ABSTRACT
The pharmacokinetics (PK) of an antibody in the brain and the spinal cord is insufficiently understood, which is an obstacle to the discovery of antibody drugs that target diseases in the central nervous system. In this study, we focused on the elimination of IgG from cerebrospinal fluid (CSF) circulating in the brain and the spinal cord in rats, and, to evaluate the influence of CSF bulk flow on the clearance of IgG, also examined the PK of inulin in CSF. To monitor their concentrations in CSF, IgG and inulin were co-administered into the lateral ventricle via a catheter, and CSF was collected from the cisterna magna via another catheter time-sequentially. Blood was also obtained from the same individuals, and the concentrations of IgG and inulin in CSF and plasma were measured. The results revealed that PK parameters of IgG were similar to those of inulin; half-life and clearance of IgG were 47.0 ± 6.49 min and 29.0 ± 15.2 mL/day/kg, and those of inulin were 52.8 ± 25.4 min and 29.0 ± 13.3 mL/day/kg. Moreover, deconvolution analysis indicated that all of the IgG administered in the lateral ventricle was transferred to plasma from CSF within 24 hours. This study demonstrated that IgG in CSF was eliminated by bulk flow and transferred totally to blood circulation.
| Abbreviations | ||
| BBB | = | blood-brain barrier |
| Cmax | = | maximum concentration |
| CNS | = | central nervous system |
| CSF | = | cerebrospinal fluid |
| ELISA | = | enzyme-linked immunosorbent assay |
| ICV | = | intracerebroventricular |
| IV | = | intravenous |
| PK | = | pharmacokinetics |
| V0 | = | volume of distribution at 0 hour |
| Vdss | = | volume of distribution at steady-state |
Introduction
Antibodies for central nervous system (CNS) diseases, such as Alzheimer disease, are currently being developed, but to date none have been granted a marketing approved. Although understanding the pharmacokinetics (PK) of an antibody in the brain and the spinal cord is a key factor in realizing antibody therapeutics for CNS diseases, current knowledge of PK in the brain and the spinal cord is not sufficient.
The brain and the spinal cord are closed spaces that are separated from blood circulation by 2 barriers, the blood-brain barrier (BBB) and the blood-cerebrospinal fluid (CSF) barrier. The CSF, which is mainly generated in the choroid plexus of the lateral and third or fourth ventricles, streams through the brain and the spinal cord, from which it finally flows out.Citation1 Because this complicated physiology restricts the transfer of large molecular drugs, such as antibodies, to the brain, research on how to deliver an antibody to the brain has been actively pursued.Citation2 There are, however, few approaches for revealing the elimination of an antibody from the brain, and thus the clearance of an antibody from CSF remains unclear.
Generally, CSF bulk flow is understood to be capable of clearing water-soluble substances, such as inulin, which have low permeability and degradability from the brain. In the case of inulin, some reports indicate that inulin administered in the lateral ventricle is cleared by bulk flow from CSF to the bloodstream and finally eliminated in urine.Citation3,4 In addition, inulin has generally been used as a clearance marker in CSF in some reports.Citation5–7
In contrast, the contribution of bulk flow to the clearance of an antibody has not yet been revealed. Bergman et al. and Rubenstein et al. demonstrated the PK of IgG in rat and monkey CSF, but did not refer to the mode of elimination.Citation8,9 Although Fujiyoshi et al. showed the elimination from CSF of a pathogenic protein,Citation7 there are so far no reports regarding the contribution of bulk flow to the disappearance of an antibody from CSF.
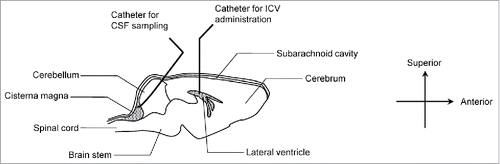
In this study, we focused on CSF in the brain and the spinal cord as a mechanism for elimination, and developed experimental methods that allow for compounds to be administered into the lateral ventricle, which generates the uppermost stream of CSF, and for CSF to be collected from the cisterna magna in a time-sequential manner from each rat (). The PK in CSF of a humanized anti-human interleukin-6 receptor IgG1 antibody referred to herein as IgG, in which the Fab does not bind to rat antigens and the Fc is wild-type human Fc, was evaluated, and compared with the PK of inulin, an impermeable marker that undergoes clearance by bulk flow.Citation3,4 In addition, the transfer of IgG from CSF to blood was also investigated.
