RE. Galactosylation variations in marketed therapeutic antibodies. MAbs 2012; 4:385-391; PMID:22531450; https://doi.org/10.4161/mabs.19868
- Butler M, Spearman M. The choice of mammalian cell host and possibilities for glycosylation engineering. Curr Opin Immunol 2014; 30:107-12; PMID:25005678; https://doi.org/10.1016/j.copbio.2014.06.010
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277:26733-40; PMID:11986321; https://doi.org/10.1074/jbc.M202069200
- Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, Nakamura K, Shitara K. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res 2005; 11:2327-36; PMID:15788684; https://doi.org/10.1158/1078-0432.CCR-04-2263
- Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods 2005; 306:151-60; PMID:16219319; https://doi.org/10.1016/j.jim.2005.08.009
- Schnueriger A, Grau R, Sondermann P, Schreitmueller T, Marti S, Zocher M. Development of a quantitative, cell-line based assay to measure ADCC activity mediated by therapeutic antibodies. Mol Immunol 2011; 48:1512-7; PMID:21570725; https://doi.org/10.1016/j.molimm.2011.04.010
- Bergelson S. Biological methods for assessment of Fc effector function of antibodies and Fc-containing molecules [oral presentation]. Presented at: Biopharmaceuticals Emerging Best Practices Association's Fifth Annual Biological Assay Conference; 2012 September 27; Lisbon, Portugal. [accessed 2017 March 8]. http://www.bebpa.org/wp-content/uploads/2012/11/Svetlana-Bergelson-BEBPA-2012-final.pdf
- Jung SK, Lee KH, Jeon JW, Lee JW, Kwon BO, Kim YJ, Bae JS, Kim DI, Lee SY, Chang SJ. Physicochemical characterization of Remsima. MAbs 2014; 6(5):1163-77; PMID:25517302; https://doi.org/10.4161/mabs.32221
- Hong J, Lee Y, Lee C, Eo S, Kim S, Lee N, Park J, Park S, Seo D, Jeong M, et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade® (infliximab). MAbs 2017; 9(2):364-82; PMID:28005456; https://doi.org/10.1080/19420862.2016.1264550
- FDA Briefing Document, Arthritis Advisory Committee Meeting. ABP 501-biosimilar candidate to adalimumab. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM511900.pdf
- FDA Briefing Document, Arthritis Advisory Committee Meeting. CT-P13, a proposed biosimilar to Remicade (infliximab), Celltrion (BLA 125544). https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm484860.pdf