Figure 1. Schematic diagram of animal experiments in rat brain. The diagram outlines the brain structure and indicates the position of indwelling catheters used for ICV administration and CSF sampling. IgG and Inulin were co-administered into the lateral ventricle. CSF was collected from the cisterna magna, in which CSF accumulates.
Results
PK of IgG and inulin in CSF after intracerebroventricular administration in rat
The CSF concentration–time profiles of IgG and inulin administered into the lateral ventricle of a rat's brain are shown in and , respectively. In both cases, more than 90% of the compounds disappeared from CSF within 6 hours after intracerebroventricular (ICV) administration and showed a 2-phase elimination curve. and show the PK parameters obtained by non-compartment model analysis. The half-life in the initial phase, clearance, volume of distribution at steady-state (Vdss), and volume of distribution at 0 hour (V0) of IgG were 47.0 ± 6.49 min, 29.0 ± 15.2 mL/day/kg, 1.78 ± 0.285 mL/kg, and 1.34 ± 0.359 mL/kg, respectively, and those of inulin were 52.8 ± 25.4 min, 29.0 ± 13.3 mL/day/kg, 1.52 ± 0.578 mL/kg, and 1.06 ± 0.421 mL/kg. All PK parameters of IgG and inulin were similar to each other, which suggests that IgG administered in CSF was cleared by bulk flow.
Figure 2. Concentration–time profiles of (A) IgG and (B) inulin in CSF after ICV administration. IgG and inulin were co-administered into the rat's lateral ventricle, and CSF was collected from the cisterna magna time-sequentially. The concentrations of IgG and inulin were measured by ligand binding assay using Gyrolab and ELISA, respectively. Each point with a vertical bar represents mean ± SD (n = 3)
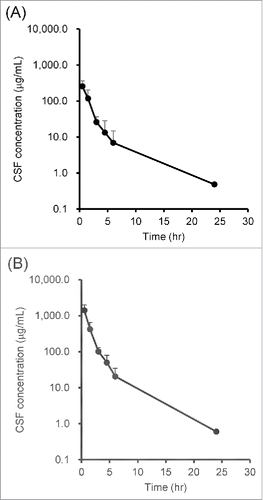
Table 1. PK parameters of IgG in CSF. CSF concentration–time profiles of IgG were analyzed with a non-compartment model. Vdss, volume of distribution at steady-state; V0, volume of distribution at 0 hour.
Table 2. PK parameters of inulin in CSF. CSF concentration–time profiles of inulin were analyzed with a non-compartment model. Vdss, volume of distribution at steady-state; V0, volume of distribution at 0 hour.
Estimation of the transfer ratio to plasma from CSF
The plasma concentration–time profiles after ICV and intravenous (IV) administration were obtained to estimate the transfer ratio from CSF to plasma. In , the plasma concentration of IgG after ICV administration at the dose of 0.5 mg/kg is shown. The plasma concentration reached Cmax (7.80 μg/mL) at 3 hours after administration, then it decreased to 5.74 μg/mL at 24 hours.
Figure 3. Plasma concentration–time profiles of IgG after (A) ICV and (B) IV administration. (A) IgG and inulin were co-administered into the rat's lateral ventricle and plasma was collected time-sequentially. (B) IgG and inulin were co-administered into the rat's tail vein, and blood was collected. The concentrations of IgG in CSF and plasma were measured by ligand binding assay. Each point represents mean ± SD (n = 3)
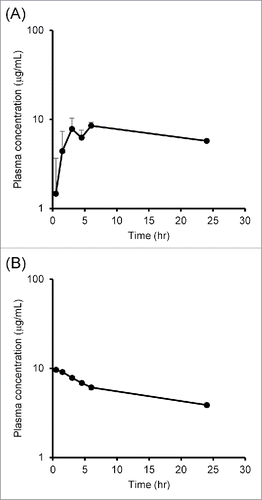
In , the plasma concentration of IgG after IV administration was plotted up to 24 hours. The concentration of IgG decreased more gradually in plasma than it did in CSF.
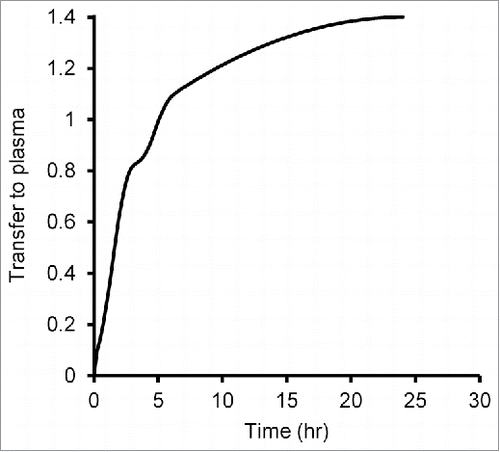
Deconvolution analysis was performed to estimate the antibody's transfer ratio from CSF to systemic circulation using the plasma concentration–time profiles of IgG after ICV () and IV () administration. As shown in , the transfer ratio from CSF to systemic circulation reached 50% at 1.5 hours and was almost complete at 24 hours, which suggests that IgG that exists in CSF is largely excreted into systemic circulation.
Figure 4. Transfer ratio from CSF to plasma calculated by deconvolution analysis. Plasma concentrations of IgG that had been administered intracerebroventricularly and systemically were used for deconvolution analysis. A weighting function that shows the relationship between time and transfer rate was determined, and then the cumulative amount of the weighting function against the dosing amount was calculated.
Discussion
The purpose of this study was to reveal the pharmacokinetic behavior of an antibody in CSF and evaluate the contribution of bulk flow to the clearance of an antibody. IgG and inulin, a marker for bulk flow clearance, were co-administered into the rat lateral ventricle and then their concentrations were measured in CSF time-sequentially.
Because there have been few reports regarding the PK of an antibody in CSF, the mechanism by which an antibody is eliminated from CSF is largely unknown. Some reports that have demonstrated clearance of an antibody and that of inulin individually show a large difference. The clearance of IgG3 and IgM in rats was reported to be 101 and 42 mL/day/kg, respectively,Citation8 whereas the clearance of inulin administered into rat CSF was reported to be either 13 or 21 mL/day/kg, depending on the report.Citation5,7 However, since each substance was evaluated separately, these data cannot be compared directly because the experimental conditions, such as the administration site, time point, and animal experimental technique, were different.
Therefore, we developed experimental methods that allow for IgG and inulin to be co-administered in the lateral ventricle, and for CSF to be collected in a time-sequential manner from the cisterna magna of each rat. Due to the difficulty of sequential sampling of CSF, generally CSF is collected using one animal per one point, which means that concentration–time profiles cannot be monitored in the same individuals. In this research, a method of time-sequential sampling made it possible to understand the concentration–time profiles of each individual, as well as the different PK in CSF between individuals.
To elucidate the contribution of bulk flow to total clearance of an antibody in CSF, standard substances that do not bind to the cellular membrane and are thus not internalized into cells and not metabolized need to be evaluated simultaneously. In this study, inulin was co-administered with IgG and, in addition to the PK of IgG, the PK of inulin as a marker for clearance of bulk flowCitation3, 4 was also evaluated, to satisfy the experimental conditions necessary to elucidate detailed information on the elimination rate of an antibody from CSF. Although the PK in CSF was studied using 4 rats, only the data obtained from 3 rats were analyzed, because the PK parameters of one rat were rejected by the Grubbs' outlier test. In this rat, clearance of IgG and inulin (IgG 103 mL/day/kg, inulin 129 mL/day/kg) was much higher than in other animals, even though the values for clearance of IgG and inulin in the individual were similar. Additionally, the concentration of IgG and inulin at time zero in that animal (IgG 180 μg/mL, inulin 782 μg/mL) was much lower than in other animals, even though the half-life, which indicates the elimination rate, was similar between IgG and inulin (IgG 36.0 min, inulin 43.2 min) in the individual. The similarity in values for IgG and inulin within the individual suggests that IgG was eliminated by bulk flow, and it is likely that some administration procedure caused lower concentration at time zero and larger clearance than in the others.
The PK described after co-administration of IgG and inulin in CSF suggests that IgG is mainly cleared by bulk flow and any contribution to clearance by another route might be minor. The similarity in the results for the concentration–time profiles of IgG and inulin ( and ) and the PK parameters obtained by non-compartmental analysis ( and ) indicate that, similarly to inulin, IgG is eliminated predominantly by bulk flow with minor contribution by other routes, such as enzymatic degradation or cellular uptake via transporters and Fc receptors. Although it is reported that the neonatal Fc receptor plays an important role in the efflux of IgG from the brain parenchyma,Citation10,11 it is difficult to compare our results with these reported results, simply because these studies focused on a different compartment. To elucidate the contribution of other routes in CSF, such as receptor-mediated transcytosis, further detailed studies are needed.
As for Vdss, the levels for IgG and inulin were similar, which indicates that IgG was not bound and internalized into cells, but instead diffused mainly into CSF and in a minor way to another sub-compartment, such as the brain compartment. Whereas the values for V0 of IgG (1.34 ± 0.359 mL/kg) and inulin (1.06 ± 0.421 mL/kg) are equivalent to about 300 μL/body, the values for Vdss of IgG (1.78 ± 0.285 mL/kg) and inulin (1.52 ± 0.578 mL/kg) are both equivalent to about 400 μL/body. The volume of CSF in rat has been variously reported as 156 μL/body (Fischer 344 rat, 3 months old),Citation12 325 μL/body (WKY rat, 3–4 months), and 240 μL/body (SHR rat, 3–4 months),Citation13 and the obtained data for V0 in this study were similar to these reported results. The difference in V0 and Vdss in this study suggests that substances administered in the lateral ventricle become diffused in the CSF compartment first, and then in another sub-compartment gradually.
In addition, the destination of IgG from CSF was investigated in a deconvolution analysis, in which the cumulative value for time-integration of the transfer ratio based on the IV and ICV concentration data sets was calculated. The analysis revealed that IgG was totally transferred to blood (). This is the first report to show the PK of an antibody in plasma after CSF elimination. CSF intersects with venous blood and lymph at a venous plexus,Citation1 so IgG might be eliminated from CSF at these points by bulk flow and transferred to systemic circulation. As for inulin, the assay sensitivity in this study was not sufficient to measure the inulin concentration for a deconvolution analysis but, as noted earlier, inulin is commonly reported as a clearance marker and is reported to be eliminated by bulk flow from CSF to the bloodstream and finally eliminated in urine after ICV administration.Citation3–7
After considering the above results comprehensively, we described the diffusion and elimination of an IgG antibody in the model shown in . The diffusion rate between CSF and brain tissue was reported to be slow,Citation14 which suggests that an antibody in CSF is not easily transferred to the brain. Thus, an antibody is mainly eliminated from CSF by bulk flow, and is finally totally transferred from CSF to plasma.
Figure 5. A model of the elimination of an antibody from CSF. Transfer clearance and diffusion rate (shown by dotted arrows) of an antibody are generally small; on the other hand, clearance via bulk flow (shown by a solid large arrow) is large.
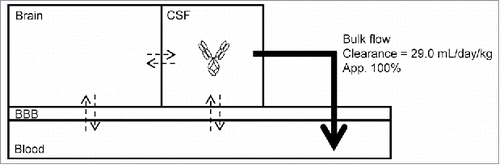
For the past decade, research has endeavored to discover compounds that can be delivered to targets in the brain by systemic administration and to evaluate the amount of transfer to the brain. Although the rates of transfer to the brain of various IgG2a antibodies in mice and rat have been reported to be much lower than the elimination clearance from CSF,Citation15,16 the effect of systemic administration needs to consider the rate of diffusion from brain to CSF and also the amount of an antibody returned directly back to blood, either by Fc receptors or receptors across the BBB, or by transcytosis. If the elimination and diffusion process from the brain is revealed in detail, the model described in may make it possible to estimate transfer clearance by measuring concentrations in CSF when an antibody is administered systemically. On these points, further studies are needed to reveal the elimination pathway in the brain because the restricted transfer of therapeutic substances to the brain would have important implications for delivery technology.
Moreover, the results in this study may be helpful for predicting antibody PK in human after deeper understanding of the clearance and diffusion from the brain is achieved. Johanson et al. reported that bulk flow clearance in human was 10 mL/day/kg.Citation1 Assuming that the mechanism of elimination from the brain does not differ across species, the model could be used to predict the concentration of an antibody in human CSF, if the results for transfer clearance are obtained in an in vitro cell-based assay and some animal experiments. Also, because CSF can be collected in clinical settings, it might be possible to estimate transfer clearance in human when the concentration in CSF has been found. However, because the estimation of transfer clearance in human is not perfect, further studies using various in vitro and in vivo methods are required.
In summary, we demonstrated that IgG was eliminated from rat CSF by bulk flow at a half-life of 47.0 ± 6.49 min and clearance of 29.0 ± 15.2 mL/day/kg, and that the eliminated IgG was totally transferred from CSF into blood circulation within 24 hours after ICV dosing.
Materials and methods
Reagents
The following materials were purchased: INULEAD®inj. (inulin, Fuji Yakuhin, #877225), Actemra® (tocilizumab, Chugai Pharmaceutical, #876399), FIT-GFR™ Kit INULIN (BioPAL, #FIT-0415), an anti-human capture antibody and a detection antibody (Antibody Solutions, #AS75-P and Southern Biotech, #9040–01), and heparin sodium for injection (Mochida Pharmaceutical, #873334). Other reagents were purchased from local commercial sources.
Animals
Crl:CD(SD) (10 weeks, female) rats were purchased from Charles River Laboratories, Japan.
Animal experiments
All animal experiments in this study were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Chugai Pharmaceutical Co., Ltd, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
PK study of IgG and inulin in rats
To administer the drug solutions, a catheter was placed into the rat's lateral ventricle, while the rat was anesthetized with isoflurane throughout the following procedure.
After an incision was made on the top of the rat's head, the head was drilled and a guide cannula 4 mm long and 0.46 mm in outer diameter (Bioresearch Center Corp., #C315GA/SPC) was set into the lateral ventricle (0.7 mm toward the cervical region from the bregma, 1.4 mm to the right side of the bregma, and 4 mm deep from the skull; see ), into which the internal cannula with an outer diameter of 0.2 mm (Bioresearch Center Corp., #C315LI/SPC) was inserted. Through this internal catheter, IgG (0.5 mg/kg) and inulin (2.5 mg/kg) were co-administered into the lateral ventricle at the volume of 50 μL/kg. Drug solution was prepared by mixing IgG and inulin with phosphate-buffered saline that included Tween80. Before and after dosing the cannula was stopped with a dummy cannula with an outer diameter of 0.2 mm (Bioresearch Center Corp., #C315DC/SPC) to prevent leakage.
To collect CSF time-sequentially, a hole was drilled in the center between the lambda and the side of the occipital bone. A catheter with an outer diameter of 0.61 mm (Becton, Dickinson and Company, #427401) was set through this hole into the cisterna magna via the cerebellum. During the experiment a cap was always fitted into the catheter. When CSF was collected, the cap was removed and a drop of CSF was collected in a tube. Consistently about 10 μL of CSF could be sampled at 30 min, 1.5 h, 3 h, 4.5 h, 6 h, and 24 h. In parallel with CSF collection, blood was obtained from the same individuals at the same time points.
The PK of IgG in plasma was evaluated by administering 0.5 mg/kg IgG in the rat tail vein. The administered volume was 10 mL/kg. At each time point, about 40 μL of blood was collected from the cervical vein and mixed with heparin sodium. Plasma was obtained by centrifugation of blood.
Measurement of IgG in samples by Gyrolab
IgG in CSF and plasma samples was measured in a sandwich ligand binding assay format using Gyrolab xP workstation (GE Healthcare, England), basically following the Gyrolab automated standard protocol. In this protocol, biotin-labeled anti-human IgG antibody at the concentration of 25 μg/mL was applied to a streptavidin-coated Gyrolab Bioaffy Disc 200 (GE Healthcare, #P0004180). The CSF and plasma samples were diluted 40-fold and used in duplicate, and finally Alexa Fluor 647-labeled anti-human Fc antibody at the concentration of 25 μg/mL was added for detection. Rexxip F and Rexxip A buffers (GE Healthcare, #P0004825 and #P0004820) were used to dilute the reagent antibodies and collected samples. Obtained signals were analyzed with Gyrolab viewer software.
Measurement of inulin in samples by enzyme-linked immunosorbent assay
Inulin was quantified with FIT-GFR™ Kit INULIN (BioPAL, #FIT-0415) using the attached protocol. The absorbance was measured by SpectraMax M2 plate reader (Molecular Devices, California).
PK analysis
PK data were analyzed using Phoenix 64 WinNonlin 6.4 (Ver. 6.4.0.768, Pharsight). Non-compartmental analysis was performed to obtain the following parameters: half-life in initial phase, area under the curve (AUC), clearance, Vdss, and concentration at 0 hour (C0). V0 was calculated using dose and C0. Weighting was performed as 1/y2.
Deconvolution analysis was performed using plasma concentration data obtained after IV and ICV administrations. The plasma concentration–time profile after IV administration was analyzed by the 2-compartmental method, and 2-compartmental PK parameters were obtained. The mean plasma concentration–time profiles after IV and ICV administration were taken as the output function and the input function, respectively. Using these PK parameters and functions, a weighting function was calculated by the deconvolution method, and its time-integration generated the bioavailability from CSF to plasma. In the analysis using WinNonlin software, the deconvolution analysis command was used, and finally the cumulative amount and ratio from CSF to plasma were obtained. The exponential terms and smoothing method were set as 2 and automatic, respectively.
Disclosure of potential conflicts of interest
All authors are employees of Chugai Pharmaceutical Co., Ltd.
Acknowledgments
The authors would like to thank Ms. Takako Sakamoto and her coworkers at Chugai Research Institute for Medical Science for their assistance in animal experiments. We also thank Ms. Sally Matsuura of Chugai Pharmaceutical for assistance in the writing of this paper.
References
- Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res 2008; 5:10; PMID:18479516; https://doi.org/10.1186/1743-8454-5-10
- Pardridge WM. Targeted delivery of protein and gene medicines through the blood-brain barrier. Clin Pharmacol Therapeutics 2015; 97:347–61; PMID:25669455; https://doi.org/10.1002/cpt.18
- Bass NH, Lundborg P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-(14 C)inulin after intrathecal infusion. Brain Res 1973; 52:323–32; PMID:4739806; https://doi.org/10.1016/0006-8993(73)90668-9
- Vladic A, Klarica M, Bulat M. Dynamics of distribution of 3H-inulin between the cerebrospinal fluid compartments. Brain Res 2009; 1248:127–35; PMID:19007752; https://doi.org/10.1016/j.brainres.2008.10.044
- Wu X, Hui AC, Giacomini KM. Formycin B elimination from the cerebrospinal fluid of the rat. Pharmaceutical Res 1993; 10:611–5; PMID:8483848; https://doi.org/10.1023/A:1018970607728
- Ito S, Ueno T, Ohtsuki S, Terasaki T. Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-beta peptide(1-40) in mouse: involvement of an LRP-1-independent pathway. J Neurochem 2010; 113:1356–63; PMID:20367755; https://doi.org/10.1111/j.1471-4159.2010.06708.x
- Fujiyoshi M, Tachikawa M, Ohtsuki S, Ito S, Uchida Y, Akanuma S, Kamiie J, Hashimoto T, Hosoya K, Iwatsubo T, et al. Amyloid-beta peptide(1-40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood-cerebrospinal fluid barrier. J Neurochem 2011; 118:407–15; PMID:21585370; https://doi.org/10.1111/j.1471-4159.2011.07311.x
- Bergman I, Burckart GJ, Pohl CR, Venkataramanan R, Barmada MA, Griffin JA, Cheung NK. Pharmacokinetics of IgG and IgM anti-ganglioside antibodies in rats and monkeys after intrathecal administration. J Pharmacol Exp Therapeutics 1998; 284:111–5; PMID:9435168
- Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 2003; 101:466–8; PMID:12393404; https://doi.org/10.1182/blood-2002-06-1636
- Cooper PR, Ciambrone GJ, Kliwinski CM, Maze E, Johnson L, Li Q, Feng Y, Hornby PJ. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res 2013; 1534:13–21; PMID:23978455; https://doi.org/10.1016/j.brainres.2013.08.035
- Caram-Salas N, Boileau E, Farrington GK, Garber E, Brunette E, Abulrob A, Stanimirovic D. In vitro and in vivo methods for assessing FcRn-mediated reverse transcytosis across the blood-brain barrier. Methods Mol Biol (Clifton, NJ) 2011; 763:383–401; PMID:21874466; https://doi.org/10.1007/978-1-61779-191-8_26
- Preston JE. Ageing choroid plexus-cerebrospinal fluid system. Microscopy Res Technique 2001; 52:31–7; PMID:11135446; https://doi.org/10.1002/1097-0029(20010101)52:1<31::AID-JEMT5>3.0.CO;2-T
- Al-Sarraf H, Philip L. Effect of hypertension on the integrity of blood brain and blood CSF barriers, cerebral blood flow and CSF secretion in the rat. Brain Res 2003; 975:179–88; PMID:12763606; https://doi.org/10.1016/S0006-8993(03)02632-5
- Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS 2011; 8:7; PMID:21349155; https://doi.org/10.1186/2045-8118-8-7
- Moos T, Morgan EH. Restricted transport of anti-transferrin receptor antibody (OX26) through the blood-brain barrier in the rat. J Neurochem 2001; 79:119–29; PMID:11595764; https://doi.org/10.1046/j.1471-4159.2001.00541.x
- Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Therapeutics 2000; 292:1048–52; PMID:10688622
